In this issue of Blood, Mayer et al describe a new method of generating high numbers of Batf3- and Irf8-dependent CD103+ conventional dendritic cells (cDCs), providing new opportunities to study this subset of antigen-presenting cells specialized in crosspresentation.1
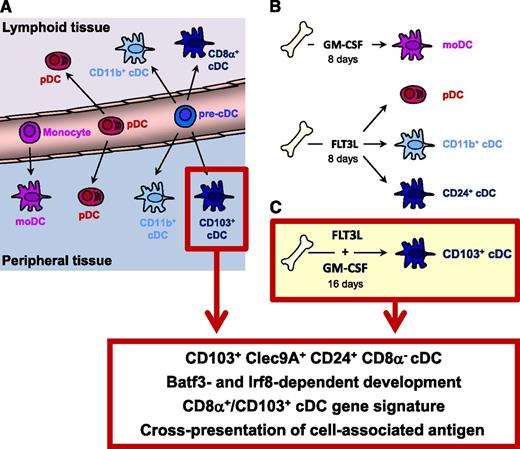
A novel culture system to efficiently generate the equivalent of peripheral tissue CD103+ cDCs from mouse bone marrow. (A) Four major DC subsets are recognized in vivo. In the steady state, plasmacytoid DCs (pDCs), CD11b+ conventional DC (cDCs) and CD8α+/CD103+ cDCs are present in lymphoid and peripheral tissues, whereas monocyte-derived DC (moDCs) are almost exclusively found in the periphery. (B) Two culture systems that are widely used to generate DC subsets from mouse bone marrow. Culture in GM-CSF produces monocyte-derived DCs. Cultures based on FLT3L yield 3 subsets: pDCs, CD11b+ cDCs, and an equivalent of the CD8α+/CD103+ cDC lineage. A drawback of this system is the limited overlap in surface markers between CD8α+/CD103+ cDCs and their in vitro equivalent. These cDCs are identified based on CD24 expression but do not express CD8α and express only very limited CD103. (C) In this issue of Blood, Mayer et al show a novel method to generate in vitro CD103+ cDCs. By 16 days of culture in the presence of FLT3L and GM-CSF, a 90% pure population of CD103+ Clec9A+ cDCs can be obtained. These cells resemble the in vivo peripheral CD103+ cDC subset based on phenotypic markers, transcription factor dependency, gene expression profile, and functional abilities.
A novel culture system to efficiently generate the equivalent of peripheral tissue CD103+ cDCs from mouse bone marrow. (A) Four major DC subsets are recognized in vivo. In the steady state, plasmacytoid DCs (pDCs), CD11b+ conventional DC (cDCs) and CD8α+/CD103+ cDCs are present in lymphoid and peripheral tissues, whereas monocyte-derived DC (moDCs) are almost exclusively found in the periphery. (B) Two culture systems that are widely used to generate DC subsets from mouse bone marrow. Culture in GM-CSF produces monocyte-derived DCs. Cultures based on FLT3L yield 3 subsets: pDCs, CD11b+ cDCs, and an equivalent of the CD8α+/CD103+ cDC lineage. A drawback of this system is the limited overlap in surface markers between CD8α+/CD103+ cDCs and their in vitro equivalent. These cDCs are identified based on CD24 expression but do not express CD8α and express only very limited CD103. (C) In this issue of Blood, Mayer et al show a novel method to generate in vitro CD103+ cDCs. By 16 days of culture in the presence of FLT3L and GM-CSF, a 90% pure population of CD103+ Clec9A+ cDCs can be obtained. These cells resemble the in vivo peripheral CD103+ cDC subset based on phenotypic markers, transcription factor dependency, gene expression profile, and functional abilities.
DCs are a heterogeneous population of hematopoietic cells with an important function in the induction and regulation of immunity. Being relatively short-lived, DCs are continuously replaced from bone marrow progenitors that are now precisely defined. Originally described as a single population, DCs are now known to comprise a large variety of subsets that can be distinguished based on transcription factor dependency, phenotype, localization, and function.2
The many subtypes of DCs are classified into 4 major subsets found in all species studied (see figure). Maybe best known to hematologists is the monocyte-derived DC that can be easily generated in vitro from human monocytes grown in granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 for DC immunotherapy but that are also found in many inflammatory disease settings (hence their alternative name of inflammatory-type DC). Plasmacytoid DCs produce large amounts of interferon when assaulted with a viral infection and reside in lymphoid tissues and blood, and in small numbers in the periphery. Plasmacytoid DCs are poor antigen presenters. The Mayer paper deals with 2 types of cDCs. In mice, CD11b+ cDCs are found in peripheral and lymphoid tissues and play an important role in the induction of Th2 and Th17 immunity in CD4 T cells,3-5 whereas CD8α+ cDCs excel in crosspresentation to CD8 T cells, for example, in the transplantation setting. The terminology of CD8α+cDCs is confusing when you are not a DC aficionado. Indeed, the term CD8α+ cDC is mainly used to delineate the crosspresenting splenic DCs and lymph node resident DCs. In peripheral tissues, this subset lacks CD8α but expresses CD103; yet based on dependency on the transcription factors Batf3, Id2, and Irf8,6,7 both lymphoid-tissue resident CD8α+ cDCs and peripheral CD103+ cDCs belong to the same lineage of crosspresenting DCs.8 Although crosspresentation is a typical characteristic of this subset, a tolerogenic role has also been suggested.9 The human equivalent of the CD8α+/CD103+ cDC expresses BDCA3 and the Clec9A (DNGR1) receptor for dead cells.
Studying the rare population of DCs ex vivo is labor intensive because it often requires enzymatic dispersion of cells from lymphoid or peripheral tissues, followed by hours of cell sorting, potentially confounding results (eg, on gene expression). Our understanding of the origin, biology, and therapeutic application of DCs has therefore greatly benefitted from the possibility to generate DCs in vitro. Currently, widely used systems include the culture of murine bone marrow cells in medium containing GM-CSF, producing monocyte-derived DCs almost exclusively. Culture of bone marrow with the hematopoietic cytokine FLT3L yields a mixed population of pDCs and cDCs that are again not subdivided based on CD8α or CD103 but rather on expression of CD11b and CD24 (see figure) because CD8α is absent and CD103 only variably expressed on Clec9a-expressing CD11b– cDCs in these cultures. The addition of GM-CSF during the last 2 days of a FLT3L culture has been shown to increase CD103 expression, but these CD103+ DCs are Batf3-independent and thus could not formally be classified as CD103+ cDC equivalents.10 Whereas monocyte-derived DCs, plasmacytoid DCs, and CD11b+ cDCs can be generated relatively well by existing methods, a culture system efficiently generating the equivalent of CD103+ cDCs was lacking. A novel method described by Mayer et al in this issue of Blood involves an extended culture of 16 days in the presence of both GM-CSF and FLT3L. This generates almost exclusively CD103+ cDCs that resemble tissue-resident CD103+ cDCs in their phenotype, transcription factor dependency, expression of signature genes, and functionality.1 The possibility to culture these cells in relatively high numbers will support further studies of this subset focusing on lineage development, terminal differentiation, and functionality. An immediate application of this method could be the adoptive transfer of large numbers of CD103+ cDCs to finally answer the outstanding question whether CD103+ cDCs can induce tolerance. As CD103+ cDCs are mainly found in peripheral tissues, and within a subfraction of migratory DCs in lymphoid tissues, it has been exceedingly difficult to obtain sufficient amounts of these cells to do these types of adoptive transfers. Another application of this protocol will be a copious source of CD103+ cDCs to perform cell biological studies—for example, to study the pathways of antigen uptake and processing—which often require large numbers of cells. Finally, this protocol could also be very useful to obtain enough DNA to be able to do chromatin immunoprecipitation–sequencing (CHIP-seq)—for example, to detect the target genes of the lineage defining transcription factors IRF8 and Batf3. A major advance of the Mayer protocol is the Batf3 dependency of the CD103+ cDC subset. One application of the protocol that could be easily tested is the use of this method to obtain a Cre-mediated recombination of floxed alleles. The classic FLT3L culture system does not lead to deletion of floxed alleles in cDCs using the Cd11cCre DC–specific deleter strain, in contrast to the GM-CSF culture system, in which moDCs are easily targeted. The reasons for this could be the length of culture and different levels in Cre expression in the 2 culture systems. Given the fact that the Mayer protocol can keep CD103+ cDCs alive and fully differentiated for at least 16 days, there is the prospect to finally be able to target floxed alleles in CD103+ cDCs in vitro. Thus, this paper further increases the armamentarium of the DC biologist and will undoubtedly lead to new insights into the biology of crosspresenting DCs.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal