Key Points
NAC increases engraftment of human hematopoietic stem cells in immunodeficient mice.
Abstract
Immunocompromised mice, such as the nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, have been widely used to examine the self-renewal and differentiation potential of human hematopoietic stem cells (HSCs) in vivo. However, the efficiency of human HSC engraftment remains very low. Here, we report that NOD/SCID mice had higher levels of reactive oxygen species (ROS) in their bone marrow (BM) than other commonly used mouse strains (C57BL/6 and BALB/C). Treatment with the antioxidant N-acetyl-l-cysteine (NAC) decreased ROS levels in the BM of NOD/SCID mice. Furthermore, the NAC-treated mice displayed a significant increase in human HSC engraftment and multilineage hematopoietic differentiation in the mice. In comparison with the control mice, NAC-treated recipients displayed a 10.8-fold increase in hematopoietic engraftment in the injected tibiae. A beneficial effect of NAC for human hematopoietic engraftment was also observed in an additional immunodeficient mouse strain, namely NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD/SCID/γc−/− or NSG). Thus, this study uncovers a previously unappreciated negative effect of ROS on human stem cell engraftment in immunodeficient mice.
Introduction
Xenotransplantation into immunodeficient mice such as nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice or NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD/SCID/γc−/− or NSG) mice has been a gold standard assay for measuring human hematopoietic stem cells (HSCs) in vivo.1 To improve human HSC engraftment in immunodeficient mice, several modifications of the transplantation protocol have been made to further deplete the remaining innate immunity of the animals.2-4 In our study, we observed that NOD/SCID mice have significantly higher levels of reactive oxygen species (ROS) in bone marrow (BM) cells than other commonly used mouse strains. Given the previous studies from our laboratory5,6 and others7-9 showing that excessive ROS impaired the function of HSCs and that antioxidants were able to overcome the exhaustion of mouse HSCs in transplant recipients, we hypothesized that poor engraftment of HSCs in NOD/SCID mice could be partially attributed to higher levels of ROS in NOD/SCID BM. We used the antioxidant N-acetyl-l-cysteine (NAC) to reduce ROS levels in the NOD/SCID BM and demonstrated significant enhancement of the human cell engraftment. Therefore, lowering the levels of ROS may serve as an effective strategy to further improve the repopulation of human HSCs in NOD/SCID or other types of immunodeficient mice.
Methods
Human cord blood processing
Human cord blood (CB) samples were obtained from Tianjin Obstetric Central Hospital (Tianjin, China), under a protocol approved by the Ethical Committee on Medical Research at the Institute of Hematology. Research was conducted in accordance with the Declaration of Helsinki, with patient informed consent. Mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation. CD34+ and lineage-negative cells were enriched by using a CD34+ microbead kit and a lineage cell depletion kit following the manufacturer’s protocols (Miltenyi Biotec). Cells were stored in liquid nitrogen until use.
Xenotransplantation
Female NOD/SCID and NSG mice were purchased from the Institute of Laboratory Animals, Chinese Academy of Medical Sciences (Beijing, China) and the Jackson Laboratory (Bar Harbor, ME), respectively, and were housed in a specific-pathogen-free facility. At 6 to 8 weeks of age, mice were irradiated at a dose of 225 cGy 24 hours prior to transplantation. Freshly thawed CB CD34+ cells were counted with trypan blue prior to transplantation, resuspended in 200 μL of phosphate-buffered saline per mouse, and injected intravenously. For limiting dilution analysis (LDA), the indicated cells (CD34+ cells: 2500, 5000, 10 000, 20 000; Lin–CD34+CD38–CD45RA–CD90+CD49f+Rholow HSCs: 100, 50, 20, 10) were injected into mouse tibiae.
Statistical analysis
The data are presented as the mean ± standard error of the mean and graphed by using Prism 5.0 software (La Jolla, CA). LDA was performed by using L-Calc software (Stem Cell Technologies). Unpaired Student t test was used to generate P values for most of the data sets.
Results and discussion
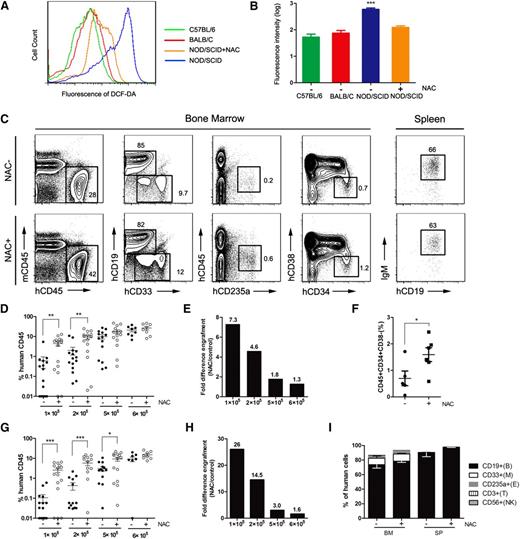
NOD/SCID mice have been widely used as a model for the enumeration of human hematopoietic repopulating cells.10 However, the engraftment of human hematopoietic cells in NOD/SCID mice is quite low, and NOD/SCID mice are not as efficient as NOD/Lt-scid/IL2Rγnull (NSG) or NOD/Shi-scid/IL2Rγnull (NOG) mice as recipients for reconstituting human HSCs because of the different immunodeficiencies among these strains.11 In this study, we detected a significant accumulation of ROS in NOD/SCID mice compared with age-matched C57BL/6 and BALB/C mice (Figure 1A). When we administered NAC into NOD/SCID mice, we observed a significant decrease of ROS (Figure 1B). Serial dilutions of CD34+ CB cells were transplanted intravenously into NOD/SCID mice, and NAC treatment significantly increased human hematopoietic cell engraftment in the BM, especially at limiting cell doses (Figure 1C-E). In particular, when 105 cells were transplanted, NAC-treated recipients had a significantly higher level of CD34+CD38– cell engraftment than the control mice (Figure 1F). When human hematopoietic cell engraftment in the spleen was evaluated, the results were similar to those in the BM (Figure 1G-H). NAC treatment had no effect on the immunophenotype of the engrafting human cells (Figure 1I).
Improved human hematopoietic cell engraftment in NOD/SCID mice by intravenous administration of NAC. NOD/SCID mice were injected with NAC or phosphate-buffered saline (control group) for 2 consecutive weeks. The NAC-injected mice also received NAC in their drinking water until the termination of the experiments. NAC-treated and untreated recipients were transplanted with human CD34+ CB cells intravenously, and human chimerism was assessed by flow cytometry in the BM and spleen. (A) Representative profile of 2′,7′-dichlorofluorescin diacetate–stained BM cells isolated from age-matched C57BL/6, BALB/C, NAC-treated, and untreated NOD/SCID mice. (B) Mean for replicates of (A) shown in BM cells (n = 10 mice per group; this analysis was consistent in 3 independent experiments). (C) Representative flow plots of human cell engraftment and multilineage differentiation in the BM and spleen of NAC-treated and untreated NOD/SCID mice engrafted with 5 × 105 CD34+ CB cells. (D,G) Mean engraftment levels of human cells in the BM and spleen of NAC-treated and untreated mice when different numbers (1 × 105, 2 × 105, 5 × 105, 6 × 105) of CD34+ CB cells were injected (for 1 × 105 and 2 × 105 cells: n = 15 mice per group, 3 independent experiments; for 5 × 105 cells: n = 14 mice per group, 3 experiments; for 6 × 105 cells: n = 8 mice per group, 2 experiments). (E,H) Bars represent the fold difference in engraftment levels between NAC-treated and untreated recipients in BM and spleen. (F) The CD34+CD38– population of the engrafting human cells (hCD45+) in NAC-treated and untreated recipient mouse BM. (I) Lineage engraftment of human cells. CD19+CD33– B cells, CD19–CD33+ myeloid cells, CD45–CD235a+ erythroid cells, CD56+ natural killer cells, and CD3+ T cells are shown. Values represent the mean ± standard error of the mean (SEM). *P < .05; **P = .01 to .001; ***P < .001. IgM, immunoglobulin M; SP, spleen.
Improved human hematopoietic cell engraftment in NOD/SCID mice by intravenous administration of NAC. NOD/SCID mice were injected with NAC or phosphate-buffered saline (control group) for 2 consecutive weeks. The NAC-injected mice also received NAC in their drinking water until the termination of the experiments. NAC-treated and untreated recipients were transplanted with human CD34+ CB cells intravenously, and human chimerism was assessed by flow cytometry in the BM and spleen. (A) Representative profile of 2′,7′-dichlorofluorescin diacetate–stained BM cells isolated from age-matched C57BL/6, BALB/C, NAC-treated, and untreated NOD/SCID mice. (B) Mean for replicates of (A) shown in BM cells (n = 10 mice per group; this analysis was consistent in 3 independent experiments). (C) Representative flow plots of human cell engraftment and multilineage differentiation in the BM and spleen of NAC-treated and untreated NOD/SCID mice engrafted with 5 × 105 CD34+ CB cells. (D,G) Mean engraftment levels of human cells in the BM and spleen of NAC-treated and untreated mice when different numbers (1 × 105, 2 × 105, 5 × 105, 6 × 105) of CD34+ CB cells were injected (for 1 × 105 and 2 × 105 cells: n = 15 mice per group, 3 independent experiments; for 5 × 105 cells: n = 14 mice per group, 3 experiments; for 6 × 105 cells: n = 8 mice per group, 2 experiments). (E,H) Bars represent the fold difference in engraftment levels between NAC-treated and untreated recipients in BM and spleen. (F) The CD34+CD38– population of the engrafting human cells (hCD45+) in NAC-treated and untreated recipient mouse BM. (I) Lineage engraftment of human cells. CD19+CD33– B cells, CD19–CD33+ myeloid cells, CD45–CD235a+ erythroid cells, CD56+ natural killer cells, and CD3+ T cells are shown. Values represent the mean ± standard error of the mean (SEM). *P < .05; **P = .01 to .001; ***P < .001. IgM, immunoglobulin M; SP, spleen.
To determine the self-renewal capacity of primary human hematopoietic cells, we performed secondary transplantation. Consistent with previous studies,12 the secondary recipients showed low levels of engraftment (supplemental Figure 1A-C available at the Blood Web site). Human cells derived from the primary mice that were treated with NAC generated higher levels of secondary engraftment than the untreated mice (supplemental Figure 1C). Engrafting human cells from 4 (36%) of 11 control mice and 9 (82%) of 11 NAC-treated primary recipient mice were able to engraft in untreated secondary recipients (supplemental Figure 1D). There was no difference in the lineage differentiation of the engrafting human cells in the secondary recipients (supplemental Figure 1E). These results suggested that NAC treatment of the primary recipients enhanced self-renewal of human HSCs and, as a result, gave rise to superior engraftment during secondary transplantation.
The functional SCID repopulation cell (SRC) assay is a quantitative measure of human HSC engraftment. We performed LDA by directly injecting human CD34+ CB cells into the right tibiae of the NOD/SCID mice. The SRC frequency in the injected tibiae (right tibiae) was approximately 3.1-fold higher in NAC-treated mice than in the control recipients (supplemental Figure 2A,D-E and supplemental Table 1). Similar increases in repopulation were detected in the noninjected bones (BM, left tibiae, 2 femurs) and spleen (supplemental Figure 2B-F).
To ascertain whether the effects of NAC treatment occurred in other immunodeficient mouse strains, we examined engraftment in NSG mice, which are more receptive to human HSCs engraftment than NOD/SCID mice, mainly because of the lack of natural killer cells in NSG mice. When 10 000 human CB CD34+ cells were transplanted into NSG mice by intratibial injection, NAC-treated recipients had 1.7-, 2.6-, and 3.5-fold higher mean engraftment in the injected tibiae, BM, and spleen, respectively (supplemental Figure 3). These increases were lower than those observed in the NOD/SCID model (3.1-, 3.9-, and 9.4-fold increases in the injected tibiae, BM, and spleen, respectively) (supplemental Figure 3C-D). Noticeably, despite NAC treatment, the engraftment level of NAC-treated NOD/SCID mice was significantly lower than that in untreated NSG mice (injected tibiae: 14.8 ± 3.1 with NAC treatment compared with 43.1 ± 21.8 for untreated mice; supplemental Figures 2A and 3B).
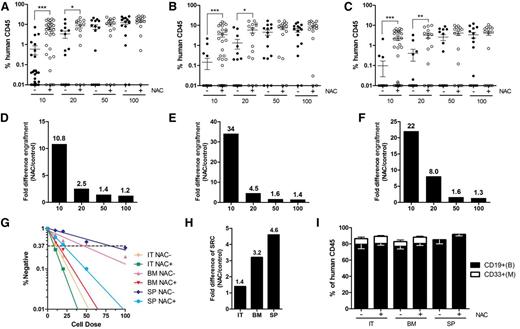
The effects of NAC treatment on highly purified human HSCs were also examined. Lin–CD34+CD38–CD45RA–CD90+CD49f+Rholow HSCs13 from human CB were transplanted into NOD/SCID mice at limiting doses (10 to 100 cells). At a dose of 10 HSCs, NAC treatment of recipient mice significantly increased engraftment in both the injected and noninjected bones, as well as in the spleen, compared with the control mice (Figure 2A-C), with fold increases of 10.8, 34, and 22, respectively (Figure 2D-F). SRC frequency, as enumerated in the injected tibiae, was approximately 1.4-fold higher in the NAC-treated NOD/SCID mice than in the control animals (Figure 2G-H and supplemental Table 2). There was no difference in multilineage differentiation of the engrafted cells between the intratibial or intravenous routes of transplantation (Figure 2I).
LDA for the engraftment of highly enriched human HSCs in NAC-treated or untreated NOD/SCID mice. NAC-treated and untreated recipients transplanted with human Lin–CD34+CD38–CD45RA–CD90+CD49f+Rholow CB cells were sacrificed 12 to 14 weeks after transplantation, and human cell chimerism was assessed by flow cytometry. (A-C) Mean human cell engraftment into the injected tibiae (IT; right tibiae), noninjected bones (BM; including left tibiae, 2 femurs) and spleen (SP) of NAC-treated or untreated NOD/SCID mice transplanted with human HSCs (for 100, 50, 20 cells: n = 15 mice per group; for 10 cells: n = 30 mice per group, 3 independent experiments with 3 CB donors). (D-F) Bars represent the fold difference in engraftment levels between NAC-treated or untreated recipients from panels A to C. (G) Frequency of human cells in NAC-treated or untreated NOD/SCID mice measured by LDA. (H) Bars represent increases in SRC frequency in the IT, BM, and SP between NAC-treated and untreated recipients. (I) Lineage potential of human cells in NAC-treated or untreated NOD/SCID recipients. Values represent the means ± SEM. *P < .05; **P = .01 to .001; ***P < .001.
