In the current issue of Blood, Degryse and coauthors1 report the transforming potential of a series of JAK3 mutations identified in primary T-cell acute lymphoblastic leukemia (T-ALL) samples and pave the way toward multitargeted JAK1 and JAK3 therapy in T-ALL.
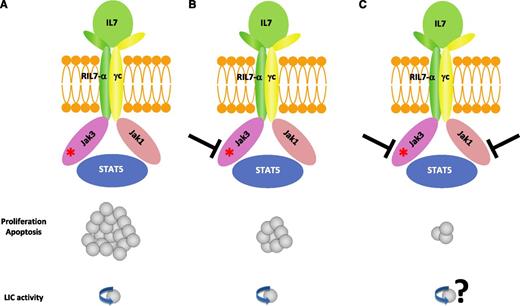
IL7R complex as a driver of T-ALL oncogenesis and a therapeutic target. (A) JAK3 mutations cause T-ALL and may or may not require JAK1 depending on whether the mutation affects the pseudokinase or the kinase domain of JAK3, respectively. JAK3 mutations require the cytokine receptor complex for transformation. (B) JAK3 inhibition can reduce the leukemic burden but could not eradicate the leukemia-initiating cells. (C) Cotargeting JAK1 and JAK3 demonstrated synergistic inhibition on the leukemic burden.
IL7R complex as a driver of T-ALL oncogenesis and a therapeutic target. (A) JAK3 mutations cause T-ALL and may or may not require JAK1 depending on whether the mutation affects the pseudokinase or the kinase domain of JAK3, respectively. JAK3 mutations require the cytokine receptor complex for transformation. (B) JAK3 inhibition can reduce the leukemic burden but could not eradicate the leukemia-initiating cells. (C) Cotargeting JAK1 and JAK3 demonstrated synergistic inhibition on the leukemic burden.
JAK3 belongs to a family of cytosolic tyrosine kinases (JAK1, JAK2, JAK3, and TYK2) that mediate cytokine and growth factors receptor signaling.2 In contrast to JAK1, JAK2, and TYK2, JAK3 is mostly restricted to the hematopoietic lineage, where it plays a major role in lymphoid development and homeostasis.3
Several JAK3 gain-of-function mutations have previously been reported in hematopoietic disorders, including T-ALL,4 but functional studies demonstrating the driver oncogenic function of these mutations in T-ALL were lacking. Degryse et al demonstrate that a majority of, but not all, JAK3 mutants transformed cytokine-dependent cell lines to cytokine-independent proliferation. Of note, only 1 of 3 JAK3 kinase domain mutations identified (L857Q) transformed cell lines in vitro. This is all the more important, as the 2 other mutations (R925S and E1106G) have been identified in 2 different patients (in the absence of other identifiable JAK mutations) and affect the potentially most important functional JAK3 domain. Using prediction protein sequence-based algorithms and sequence conservation analysis, the authors suggested that these 2 kinase domain JAK3 mutations are “passenger” and not “driver” mutations. This is consistent with other previous reports and could be expected from large-scale sequencing studies,5 because random passenger mutations occur all over the genome and as such can “accidentally” affect oncogenes such as JAK3. Oncogene alteration does not necessarily signify a leukemogenic driver, which must be determined by functional studies, as undertaken here.
Among the cytokine receptors whose signaling is mediated by the JAK kinases, interleukin 7 (IL7) plays a major role in the regulation of T-cell lymphoid differentiation and homeostasis. JAK1 and JAK3 are essential components of the heterodimeric IL7 receptor (RIL7), in which IL7Ra signals via Jak1 and the common γ chain through Jak3. Activation of the receptor upon ligand binding results in phosphorylation of both JAK1 and JAK3, with subsequent phosphorylation of STAT5, which translocates to the nucleus to regulate gene expression (see figure panel A).6 Gain-of-function mutations leading to constitutively active forms of RIL7, JAK1, and JAK3 have been reported in up to 25% of T-ALLs, primarily of early thymic phenotype (ETP-ALL).4 It has recently been shown that activating mutations in RIL7 are sufficient to generate ETP-ALL in mice and that the oncogenic cellular mechanism is a block of thymocyte differentiation at the double-negative (DN) 2 stage at which both lymphoid and myeloid developmental potential coexist.7 The in vivo data (mouse bone marrow transplant model) of JAK3-induced T-ALL presented by Degryse et al support a transforming role of mutant JAK3, with a predominant immature CD4/8 DN phenotype close to that observed in RIL7 mutant models. Of note, the authors demonstrated that the use of tofacitinib, a JAK3 inhibitor, can reduce the leukemic burden within their mouse JAK3-induced T-ALL model. Tofacitinib as a single agent inhibited leukemic cell proliferation and induced apoptosis. However, after tofacitinib was stopped, an increase in the white blood cell count and disease progression occurred, indicating that tofacitinib alone could not eradicate the leukemia initiating cells (panel B). This does not exclude the possibility that the tofacitinib inhibitory effect observed could make it a useful addition to combination chemotherapeutic regimens, particularly in relapsed patients or poor prognostic subgroups such as ETP-ALL.8 However, the use of tofacitinib, the first-in-class JAK3 inhibitor, has been hindered by undesirable pan-JAK inhibitory side effects that include anemia and neutropenia, possibly related to JAK2 inhibition. It will therefore probably be necessary to test new more selective drugs, such as the EP009 molecule recently reported to be active in T-ALL.9
A key finding from Degryse et al is that the driver JAK3 mutations require the cytokine receptor complex for transformation, because JAK1 kinase activity remains necessary to most (pseudokinase) JAK3 mutants. When the pseudokinase JAK3 mutant-transformed cells were treated by tofacitinib, ruxolitinib (a JAK1 inhibitor), or a combination of both drugs, there was clear synergistic inhibition (panel C). Conversely, the kinase domain JAK3 mutant remained independent of JAK1 inhibition. These finding indicate that different JAK3 mutations may have different signaling properties, which may affect their sensitivity to JAK kinase inhibitors. Interestingly, this study also suggests that cotargeting strategies, with concomitant inhibition of JAK1 and JAK3, may be a relevant therapeutic option in T-ALL. This concept was recently suggested in T-ALL by cotargeting FLT3 and c-Kit.10 Multitargeting of the RIL7 signaling pathway or, alternatively, RIL7 antagonist antibodies should be investigated in future preclinical and clinical studies.
Conflict-of-interest disclosure: The author declares no competing financial interests.