Key Points
After being killed by artesunate, malaria parasites are expelled from red cells and then these pitted red cells reenter the circulation.
When many pitted red cells are produced during therapy, their delayed clearance a few weeks later triggers hemolytic episodes.
Abstract
Patients with severe malaria treated with artesunate sometimes experience a delayed hemolytic episode. Artesunate (AS) induces pitting, a splenic process whereby dead parasites are expelled from their host erythrocytes. These once-infected erythrocytes then return to the circulation. We analyzed hematologic parameters in 123 travelers treated with AS for severe malaria. Among 60 nontransfused patients observed for more than 8 days, 13 (22%) had delayed hemolysis. The peak concentration of circulating once-infected erythrocytes was measured during the first week in 21 patients and was significantly higher in 9 patients with delayed hemolysis than in 12 with other patterns of anemia (0.30 vs 0.07; P = .0001). The threshold of 180 million once-infected erythrocytes per liter discriminated patients with delayed hemolysis with 89% sensitivity and 83% specificity. Once-infected erythrocyte morphology analyzed by using ImageStream in 4 patients showed an 8.9% reduction in their projected area, an alteration likely contributing to their shorter lifespan. Delayed clearance of infected erythrocytes spared by pitting during AS treatment is an original mechanism of hemolytic anemia. Our findings consolidate a disease framework for posttreatment anemia in malaria in which delayed hemolysis is a new entity. The early concentration of once-infected erythrocytes is a solid candidate marker to predict post-AS delayed hemolysis
Introduction
Parenteral artesunate (AS) is the first-line treatment for severe malaria worldwide.1 Compared with quinine, AS further reduces mortality resulting from severe malaria by 22.5% to 35%2,3 and induces fewer adverse cardiac events or hypoglycemic episodes.2-5 However, delayed anemic episodes were recently reported in 20% to 25% of travelers with severe malaria treated with AS,6-12 a high, and possibly overestimated, proportion. All of these patients survived, but 60% of them required transfusions.6-12 Hence, although it does not jeopardize the lifesaving effect of AS, post-AS anemia does complicate patient management. Furthermore, being currently unpredictable, this delayed post-AS hemolysis and anemia require close monitoring for all patients with severe malaria treated with AS, although it will occur in only 20% to 25% of them. It may also negatively affect the clinical utility of AS, particularly in medically underserved endemic areas, and it creates preapproval regulatory concerns in nonendemic countries. Because both of these potentialities might threaten the control of malaria-induced mortality, it is crucial to understand the mechanisms of post-AS anemia and to identify an early predictive marker to optimize patient follow-up.13
Although AS can cause reversible bone marrow depression,14 transient dyserythropoiesis probably contributes only mildly to post-AS anemia. Most episodes have included a marked extravascular hemolysis component with high lactate dehydrogenase (LDH) and low plasma haptoglobin.6-12 Conversely, intense, acute intravascular hemolysis (as in blackwater fever) has been equally infrequent in quinine- and in AS-treated patients.2,3 The search for conventional causes or markers of immune-mediated or drug-induced hemolytic anemia, such as glucose-6-phosphate dehydrogenase deficiency or the direct antiglobulin test, has been generally inconclusive so far.6,9,12 Post-AS delayed hemolysis (PADH) has attracted attention because it often occurs well after resolution of malaria-related symptoms and at least several days after complete parasite clearance.6 Erythrocyte loss during post-AS hemolysis is therefore not directly related to active infection or to the presence of parasites. Typical delayed episodes are defined by a greater than 10% decline in hemoglobin levels associated with markers of hemolysis occurring more than 7 days after the initiation of treatment.12 Their incidence is significantly higher in AS- than in quinine-treated patients.5
Because PADH is AS related,5 its mechanism may be linked to a specific effect of the artemisinin class of drugs. The rapid parasite clearing action of AS is based on pitting,15-19 a process whereby artemisinin-exposed rings are expelled from their host erythrocytes in the spleen. The erythrocytes then reseal rather than lyse.15,19 As a mechanism of microbial clearance, pitting is unique because it initially spares the host cell.20 After being pitted, once-infected erythrocytes reenter the circulation, now parasite free but with a reduced lifespan.15,17,18 Thus, although pitting initially spares ring-hosting erythrocytes, this positive effect is not sustained.18 The delayed clearance of once-infected erythrocytes would explain major features of PADH, specifically the syndrome’s delayed occurrence in parasitologically cured patients and the observed hemolytic profile.
The major goal of this study was to assess the potential link between the clearance of once-infected erythrocytes and PADH.
Materials and methods
General description of the program
This program is a prospective analysis of clinical and hematologic characteristics in patients receiving AS through an existing AS surveillance process in France.
Patient surveillance program and treatment
Intravenous AS has been available in France since May 2011 as part of a named-patient program for patients with imported severe malaria. Quinine remains available for treatment of severe malaria; it is still used according to standard procedures in approximately 40% of patients. AS (vial of 60 mg of powder and solvent; Guilin Laboratories, Shanghai, China) was stocked and then released in real time by pharmacists in hospitals that participated in the named-patient program upon receipt of forms completed by attending physicians that confirmed the presence of at least one criterion of severe malaria in patients with proven Plasmodium falciparum infection. AS was administered in the next few hours from the local stock. A post hoc control for the accuracy of the indication was performed on the next working day by the National Agency of Medicine and Health Product Safety (ANSM [L’Agence nationale de sécurité du médicament et des produits de santé]) based on the form filled out by the attending physician.21 Forms, data, and samples from all patients were collected in the setting of an observational program (called here the “named-patient program”) implemented by the National Reference Center for Malaria (CNR [Centre National de Référence]), on the behalf of the ANSM. The Ile de France II Institutional Review Board has approved this approach as a non-research process (Article L1121-1 of the French Code for Public Health) embedded in the surveillance missions of the CNR, officially empowered to collect information and biological samples (Article L1413-5 of the French Law No. 2004-806 9th of August 2004-http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000025055544&dateTexte=&categorieLien=id). Patients provided consent according to a procedure common to all French National Reference Centers (http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000810056&date%20Texte=). The analysis of pre- and posttreatment forms was completed by a proactive review of medical charts by the CNR team. Laboratory parameters were retrieved by phoning or e-mailing health care providers directly. The study was conducted in accordance with the Declaration of Helsinki.
Case definitions
Within the setting of the French AS surveillance program, we selected patients who had received both an appropriate initial clinical evaluation at day 0 and follow-up that included the analysis of two sets of laboratory parameters, both before and after day 8. Selected clinical and biological criteria relevant to the diagnosis of severe malaria (as previously defined) were retrieved.22,23 Briefly, a severe case was defined by a positive blood smear with asexual parasite forms of P falciparum associated with at least 1 of the following criteria of severity: neurologic impairment (lethargy, confusion, multiple convulsions, and/or coma [with Glasgow coma scale]),24 respiratory distress, cardiocirculatory impairment, spontaneous bleeding, purpuric lesions, macroscopic hemoglobinuria, jaundice and/or plasma bilirubin >50 μmol/L, hypoglycemia (glucose <2.2 mmol/L), acidosis (plasma bicarbonate <15 mmol/L and/or acidemia pH <7.35), hyperlactatemia (arterial lactate >1.8 mmol/L), renal impairment, anemia (hemoglobin <7 g/dL or hematocrit <20%), or hyperparasitemia (>4% parasitized red cells).
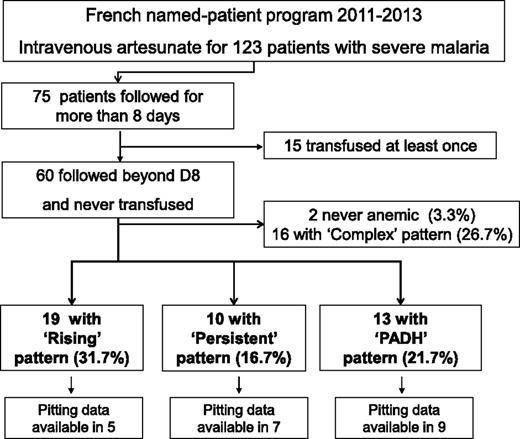
Anemia was defined as blood hemoglobin below 12 g/dL for females and 13g/dL for males. Hemolysis was defined as plasma haptoglobin below 0.1 g/L, and/or plasma LDH above 390 IU/L. For an accurate interpretation of the patterns and mechanisms of anemia, transfused patients were excluded from the analysis. We defined 4 patterns of anemia based on previous proposals including a median follow up of 28 days interquartile range (Q1-Q3 = 25 to 31).5,6,12 The “rising” pattern was defined by a nadir of hemoglobin and a peak of hemolysis occurring before day 8 without positive markers of hemolysis from day 8 to the end of follow-up. The delayed “PADH” pattern was defined by a >10% drop in hemoglobin level or a >10% rise in LDH concentrations occurring anytime between day 8 and the end of follow-up. The “persistent” pattern was defined by the presence of anemia and positive hemolysis markers both before and after day 8 and a pattern not fitting the definition of PADH patterns. All other cases of anemia that lacked information or that did not fit any of the 3 previously defined patterns so they could be strictly classified into one of them were defined as “complex” pattern. The complex group (16 patients; Figure 1) was excluded from further analyses.
Program flowchart. Named-patient program 2011-2013; 123 patients with severe malaria treated with artesunate. D, day.
Program flowchart. Named-patient program 2011-2013; 123 patients with severe malaria treated with artesunate. D, day.
Sample collection
Small blood samples from quinine- and AS-treated patients were retrieved following collection in the setting of medical care at day 0, day 2 ± 1, day 7 ± 2, day 14 ± 3, day 21 ± 3, and day 28 ± 3 per the recommendations of the French High Committee for Public Health and ANSM.21,25 These samples were routinely analyzed by attending teams for hemoglobin, reticulocyte count, total bilirubin, glucose, plasma bicarbonate, lactate, plasma creatinine, blood urea nitrogen, LDH, haptoglobin, and parasitemia.
Parasite clearance and pitting rates
Parasite clearance and pitting rates were determined for AS-treated patients and for a subset of quinine-treated patients (when available) as a control group. For homogeneity, the kinetics of parasitemia were expressed in all patients as a proportion of the initial parasitemia (number of infected erythrocytes/number of infected erythrocytes at day 0 × 100). The kinetics of once-infected erythrocytes (pitting rate) were expressed similarly, after normalization against initial parasitemia (number of once-infected erythrocytes/number of infected erythrocytes at day 0 × 100). Once-infected erythrocytes were quantified by conventional fluorescence microscopy and flow cytometry using erythrocyte membrane immunofluorescence and Hoechst staining. Immunofluorescent assay (IFA) slides were prepared as previously described.19 Briefly, erythrocytes were washed with tris(hydroxymethyl)aminomethane (Tris)–buffered Hanks solution, deposited in IFA slide wells previously coated with coating buffer, washed, fixed in a 1% glutaraldehyde solution in phosphate-buffered saline (PBS), and then frozen until use. Erythrocytes fixed in IFA wells were stained by using an African polyclonal hyperimmune serum (from Dr. David, Institute Pasteur, Paris, France) and a goat anti-human immunoglobulin G coupled with Alexa-Fluor 488 (Life Technologies, Saint Aubin, France) as a secondary antibody, as previously described, and Hoechst dye for parasite DNA detection.19,26 Images were acquired by using a Leica DMI3000 microscope and Leica camera (Leica Microsystèmes SAS, Nanterre, France). Parasitemia and concentrations of once-infected erythrocytes were also assessed by flow cytometry (Accuri C6, BD Biosciences, Le Pont de Claix, France). Briefly, samples were fixed as above with PBS and 1% glutaraldehyde and incubated with the polyclonal hyperimmune serum at 1:10 in a suspension of PBS 1% AlbuMAX II (Life Technologies) after permeation with Triton X-100 (Sigma-Aldrich). Samples were then washed and incubated with secondary antibody (goat anti-human immunoglobulin G coupled with Alexa-Fluor 568 [Life Technologies] and SYBR green [Life Technologies]) for DNA labeling before analysis. For each patient, 1 to 3 samples were collected during the first week of treatment and analyzed to assess early concentrations of once-infected erythrocytes.
Erythrocyte dimension
Fluorescence flow microscopy using ImageStream technology (Inspire v4.0 and Ideas v4.0, Amnis Corporation, Seattle, WA) was performed to determine the projected surface area of erythrocytes by using bright filter images processed with Ideas software (Ideas v4.0), as previously described.27,28 Infected, once-infected, and uninfected erythrocytes were identified by combining membrane- and DNA-staining methods as described for flow cytometry (SYBR green was replaced by Hoechst for DNA staining). Projected surface area was determined for infected, once-infected, and never-infected erythrocytes. The percentage of reduced projected surface area of infected erythrocytes or once-infected erythrocytes compared with uninfected erythrocytes from the same sample was then calculated by using the formula (1– [median values of projected surface area of infected erythrocytes or once-infected erythrocytes /median values of projected surface area of uninfected erythrocytes] × 100).
Statistical analysis
Demographic, clinical, and laboratory variables were evaluated. Quantitative variables were expressed as the median and interquartile ranges or by the mean with standard error of the mean when appropriate. Qualitative variables were expressed as percentages. Differences among the anemia patterns were analyzed with the Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables. When a significant difference was detected among the patterns, two-by-two comparisons were performed, applying the Bonferroni correction. Statistical analyses were performed by using SPSS software, version 20 for Windows. All reported P values are two-tailed.
Results
From May 2011 through June 2013, 123 patients were involved in our surveillance program, of whom 75 could be observed for more than 8 days.
PADH occurred in 21% of analyzable patients
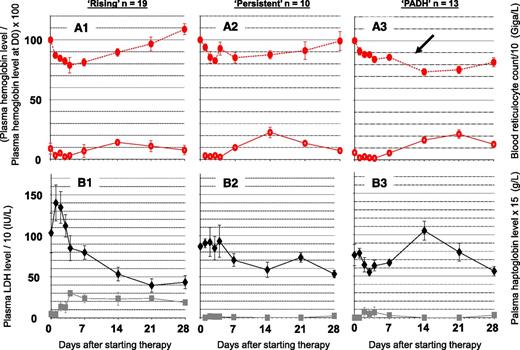
In 60 patients without transfusion and with follow-up biological data beyond day 8, the rising, persistent, and PADH patterns were observed in 32% (19 of 60), 17% (10 of 60), and 22% (13 of 60) of patients, respectively (Figure 1). The rising pattern was characterized by a 21% decline in red blood cell (RBC) hemoglobin levels with marked hemolysis until day 4. Blood reticulocyte count then increased, and RBC hemoglobin levels rose to 109% of pretreatment values at day 28 (Figure 2). The persistent pattern was characterized by a 17% decline in plasma hemoglobin levels until day 4, associated with moderate hemolysis. Beyond day 8, mean hemoglobin levels rose at a rate of 5% per week. Hemolysis persisted until day 28 (Figure 2). The PADH pattern was characterized by a 16% mean decline in hemoglobin levels until day 4 (Figure 2A3), then stabilization, and finally a 12% renewed decline between day 7 and day 14, with this drop being accompanied by relapsing hemolysis. Comparative analysis of demographic, clinical, and laboratory features in the different patterns showed that PADH patients had the highest median parasitemia on admission: 8% vs 3.9% and 2.4% for the persistent and rising anemia patterns, respectively (Table 1). The proportion of patients with hyperparasitemia was higher in the PADH group (92%; 12) than in the rising (37%; 7) or persistent (50%; 5) groups (P = .006 for Fisher exact test among the 3 groups).
Three typical patterns of posttreatment anemia in 42 nontransfused patients with severe malaria treated with AS and monitored for more than 8 days. (A1-A3). Mean and standard error of the mean (SEM; vertical bars) of (solid red circles) blood hemoglobin level and (empty red circles) blood reticulocyte count per 10 g/L on day 0, day 2 ± 1, day 7 ± 2, day 14 ± 3, day 21 ± 3, and day 28 ± 3 in subgroups of patients categorized according to their evolution pattern as (A1) rising, (A2) persistent, or (A3) PADH, as defined in the “Materials and methods” section. At least 2 samples were collected before and after day 8 in each patient. (B1-B3) Mean and SEM of (solid black diamonds) plasma LDH 10 IU/L and (solid grey squares) haptoglobin levels (×15 g/L) in the same subgroups. The blood hemoglobin level was normalized against the hemoglobin level on day 0. The delayed drop in hemoglobin levels in patients with PADH is indicated by a black arrow.
Three typical patterns of posttreatment anemia in 42 nontransfused patients with severe malaria treated with AS and monitored for more than 8 days. (A1-A3). Mean and standard error of the mean (SEM; vertical bars) of (solid red circles) blood hemoglobin level and (empty red circles) blood reticulocyte count per 10 g/L on day 0, day 2 ± 1, day 7 ± 2, day 14 ± 3, day 21 ± 3, and day 28 ± 3 in subgroups of patients categorized according to their evolution pattern as (A1) rising, (A2) persistent, or (A3) PADH, as defined in the “Materials and methods” section. At least 2 samples were collected before and after day 8 in each patient. (B1-B3) Mean and SEM of (solid black diamonds) plasma LDH 10 IU/L and (solid grey squares) haptoglobin levels (×15 g/L) in the same subgroups. The blood hemoglobin level was normalized against the hemoglobin level on day 0. The delayed drop in hemoglobin levels in patients with PADH is indicated by a black arrow.
Demographic and clinical characteristics of patients with 3 typical patterns of post-AS anemia
| Characteristic . | Rising pattern n = 19 . | Persistent pattern n = 10 . | PADH pattern n = 13 . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Male gender | 12 | 63 | 8 | 80 | 7 | 54 | .46 |
| Median (IQR) age, years | 38 (21-49) | 39 (25-47) | 42 (34-53) | .58 | |||
| Area of birth | .48 | ||||||
| Africa | 13 | 68 | 6 | 60 | 6 | 46 | |
| Europe and North America | 5 | 26 | 4 | 40 | 7 | 54 | |
| South and Central America | 1 | 5 | 0 | 0 | 0 | 0 | |
| Asia | 0 | 0 | 0 | 0 | 0 | 0 | |
| Median (IQR) time of illness before AS, days | 4 (3-5) | 4 (3-9) | 4 (3-4) | .65 | |||
| Selected severe malaria criteria at day 0 | |||||||
| Neurologic impairment (any signs) | 8 | 42 | 4 | 40 | 4 | 31 | .84 |
| At least lethargy, confusion, multiple convulsion, coma Glasgow Scale <11 | 4 | 21 | 0 | 0 | 0 | 0 | .13 |
| Respiratory distress (any signs) | 0 | 0 | 0 | 0 | 1 | 8 | .54 |
| At least respiratory frequency >32/mn, crackles, pulmonary infiltrates (chest x-ray), PaO2 <60 mmHg, SpO2 <91% | |||||||
| Cardiocirculatory impairment (any signs) | 3 | 16 | 1 | 10 | 4 | 31 | .53 |
| At least shock (systolic blood pressure ≤80 mmHg), peripheral signs of hypotension* | |||||||
| Vasopressive supportive drug | 0 | 0 | 0 | 0 | 0 | 0 | |
| Spontaneous bleeding | 0 | 0 | 0 | 0 | 0 | 0 | |
| Purpuric lesions | 0 | 0 | 0 | 0 | 0 | 0 | |
| Macroscopic hemoglobinuria | 3 | 16 | 1 | 10 | 0 | 0 | .33 |
| Jaundice and/or bilirubin >50 μmol/L | 9 | 47 | 7 | 70 | 7 | 54 | .57 |
| Hypoglycemia (glucose <2.2 mmol/L) | 0 | 0 | 0 | 0 | 1 | 8 | .54 |
| Acidosis (any signs) | 1 | 5 | 0 | 0 | 0 | 0 | 1.00 |
| At least plasma bicarbonate <15 mmol/L, acidemia pH <7.35 | |||||||
| Hyperlactatemia (arterial lactate >1.8 mmol/L) | 4 | 21 | 2 | 20 | 7 | 54 | .12 |
| Renal impairment | |||||||
| Creatinine >120 μmol/L | 3 | 16 | 0 | 0 | 2 | 15 | .57 |
| Creatinine >265 μmol/L and/or blood urea nitrogen >17 mmol/L | 2 | 11 | 0 | 0 | 0 | 0 | .49 |
| Anemia (Hb <7 g/dL or hematocrit <20%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Median (IQR) hemoglobin at day 0 (g/dL) | 11.4 (10-13) | 11.8 (11-14) | 13.6 (12-15) | .019† | |||
| Median (IQR) nadir of hemoglobin before day 8 (g/dL) | 9.0 (7-11) | 9.9 (9-12) | 11.2 (10-13) | .004† | |||
| Median (IQR) loss of hemoglobin between day 0 and the day of the nadir before day 8 (g/dL) | 2.3 (1-3) | 1.9 (1-3) | 2.3 (2-3) | .91 | |||
| Median RPI | 1.1 (1-1.4)‡ | 1.5 (1-2)|| | 1.6 (1-3)¶ | .1 | |||
| Hyperparasitemia (>4%) | 7 | 37 | 5 | 50 | 12 | 92 | .006# |
| Median (IQR) parasitemia at day 0 (% of infected red blood cells) | 2.4 (0.4-7) | 3.9 (0.4-12) | 8.0 (6-14) | .013† | |||
| Total dose of AS (mg) | |||||||
| Median (IQR) total dose | 755 (540-1020) | 844 (577-970) | 800 (603-925) | .92 | |||
| Complementary qualitative features when available | |||||||
| DAT +/DAT realized | 0 | 1 | 1** | 2 | 1 | 3†† | |
| G6PD deficiency/G6PD realized | 0 | 1 | 0 | 0 | 0 | 5 | |
| Abnormal Hb electrophoresis/Hb electrophoresis realized | 1 | 2‡‡ | 0 | 1 | 1 | 4a | |
| Characteristic . | Rising pattern n = 19 . | Persistent pattern n = 10 . | PADH pattern n = 13 . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Male gender | 12 | 63 | 8 | 80 | 7 | 54 | .46 |
| Median (IQR) age, years | 38 (21-49) | 39 (25-47) | 42 (34-53) | .58 | |||
| Area of birth | .48 | ||||||
| Africa | 13 | 68 | 6 | 60 | 6 | 46 | |
| Europe and North America | 5 | 26 | 4 | 40 | 7 | 54 | |
| South and Central America | 1 | 5 | 0 | 0 | 0 | 0 | |
| Asia | 0 | 0 | 0 | 0 | 0 | 0 | |
| Median (IQR) time of illness before AS, days | 4 (3-5) | 4 (3-9) | 4 (3-4) | .65 | |||
| Selected severe malaria criteria at day 0 | |||||||
| Neurologic impairment (any signs) | 8 | 42 | 4 | 40 | 4 | 31 | .84 |
| At least lethargy, confusion, multiple convulsion, coma Glasgow Scale <11 | 4 | 21 | 0 | 0 | 0 | 0 | .13 |
| Respiratory distress (any signs) | 0 | 0 | 0 | 0 | 1 | 8 | .54 |
| At least respiratory frequency >32/mn, crackles, pulmonary infiltrates (chest x-ray), PaO2 <60 mmHg, SpO2 <91% | |||||||
| Cardiocirculatory impairment (any signs) | 3 | 16 | 1 | 10 | 4 | 31 | .53 |
| At least shock (systolic blood pressure ≤80 mmHg), peripheral signs of hypotension* | |||||||
| Vasopressive supportive drug | 0 | 0 | 0 | 0 | 0 | 0 | |
| Spontaneous bleeding | 0 | 0 | 0 | 0 | 0 | 0 | |
| Purpuric lesions | 0 | 0 | 0 | 0 | 0 | 0 | |
| Macroscopic hemoglobinuria | 3 | 16 | 1 | 10 | 0 | 0 | .33 |
| Jaundice and/or bilirubin >50 μmol/L | 9 | 47 | 7 | 70 | 7 | 54 | .57 |
| Hypoglycemia (glucose <2.2 mmol/L) | 0 | 0 | 0 | 0 | 1 | 8 | .54 |
| Acidosis (any signs) | 1 | 5 | 0 | 0 | 0 | 0 | 1.00 |
| At least plasma bicarbonate <15 mmol/L, acidemia pH <7.35 | |||||||
| Hyperlactatemia (arterial lactate >1.8 mmol/L) | 4 | 21 | 2 | 20 | 7 | 54 | .12 |
| Renal impairment | |||||||
| Creatinine >120 μmol/L | 3 | 16 | 0 | 0 | 2 | 15 | .57 |
| Creatinine >265 μmol/L and/or blood urea nitrogen >17 mmol/L | 2 | 11 | 0 | 0 | 0 | 0 | .49 |
| Anemia (Hb <7 g/dL or hematocrit <20%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Median (IQR) hemoglobin at day 0 (g/dL) | 11.4 (10-13) | 11.8 (11-14) | 13.6 (12-15) | .019† | |||
| Median (IQR) nadir of hemoglobin before day 8 (g/dL) | 9.0 (7-11) | 9.9 (9-12) | 11.2 (10-13) | .004† | |||
| Median (IQR) loss of hemoglobin between day 0 and the day of the nadir before day 8 (g/dL) | 2.3 (1-3) | 1.9 (1-3) | 2.3 (2-3) | .91 | |||
| Median RPI | 1.1 (1-1.4)‡ | 1.5 (1-2)|| | 1.6 (1-3)¶ | .1 | |||
| Hyperparasitemia (>4%) | 7 | 37 | 5 | 50 | 12 | 92 | .006# |
| Median (IQR) parasitemia at day 0 (% of infected red blood cells) | 2.4 (0.4-7) | 3.9 (0.4-12) | 8.0 (6-14) | .013† | |||
| Total dose of AS (mg) | |||||||
| Median (IQR) total dose | 755 (540-1020) | 844 (577-970) | 800 (603-925) | .92 | |||
| Complementary qualitative features when available | |||||||
| DAT +/DAT realized | 0 | 1 | 1** | 2 | 1 | 3†† | |
| G6PD deficiency/G6PD realized | 0 | 1 | 0 | 0 | 0 | 5 | |
| Abnormal Hb electrophoresis/Hb electrophoresis realized | 1 | 2‡‡ | 0 | 1 | 1 | 4a | |
Numbers shown in bold reach significance.
DAT, direct antiglobulin test; G6PD, glucose-6-phosphate dehydrogenase; Hb, hemoglobin; Ht, hematocrit; mn, minutes; Pa02, arterial partial pressure of oxygen; RPI, reticulocyte production index, defined by [% Reticulocyte at peak × hematocrit value at nadir]/45] ×[1/Correction factor]40; SPo2, oxygen saturation.
peripheral signs of hypotension with systolic blood pressure >80 mmHg.
n = 7.
n = 6.
n = 11.
Mann-Whitney U test, between patterns Rising and PADH.
Fisher exact test.
DAT + for IgG.
DAT + for IgM.
Minor α thalassemia.
Sickle cell disease A/S hemoglobin.
Pitting rates were higher in AS-treated travelers than in quinine-treated travelers
The kinetics of once-infected erythrocytes was established in 11 quinine-treated and 16 AS-treated patients with severe malaria (Figure 3A). At day 3, the mean concentration of once-infected erythrocytes was significantly higher in AS-treated patients (78% vs 15% of initial parasitemia in AS-treated and quinine-treated patients, respectively; P = .01 for Mann-Whitney U test). A wide range of pitting rates (10% to 160% at day 3; Figure 3A) was observed in AS-treated patients.
Pitting and surface area loss. (A) Quinine- vs AS-induced parasite clearance and pitting rates. Mean and SEM for (solid circles) parasitemia and concentration of (open circles) once-infected erythrocytes (both normalized against parasitemia at day 0) in patients with severe malaria treated either with quinine (n = 11) or AS (n = 16). (B) Concentration of circulating once-infected erythrocytes (billions of cells per liter) in patients with severe malaria treated with AS for whom posttreatment evolution of anemia could be categorized as (solid red circles) PADH (9 patients) and (solid black squares) non-PADH (12 patients). (C) Population frequency of erythrocytes—uninfected erythrocytes (uEs, yellow line; C2-C3), infected erythrocytes (iEs, blue line; C2), or once-infected erythrocytes (o-iEs, orange line; C3)—according to the projected surface areas (μm2) estimated on the bright field picture after identifying erythrocyte subtype by using a combination of immunofluorescent (IF) and Hoechst staining for parasite DNA. Uninfected erythrocytes were IF- and DNA-negative, infected erythrocytes were both IF- and DNA-positive, and once-infected erythrocytes were IF-positive and DNA-negative. Typical aspects are shown in panel C1. Samples were collected from the same patient at (C2) day 0 or (C3) day 4. At least 5000 infected or once-infected erythrocytes and 100 000 never-infected erythrocytes were analyzed. (D) (open circles) Individual values and (open bars) mean projected surface of uninfected and infected erythrocytes in samples from 4 patients on day 0 and of uninfected and once-infected erythrocytes from 4 patients on day 3 ± 1. The lines connect 2 subpopulations analyzed simultaneously in the same sample. The percentages indicate the mean decrease in projected surface area between uninfected and infected erythrocytes at day 0 or between uninfected and once-infected erythrocytes at day 3 ± 1.
Pitting and surface area loss. (A) Quinine- vs AS-induced parasite clearance and pitting rates. Mean and SEM for (solid circles) parasitemia and concentration of (open circles) once-infected erythrocytes (both normalized against parasitemia at day 0) in patients with severe malaria treated either with quinine (n = 11) or AS (n = 16). (B) Concentration of circulating once-infected erythrocytes (billions of cells per liter) in patients with severe malaria treated with AS for whom posttreatment evolution of anemia could be categorized as (solid red circles) PADH (9 patients) and (solid black squares) non-PADH (12 patients). (C) Population frequency of erythrocytes—uninfected erythrocytes (uEs, yellow line; C2-C3), infected erythrocytes (iEs, blue line; C2), or once-infected erythrocytes (o-iEs, orange line; C3)—according to the projected surface areas (μm2) estimated on the bright field picture after identifying erythrocyte subtype by using a combination of immunofluorescent (IF) and Hoechst staining for parasite DNA. Uninfected erythrocytes were IF- and DNA-negative, infected erythrocytes were both IF- and DNA-positive, and once-infected erythrocytes were IF-positive and DNA-negative. Typical aspects are shown in panel C1. Samples were collected from the same patient at (C2) day 0 or (C3) day 4. At least 5000 infected or once-infected erythrocytes and 100 000 never-infected erythrocytes were analyzed. (D) (open circles) Individual values and (open bars) mean projected surface of uninfected and infected erythrocytes in samples from 4 patients on day 0 and of uninfected and once-infected erythrocytes from 4 patients on day 3 ± 1. The lines connect 2 subpopulations analyzed simultaneously in the same sample. The percentages indicate the mean decrease in projected surface area between uninfected and infected erythrocytes at day 0 or between uninfected and once-infected erythrocytes at day 3 ± 1.
PADH is related to pitting
The kinetics of once-infected erythrocytes could be established before and after day 8 in 5, 7, and 9 patients with rising, persistent, and PADH patterns of anemia, respectively (for whom at least two blood samples were received in a timely manner by the central laboratory for this specialized analysis). The number of once-infected erythrocytes peaked before day 8 in all patients: in 12 on day 2 ± 1 and in 9 on day 7 ± 2. In patients with PADH, the number of circulating once-infected erythrocytes and hemoglobin levels declined simultaneously during the second and/or third weeks after initiation of AS (Figures 2A3 and 3B). The peak pitting rate (normalized against initial parasitemia) was higher than 45% in 100% and 47% of samples from patients with PADH and non-PADH patterns of anemia, respectively (Figure 4B). During the first week after initiation of therapy, the concentration of circulating once-infected erythrocytes (in billions of cells per liter) was significantly higher in patients with PADH than in those with other patterns of anemia (0.30 vs 0.07; P = .0001 for Mann-Whitney U test). Indeed, in samples collected between day 2 and day 8, this concentration was higher than 0.18 g/L in all PADH patients except 1 (8 of 9 patients; 15 of 16 samples) and lower than this threshold in all patients with non-PADH patterns except 2 (2 of 12 patients; 2 of 19 samples) (Figure 4C). In patients with PADH, the mean peak in once-infected erythrocytes was 7.93% (range, 1.6% to 17.5%) of circulating RBCs.
Parasitemia, pitting rate, concentration of once-infected erythrocytes, and the risk of evolution toward PADH or other patterns of post-AS anemia in patients with severe malaria. (A) Individual values of parasitemia (as a percentage of infected erythrocytes) in patients with severe malaria treated with AS in whom posttreatment evolution of anemia could be categorized as rising (19 patients), persistent (10 patients), or PADH (13 patients). The dotted line corresponds to the threshold of 4% parasitemia, which provides the best discrimination between PADH and other patterns of post-AS anemia. Percentages at the top of the panel are the proportion of patients above this 4% threshold for each pattern of anemia. (B-C) Individual values of pitting rate (normalized against initial parasitemia [B]), and concentration of once-infected erythrocytes (g/L [C]) in 35 samples from 21 patients with severe malaria treated with AS in whom posttreatment evolution of anemia could be categorized as rising, persistent, or PADH. The dotted lines correspond to thresholds providing the best discrimination between PADH (9 patients) and other patterns of post-AS anemia (12 patients). Percentages at the top of the panel are the proportion of samples above this threshold of 45% pitting rate and above 0.18 billion once-infected erythrocytes per liter for each pattern of anemia. (D) Graphic representation of the relative influences of parasitemia on admission, peak pitting rate, and peak concentration of once-infected erythrocytes on the risk of evolution toward the different patterns of post-AS anemia. The concentration of circulating once-infected erythrocytes was computed by multiplying parasitemia by the pitting rate. Patients with high initial parasitemia and a high pitting rate harbor a high concentration of once-infected erythrocytes (upper right zone of the panel) and are at high risk of intense PADH in the following days or weeks. On the basis of these markers, the risk of subsequent PADH can be predicted before the end of the first week. The risk becomes significant when parasitemia on admission is above 4% and the peak pitting rate on days 2 through 8 is above 45%. On the basis of this data set (panels A-C), a concentration of circulating once-infected erythrocytes on days 2 to 8 greater than 0.18 g/L discriminates patients with PADH from patients with other patterns of anemia more effectively than do parasitemia or the peak pitting rate taken separately. This model also explains why delayed hemolysis has not been observed in severe malaria patients treated with quinine, a compound that induces low pitting rates.
Parasitemia, pitting rate, concentration of once-infected erythrocytes, and the risk of evolution toward PADH or other patterns of post-AS anemia in patients with severe malaria. (A) Individual values of parasitemia (as a percentage of infected erythrocytes) in patients with severe malaria treated with AS in whom posttreatment evolution of anemia could be categorized as rising (19 patients), persistent (10 patients), or PADH (13 patients). The dotted line corresponds to the threshold of 4% parasitemia, which provides the best discrimination between PADH and other patterns of post-AS anemia. Percentages at the top of the panel are the proportion of patients above this 4% threshold for each pattern of anemia. (B-C) Individual values of pitting rate (normalized against initial parasitemia [B]), and concentration of once-infected erythrocytes (g/L [C]) in 35 samples from 21 patients with severe malaria treated with AS in whom posttreatment evolution of anemia could be categorized as rising, persistent, or PADH. The dotted lines correspond to thresholds providing the best discrimination between PADH (9 patients) and other patterns of post-AS anemia (12 patients). Percentages at the top of the panel are the proportion of samples above this threshold of 45% pitting rate and above 0.18 billion once-infected erythrocytes per liter for each pattern of anemia. (D) Graphic representation of the relative influences of parasitemia on admission, peak pitting rate, and peak concentration of once-infected erythrocytes on the risk of evolution toward the different patterns of post-AS anemia. The concentration of circulating once-infected erythrocytes was computed by multiplying parasitemia by the pitting rate. Patients with high initial parasitemia and a high pitting rate harbor a high concentration of once-infected erythrocytes (upper right zone of the panel) and are at high risk of intense PADH in the following days or weeks. On the basis of these markers, the risk of subsequent PADH can be predicted before the end of the first week. The risk becomes significant when parasitemia on admission is above 4% and the peak pitting rate on days 2 through 8 is above 45%. On the basis of this data set (panels A-C), a concentration of circulating once-infected erythrocytes on days 2 to 8 greater than 0.18 g/L discriminates patients with PADH from patients with other patterns of anemia more effectively than do parasitemia or the peak pitting rate taken separately. This model also explains why delayed hemolysis has not been observed in severe malaria patients treated with quinine, a compound that induces low pitting rates.
Once-infected erythrocytes are smaller than uninfected erythrocytes in AS-treated patients
Upon treatment initiation, assuming that the fixation-permeation process used to label RBCs induces similar modifications on the different RBC subpopulations processed in the same way in the same sample, the mean reduction in projected surface area of parasitized erythrocytes compared with uninfected erythrocytes (ie, erythrocytes that had never been infected) was 3% (range, 1.7% to 4.5%; 4 patients; Figure 3D). At day 3 ± 1 after initiation of therapy, the mean reduction in projected surface of once-infected erythrocytes again compared with uninfected erythrocytes (ie, erythrocytes that had never been infected) in the same sample, was 8.9% (range, 4.6% to 12.5%; 4 patients; Figure 3D).
Discussion
In this study, we linked typical PADH events to the delayed loss of once-infected erythrocytes. Compared with patients with other patterns of anemia, those with PADH indeed had a higher peak concentration of circulating once-infected erythrocytes; this concentration then declined a few weeks later as PADH developed. In keeping with this observation, the level of once-infected erythrocytes circulating during the first week of follow-up was the best predictor of subsequent PADH. Thus, circulating infected erythrocytes spared by pitting during AS treatment were subsequently cleared a few days or weeks later, a process that contributed to PADH. This observation is in line with the hemolytic component of PADH and with its occurrence in asymptomatic, parasite-free patients a few weeks after effective treatment. The link between pitting and PADH is also consistent with the occurrence of typical episodes in patients treated with artemisinin but not in those treated with quinine.5 Pitting rates are indeed generally high in AS-treated patients and low in quinine-treated patients (Figure 3).15-19 PADH is thus related to the original cellular mechanism (pitting) that contributes to the superior efficacy of AS in malaria and does not significantly alter the positive impact of AS in terms of mortality. PADH is indeed rarely (if ever) fatal and is becoming predictable. Our results should therefore encourage programs and physicians to maintain the momentum for a rapid implementation of this lifesaving therapeutic approach for severe malaria worldwide.
Our approach has some limitations. First, the study was not randomized. This is because, in the context of imported severe malaria, it has generally been considered unethical to repeat AS vs quinine trials already performed in endemic countries.2 Second, our conclusions are based on the analysis of a relatively limited number of patients and samples. This weakness is compensated by the strong statistical significance of differences observed between early concentrations of once-infected erythrocytes in the different patterns of anemia (Figure 4). Our cohort is also the largest reported to date on the safety of AS in the treatment of severe malaria in travelers that includes the analysis of specific cellular parameters obtained between day 8 and day 28 after initiation of therapy, a demanding task. Because deciphering the mechanisms of PADH requires a robust analysis of the kinetics of erythrocyte subpopulations, we excluded transfused patients. General efficacy and safety data from the whole cohort, including transfused patients will be reported elsewhere. Finally, although our data show a strong link between PADH and the loss of once-infected erythrocytes, we cannot definitely exclude the intervention of a complementary mechanism of hemolysis. In PADH patients, the mean delayed decline in hemoglobin was 12% (Figure 2), and the median peak concentration of once-infected erythrocytes was 7.93% (range, 1.6% to 17.5%) of circulating erythrocytes. These numbers match reasonably well, but in some individuals, other subpopulations of erythrocytes (that had never been infected) may have been lost simultaneously. In malarial anemia occurring before antimalarial therapy, the loss of infected erythrocytes is accompanied by the loss of uninfected erythrocytes.29 A similar amplifying mechanism may occur in PADH. Alternatively, the loss of once-infected erythrocytes may have triggered a by stander erythrocyte loss as in the posttransfusion hyperhemolysis syndrome.30,31 In both cases, the precise amplifying or bystander mechanism has not yet been established.
Once-infected erythrocytes are initially spared by pitting. Why and how they are ultimately cleared a few weeks later remains to be fully elucidated. PADH typically occurs 2 weeks after treatment initiation. This is consistent with the intervention of an adaptive immunologic process such as a specific humoral response targeted against a potential alteration of once-infected erythrocyte surface. However, to the best of our knowledge, no serum reactivity to AS-exposed parasitized erythrocytes has been observed to date (P.A.N. and C.R. unpublished observations).17 This negative observation does not definitively exclude the intervention of opsonization because antibodies may be very rapidly removed from the circulation (with their pitted RBC target) by the activated post-malarial spleen. Yet, of 72 children treated with AS in Gabon and Ghana for severe malaria, 5 experienced delayed hemolysis; associated risks factors were high parasitemia on admission and younger age (median, 24 months).32 These features do not support the hypothesis of a hemolytic process strongly related to acquired immunity. More sensitive explorations on a larger number of patients are required for a conclusion on this point. By using the ImageStream technology recently adapted to analyze erythrocyte morphology,27,28,33 we observed an 8.9% reduction in projected surface area of once-infected erythrocytes compared with uninfected erythrocytes in the same sample. A 17% surface loss immediately induces the mechanical retention of erythrocytes in the spleen.27,28,33 We suspect that infection of erythrocytes by P falciparum followed by pitting may have mimicked premature aging, thus reducing the lifespan of once-infected erythrocytes. The moderately reduced size of once-infected erythrocytes is thus consistent with a premature, immune-independent mechanical clearance a few days or weeks after AS administration. Analysis of this marker in a larger group of patients is needed.
PADH has affected approximately 20% to 25% of travelers with severe malaria6-8 and a smaller proportion of African children treated with AS.32 If PADH is indeed caused by the delayed clearance of once-infected erythrocytes, it is by definition linked to drug activity (ie, pitting that operates in a high proportion of AS-treated patients). This model thus convincingly explains why the incidence of PADH is higher than that of most idiosyncratic reactions to drugs (Figure 3A). The inconstant occurrence of PADH is also predicted by the delayed loss of once-infected erythrocytes. High initial parasitemia and high pitting rates are indeed both required to generate a sufficient volume of once-infected erythrocytes, the clearance of which will trigger delayed anemia (Figure 4). Significant variations in these parameters were observed across individuals (Figures 3A and 4)34 ; for example, parasitemia was lower than 4% in 44% of patients (18 of 43) whose anemia patterns were analyzed in detail (Figure 4). In this nonhyperparasitemic subgroup, PADH was infrequent (1 of 18; 6%). Pitting rates were also variable (Figure 4B) in our study. This too had role to play because we observed that some hyperparasitemic patients with low pitting rates did not have PADH.
Patterns of post-AS anemia other than PADH were frequent in our study but generally exposed patients to a threat more easily manageable than that of PADH. Because the rising pattern of anemia corresponded to an early hemolytic episode followed by a sustained recovery without delayed anemia, the conventional follow-up recommended by the World Health Organization (day 0, day 3, day 7, day 28) was well suited for the detection of early complications or late relapse of the malaria attack. This pattern has been observed in thousands of uncomplicated malaria cases in both adults and children treated with artemisinin combined therapy.35,36 Our observations also suggest that the persistent pattern may result from a more complex overlap of mechanisms that comprise those causing the 2 polar rising and PADH patterns (Figures 2 and 4). A monophasic, accelerated clearance of normal or uninfected erythrocytes has been observed in malaria patients in Thailand.37 It lasts several weeks and is more intense in severe as compared with uncomplicated malaria, thus fitting the characteristics of the persistent pattern. Either nonspecific splenic activation or decoration of uninfected erythrocytes by P falciparum proteins such as RSP-2 and/or RAP-2 may induce this prolonged extravascular hemolysis, as already observed in quinine-treated patients.37-39 Via the division of post-AS anemia into distinct patterns and the quantification of once-infected erythrocytes, we confirmed that PADH is a separate entity related to a novel mechanism of anemia. Our findings markedly strengthen a new disease framework for post-AS anemia and open the way to the development of early predictive and prognosis markers. We propose that the combination of 2 parameters—initial parasitemia and pitting rate—determine the number of once-infected erythrocytes produced and the risk of PADH (Figure 4D). Although no strongly predictive threshold of parasitemia could be identified, a concentration of once-infected erythrocytes greater than 180 million cells per liter during the first week effectively discriminated PADH from other patterns of post-AS anemia (Figure 4). Because the highest concentrations of once-infected erythrocytes were observed between day 3 and day 7, this marker should be a strong candidate for providing an accurate prediction of subsequent PADH before patients are discharged from the hospital. Currently, quantification of once-infected erythrocytes can be performed via flow cytometry at reference centers, but a point-of-care test should also be developed for deployment in the field. The approach described here should ultimately facilitate the management of patients treated with any endoperoxide-containing antimalarial compound (including synthetic trioxolane ozonide compounds) for a malaria attack with initial parasitemia greater than 4%. The test would identify not only patients who require very close follow-up during the 2 to 4 weeks following therapy but also those who are at low risk of subsequent PADH and for whom the conventional World Health Organization follow-up schedule (day 7 and day 28) would suffice. This approach would ensure that all patients benefit from more pertinent follow-up while reducing burdens on health care systems.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Arjen Dondorp from the Mahidol Oxford Research Unit; Thomas Zoller from the Charité Universitätsmedizin, Berlin, Germany; and Rick Fairhurst from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Rockville, MD for the fruitful discussions.
This work was supported by grants from the Domaine d’Intérêt Majeur Mal Inf Région Ile de France and the Worldwide Antimalarial Resistance Network, and by the Bill & Melinda Gates Foundation (P.A.N.), by the Follereau Foundation (L.C.), by an INSERM-AP-HP France interface contract (S.J.), and by a grant from the National Institutes of Health, National Heart, Lung and Blood Institute (K.H.). Support for equipment was provided by Oxford University (Project No. 5P01HL078826-06). Labex GR-Ex is funded by the program “Investissements d'avenir.”
Authorship
Contribution: S.J., P.A.N., and P.B. designed and performed research, contributed vital analytical tools, analyzed data, and wrote the paper; C.R. and M.T. performed research, contributed vital analytical tools, analyzed data, and wrote the paper; I.S., M.N., M.V., K.H., and D.M. contributed vital analytical tools, analyzed data, and wrote the paper; E.K. contributed vital analytical tools and performed research; M.D. designed research; F.A., S.B., L.C., O.M., A.A., F.B., and J.M. performed research; and E.C. wrote the paper.
Conflict of interest: S.J. collaborates with Guilin Laboratories; P.B. provided expertise and collaborates with Fast-Track Drugs & Biologics LLC and Sigma-Tau Pharmaceuticals, is engaged in a collaboration with Guilin Laboratories, and has provided expertise to Sanofi Aventis Research & Development. The remaining authors declare no competing financial interests.
A complete list of the members of the French Artesunate Working Group appears in the online data supplement, available on the Blood Web site.
Correspondence: Pierre Buffet, Service de Parasitologie-Mycologie Groupe Hôpital Pitie-Salpêtrière, 47 Boulevard de l’hôpital, 75651 Paris Cedex 13, France; e-mail: pabuffet@gmail.com.
References
Author notes
S.J. and P.A.N. contributed equally to this study.


![Figure 4. Parasitemia, pitting rate, concentration of once-infected erythrocytes, and the risk of evolution toward PADH or other patterns of post-AS anemia in patients with severe malaria. (A) Individual values of parasitemia (as a percentage of infected erythrocytes) in patients with severe malaria treated with AS in whom posttreatment evolution of anemia could be categorized as rising (19 patients), persistent (10 patients), or PADH (13 patients). The dotted line corresponds to the threshold of 4% parasitemia, which provides the best discrimination between PADH and other patterns of post-AS anemia. Percentages at the top of the panel are the proportion of patients above this 4% threshold for each pattern of anemia. (B-C) Individual values of pitting rate (normalized against initial parasitemia [B]), and concentration of once-infected erythrocytes (g/L [C]) in 35 samples from 21 patients with severe malaria treated with AS in whom posttreatment evolution of anemia could be categorized as rising, persistent, or PADH. The dotted lines correspond to thresholds providing the best discrimination between PADH (9 patients) and other patterns of post-AS anemia (12 patients). Percentages at the top of the panel are the proportion of samples above this threshold of 45% pitting rate and above 0.18 billion once-infected erythrocytes per liter for each pattern of anemia. (D) Graphic representation of the relative influences of parasitemia on admission, peak pitting rate, and peak concentration of once-infected erythrocytes on the risk of evolution toward the different patterns of post-AS anemia. The concentration of circulating once-infected erythrocytes was computed by multiplying parasitemia by the pitting rate. Patients with high initial parasitemia and a high pitting rate harbor a high concentration of once-infected erythrocytes (upper right zone of the panel) and are at high risk of intense PADH in the following days or weeks. On the basis of these markers, the risk of subsequent PADH can be predicted before the end of the first week. The risk becomes significant when parasitemia on admission is above 4% and the peak pitting rate on days 2 through 8 is above 45%. On the basis of this data set (panels A-C), a concentration of circulating once-infected erythrocytes on days 2 to 8 greater than 0.18 g/L discriminates patients with PADH from patients with other patterns of anemia more effectively than do parasitemia or the peak pitting rate taken separately. This model also explains why delayed hemolysis has not been observed in severe malaria patients treated with quinine, a compound that induces low pitting rates.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/2/10.1182_blood-2014-02-555953/4/m_167f4.jpeg?Expires=1763477892&Signature=xXH6CBKzMOCA0UC3OlWlWbaqQwKLs2M3Kzvuvx2WywvozP8m2mkxvLIDKfYX~3CDnAX4iVzsx9oesEkjBVChJ~4~VcdaycTp2gqmC6IRCopkBVGkjUSkstqM3U4t3VYu2jjXqIGUkssW5gmrvY3lgUBw0L~9jd44AeZv-bQQBM~LfTCILfdT6dMJTeDH~W6JByNT8N8JJkEZ9n8IfEd2CWNg4A5b~MXnBnFtXr-5eN9z2YZO53yZHsnqrlVXIAmGK4iddqrB96PY0JmDbaZSJg0WP0ZR7uhivNg8xb5HXov1pJq1BwNzDb4H46tqAu1uSbaQUnvr4wTYHArS4qZK-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)