LDA for the engraftment of highly enriched human HSCs in NAC-treated or untreated NOD/SCID mice. NAC-treated and untreated recipients transplanted with human Lin–CD34+CD38–CD45RA–CD90+CD49f+Rholow CB cells were sacrificed 12 to 14 weeks after transplantation, and human cell chimerism was assessed by flow cytometry. (A-C) Mean human cell engraftment into the injected tibiae (IT; right tibiae), noninjected bones (BM; including left tibiae, 2 femurs) and spleen (SP) of NAC-treated or untreated NOD/SCID mice transplanted with human HSCs (for 100, 50, 20 cells: n = 15 mice per group; for 10 cells: n = 30 mice per group, 3 independent experiments with 3 CB donors). (D-F) Bars represent the fold difference in engraftment levels between NAC-treated or untreated recipients from panels A to C. (G) Frequency of human cells in NAC-treated or untreated NOD/SCID mice measured by LDA. (H) Bars represent increases in SRC frequency in the IT, BM, and SP between NAC-treated and untreated recipients. (I) Lineage potential of human cells in NAC-treated or untreated NOD/SCID recipients. Values represent the means ± SEM. *P < .05; **P = .01 to .001; ***P < .001.
Recently, it has been reported that a single, long-term human HSC can sustain life-long hematopoiesis in an NSG mouse.13 We isolated Lin–CD34+CD38–CD45RA–CD90+CD49f+Rholow CB cells and transplanted a single cell into NOD/SCID mice. In our first and second experiments, the levels of engraftment were too low to isolate engrafted human cells (supplemental Figure 4A-B and supplemental Table 3). However, in the third experiment, significant engraftments (supplemental Figure 4C) were observed in 2 (4.4%) of 45 control mice and 4 (12.5%) of 32 NAC-treated recipients (supplemental Table 3), of which engrafted cells from 1 of the 2 control mice and 3 of the 4 NAC-treated mice were able to engraft in secondary recipients (supplemental Figure 4C). These data supported the conclusion that NAC-treated recipients exhibited superior self-renewal of human HSCs at the single-cell level.
HSCs are believed to grow in low oxygen milieu, and hypoxia plays a crucial role in regulating HSC functions.9 We evaluated the ROS levels in both the recipient BM cells and the engrafted human cells at 12 weeks after transplantation. The ROS levels in the mouse BM cells of NAC-treated recipients were significantly lower than in the control mice (supplemental Figure 5A). However, to our surprise, the ROS levels in the engrafting human cells showed only a slight reduction upon NAC treatment (supplemental Figure 5B). Therefore, NAC might increase human engraftment primarily by improving the recipient BM niche, thereby providing a better microenvironment for the survival and function of transplanted human cells. NAC treatment of the recipient animals resulted in a higher fraction of Ki67-negative engrafted human HSCs (CD34+CD38– fraction), suggesting that they are more quiescent in the cell cycle (supplemental Figure 5C-D).
In conclusion, this study showed that ROS accumulated in NOD/SCID mice and that scavenging excessive ROS with NAC enhanced human HSC engraftment in the recipients, thereby providing a new strategy for improving the efficiency of human cell transplantation in immunodeficient rodent models. As many hematological disorders with immunodeficiencies, especially following preconditioning with total body irradiation or cytotoxic agents, may result in high levels of ROS, our study also implies a potential value of some antioxidants in HSC transplantation for patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2011CB964801, 2012CB966604, 2013CB966902, 2012CB966504), the National Science Foundation of China (81090411, 81400150, 81400077, 30825017, 81170465, 81130074, 81328004, 81421002), and the National “Twelfth Five-Year” Plan for Science and Technology Support (2013BAI01B09). A.Y.-H.L. is the Li Shu Fan Medical Foundation Professor in Hematology and has received funding from its endowment.
Authorship
Contribution: L.H. performed all the experiments, analyzed data, and wrote the paper; H.C. helped with the experiments, analyzed data, and wrote the paper; Y.G. designed the research; M.S., Y.L., Z.H., and J.X. helped with the transplantation assay; A.Y.-H.L., L.Q., W.Y., and Y.-G.Y. analyzed the data; and T.C. conceived the study, designed the experiments, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tao Cheng, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing Rd, Heping District, Tianjin, No. 288, Tianjin 300020, China; e-mail: chengt@pumc.edu.cn.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal