Key Points
HRS cell-derived LTα activates the endothelium to enhance T-cell recruitment.
COX/NF-κB/AP1 pathways act in concert to regulate LTα production.
Abstract
It is known that cells within the inflammatory background in classical Hodgkin lymphoma (cHL) provide signals essential for the continual survival of the neoplastic Hodgkin and Reed-Sternberg (HRS) cells. However, the mechanisms underlying the recruitment of this inflammatory infiltrate into the involved lymph nodes are less well understood. In this study, we show in vitro that HRS cells secrete lymphotoxin-α (LTα) which acts on endothelial cells to upregulate the expression of adhesion molecules that are important for T cell recruitment. LTα also enhances the expression of hyaluronan which preferentially contributes to the recruitment of CD4+ CD45RA+ naïve T cells under in vitro defined flow conditions. Enhanced expression of LTα in HRS cells and tissue stroma; and hyaluronan on endothelial cells are readily detected in involved lymph nodes from cHL patients. Our study also shows that although NF-κB and AP-1 are involved, the cyclooxygenase (COX) pathway is the dominant regulator of LTα production in HRS cells. Using pharmacological inhibitors, our data suggest that activity of COX1, but not of COX2, directly regulates the expression of nuclear c-Fos in HRS cells. Our findings suggest that HRS cell-derived LTα is an important mediator that contributes to T cell recruitment into lesional lymph nodes in cHL.
Introduction
Classical Hodgkin lymphoma (cHL) is characterized by a paucity of malignant Hodgkin and Reed-Sternberg cells (HRS cells) embedded within an inflammatory infiltrate comprising CD4+ T cells, B cells, macrophages, granulocytes, and fibroblasts. HRS cells secrete numerous cytokines and chemokines that act collectively to recruit this cellular infiltrate, which, in turn, is the source of various soluble factor-derived and membrane bound molecule-derived signals essential for the continued survival of the neoplastic cells.1-3
HRS cells originate from crippled postgerminal center B cells that have escaped apoptosis.4,5 They show aberrant signaling activities involving nuclear factor-κB (NF-κB),6,7 Janus kinase-signal transducer and activator of transcription,8-11 activator protein 1 (AP-1),12 PI3K-Akt,13,14 and multiple receptor tyrosine kinases.15 Deregulated NF-κB and AP-1 pathways contribute significantly to the survival of HRS cells and the maintenance of an immunosuppressive microenvironment. Overexpression of components of the AP-1 transcription complex, such as JunB and c-Jun in HRS cells, results in the induction of many target genes to suppress antitumor T-cell activity.16,17 Similarly, overexpression of the cyclooxygenase-2 (COX2) enzyme is associated with enhanced angiogenesis in HL.18
Lymph node architecture and the protein microenvironment are important for regulation of lymphocyte recirculation and optimal immune response.19 Although tissue architecture is effaced in cHL lymph nodes, cell trafficking into the lesion is unaffected. Machado et al detected several T-cell homing molecules such as CXCL12, CCL21 and peripheral node addressin (PNAd) within the vasculature of HL lesions.20 Estrada-Bernal et al reported that HRS cell-derived soluble factors can activate endothelial cells to enhance leukocyte binding in vitro.21 This suggests that HRS cells are able to modulate endothelial function in the affected lymph node to enhance inflammatory cell recruitment. However, neither study identified the HRS cell-derived soluble factor(s) responsible.
Lymphotoxin-α (LTα) is a member of the tumor necrosis factor (TNF) superfamily, existing either as the soluble homotrimer, LTα3, or as a membrane-bound heterodimer, LTα1β2. LTα3 is secreted by CD4+ T helper (Th)1, CD8+, natural killer, B, and lymphoid tissue inducer cells.22-24 LTα3 binds to and signals through the TNF receptors TNFRI and TNFRII and exhibits overlapping biological activities with TNF-α. LTα can induce intercellular adhesion molecule (ICAM)-1 and E-selectin on endothelial cells in vitro25 or vascular cell adhesion molecule (VCAM)-1 in vivo.26 LTα is also required for the maintenance of lymphoid architecture and segregation of T and B cells in adult lymph nodes.27,28 LTα gene expression has been found in cHL lymph nodes29 and HRS cells identified as a source of LTα.30 However, the serum level of LTα is not elevated in cHL patients, nor is expression of LTα associated with B symptoms.31
In this study, we identify in vitro HRS cell-derived LTα as the mediator that induces endothelial cell activation to enhance interaction with T cells. Binding of naive T cells to the LTα-activated endothelial monolayer is mediated by l-selectin-l-selectin ligand and CD44-hyaluronan adhesive pathways. We also identify NF-κB, c-Jun N-terminal kinase (JNK)/AP-1, and COX enzymatic pathways as the signaling pathways involved in LTα production in HRS cells. Our data are corroborated by demonstration of positive immunohistochemical staining (IHC) for LTα expression in HRS cells and of overexpression of hyaluronan in CD31+ endothelial cells within cHL lymph nodes.
Materials and methods
Cells and lymph nodes
HRS cell lines, KM-H2, L1236, L428, and L540, obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, were maintained in supplemented RPMI 1640 media.
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cords from consented donors as described previously.32 The cells were maintained in Clonetics EBM2 Medium (Lonza) and used at passages 3 to 6.
CD4+ T cells were harvested from buffy coats of healthy donors using whole blood CD4 microbeads (Miltenyi Biotec). Naïve and memory T cells were then purified by negative selection using, respectively, anti-CD45RO–coated and anti-CD45RA–coated immunomagnetic beads (Invitrogen) as previously described.33
The use of freshly isolated cells and paraffinized lymph node sections from archival tissues of cHL patients from Singapore and United Arabs of Emirates was approved by ethics committees of respective institutions in accordance with the principles of the Declaration of Helsinki.
Preparation of culture supernatant
HRS cells (2 × 106) were cultured in 1 mL of fresh RPMI 1640 complete media for 24 hours. Culture supernatant (C/S) was used at a dilution of 1 in 2 or 1 in 8 for activation of endothelial cell monolayers.
To investigate the signaling pathways involved, KM-H2 cells were treated with Bay11-7085 (inhibitor of IkBα phosphorylation; Merck), SP600125 (inhibitor of JNK phosphorylation; Cayman Company), Celecoxib (selective Cox-2 inhibitor; Cayman Company), or indomethacin (nonselective COX inhibitor; Sigma Aldrich) for 12 hours at the concentrations stated. Controls were untreated KM-H2 cells or KM-H2 cells treated with dimethylsulfoxide (DMSO; vehicle). LTα concentration in the C/S was measured by enzyme-linked immunosorbent assay (eBioscience). Lysate from treated cells was analyzed by western blot.
Western blot
Cytoplasmic or nuclear protein (25 μg/lane) was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel for p65 and c-Fos or 12% gel for JNK and c-Jun detection. Protein separation was carried out at 100 V. Following protein transfer and blocking with 5% nonfat milk, blots were incubated overnight at 4°C with diluted primary antibodies. Incubation with secondary anti-rabbit or anti-mouse IgG horseradish peroxidase conjugate (Santa Cruz) was for 1 hour at room temperature prior to visualization by chemiluminescence (Merck Milipore). The following antibodies were used: anti-p65 and anti-phosphorylated c-Fos (Santa Cruz) anti-total c-Fos, anti-phosphorylated JNK, anti-total JNK, anti-phosphorylated c-Jun, anti-total c-Jun (Cell Signaling). Blots were reprobed with an anti–β-actin antibody (Santa Cruz) as a cytoplasmic loading control and anti-TATA box antibody (Abcam) as a nuclear loading control.
TNF-α/LTα bioassay
The mouse fibroblast cell line, L929, which is sensitive to both TNF-α and LTα, was seeded into 96-well plates (5 × 103 cells/100 µL/well) and maintained for 24 hours. Culture medium was replaced with diluted C/S from HRS cell lines at the dilutions indicated and supplemented with actinomycin D (0.2 µg/mL). Cell viability was assessed after 12 hours by Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega Corporation); 10 ng/mL TNF-α was included as a positive control. Neutralization of TNF-α and LTα was achieved by pretreating the C/S with anti–TNF-α or anti-LTα neutralizing antibodies (R&D Systems) for 30 minutes at 37°C.
In vitro flow T cell-HUVEC interaction analysis
T cell-endothelial cell interactions under defined flow conditions were examined using the parallel plate flow chamber system as described previously.32-34 The experimental setup and conditions used are detailed in the supplemental Methods available on the Blood Web site.
Statistical analysis
All statistical analyses between 2 different conditions were performed using the Mann-Whitney U test. P ≤ .05 was considered to indicate a statistically significant difference.
Protocols for cell-based enzyme-linked immunosorbent assay and IHC are as described in the supplemental Methods.
Results
LTα is a potent endothelial cell stimulant spontaneously secreted by HRS cells
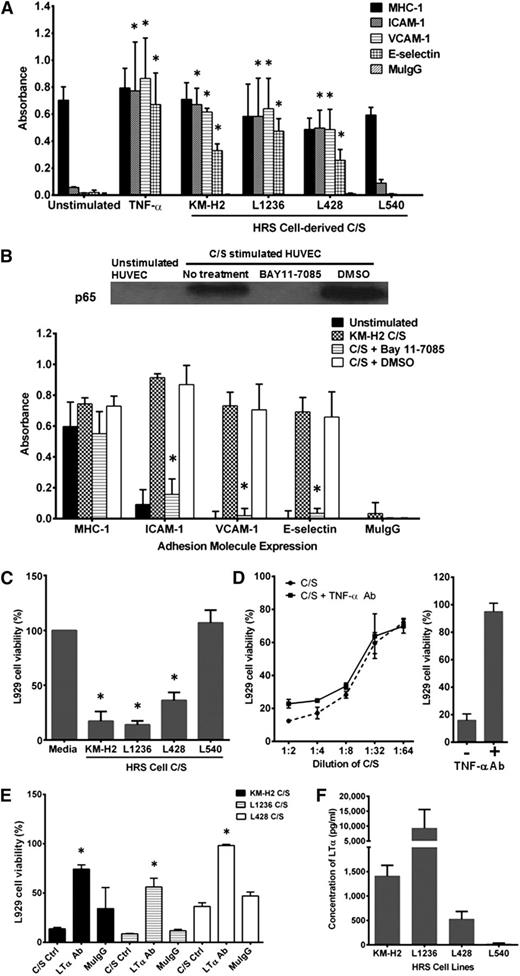
Figure 1A shows that C/S from 3 of 4 HRS cell lines, KM-H2, L1236, and L428, stimulated HUVECs to upregulate expression of ICAM-1, VCAM-1, and E-selectin to levels comparable to that seen in TNF-α–activated HUVECs. The stimulating factor(s) present in the C/S is secreted spontaneously and is of high efficacy (supplemental Figure 1A-B). This stimulatory effect is not due to endotoxin contamination or to serum components, as added polymyxin B and replacement with serum-free culture medium did not inhibit its potency (supplemental Figure 1C-D). It is, however, heat sensitive, as boiling the C/S (5 minutes) completely negated its effect on HUVECs (supplemental Figure 1D).
HRS cell-derived LTα-induced upregulation of adhesion molecule expression in HUVECs is dependent on NF-κB activation. (A) Incubation of HUVECs with C/S from KM-H2, L1236, and L428 cells but not L540 cells for 4 hours induced upregulation of ICAM-1, VCAM-1, and E-selectin to levels comparable to that seen with TNF-α–stimulated HUVECs. C/S-activated HUVECs incubated with purified mouse IgG (MuIgG) and anti-MHC class I mAb were used, respectively, as negative and positive controls. (B) (Upper) Enhanced p65 nuclear translocation in C/S-stimulated HUVECs is completely inhibited by pretreatment of the HUVEC monolayer with 20 μM Bay11-7085 for 30 minutes before C/S stimulation. (Lower) Bay11-7085 pretreatment also prevented the upregulation of ICAM-1, VCAM-1, and E-selectin expression on C/S-stimulated HUVECs. (C) Although C/S derived from KM-H2, L1236, and L428 exhibits significant cytotoxicity in L929 cells, L540-derived C/S did not induce any L929 cell death. (D) (Left) Neutralization of TNF-α in KM-H2 C/S did not improve L929 cell viability, (right) although the dosage used can effectively neutralize 10 ng/mL of recombinant TNF-α in vitro. (E) Neutralization of LTα in the various HRS cell-derived C/S successfully reduced their cytotoxic effects in L929 cells compared with untreated C/S and MuIgG-treated C/S. (F) The highest concentration of LTα was detected in L1236 C/S, whereas negligible amounts of LTα were present in L540-derived C/S. Values shown are mean ± standard error of the mean (SEM) from 3 experiments. *P ≤ .05 compared with unstimulated control or C/S-stimulated HUVECs for A and B; *P ≤ .05 compared with untreated C/S for C-F. Western blot is representative of 3 independent experiments.
HRS cell-derived LTα-induced upregulation of adhesion molecule expression in HUVECs is dependent on NF-κB activation. (A) Incubation of HUVECs with C/S from KM-H2, L1236, and L428 cells but not L540 cells for 4 hours induced upregulation of ICAM-1, VCAM-1, and E-selectin to levels comparable to that seen with TNF-α–stimulated HUVECs. C/S-activated HUVECs incubated with purified mouse IgG (MuIgG) and anti-MHC class I mAb were used, respectively, as negative and positive controls. (B) (Upper) Enhanced p65 nuclear translocation in C/S-stimulated HUVECs is completely inhibited by pretreatment of the HUVEC monolayer with 20 μM Bay11-7085 for 30 minutes before C/S stimulation. (Lower) Bay11-7085 pretreatment also prevented the upregulation of ICAM-1, VCAM-1, and E-selectin expression on C/S-stimulated HUVECs. (C) Although C/S derived from KM-H2, L1236, and L428 exhibits significant cytotoxicity in L929 cells, L540-derived C/S did not induce any L929 cell death. (D) (Left) Neutralization of TNF-α in KM-H2 C/S did not improve L929 cell viability, (right) although the dosage used can effectively neutralize 10 ng/mL of recombinant TNF-α in vitro. (E) Neutralization of LTα in the various HRS cell-derived C/S successfully reduced their cytotoxic effects in L929 cells compared with untreated C/S and MuIgG-treated C/S. (F) The highest concentration of LTα was detected in L1236 C/S, whereas negligible amounts of LTα were present in L540-derived C/S. Values shown are mean ± standard error of the mean (SEM) from 3 experiments. *P ≤ .05 compared with unstimulated control or C/S-stimulated HUVECs for A and B; *P ≤ .05 compared with untreated C/S for C-F. Western blot is representative of 3 independent experiments.
Pretreatment of HUVECs with NF-ĸB inhibitor, Bay11-7085, prior to C/S stimulation, reduced nuclear p65 expression with concurrent attenuation of ICAM-1, VCAM-1, and E-selectin expression on these cells compared with DMSO-treated HUVECs (Figure 1B). This suggests that activation of HUVECs by C/S is mediated via the NF-κB pathway.
Because the C/S-stimulated endothelial cells showed functional similarity to their TNF-α–stimulated counterparts, the L929 mouse fibroblast cytotoxic assay was used to determine the presence of biologically active TNF-α in the HRS cell C/S. Interestingly, the C/S stimulatory effects of each HRS cell-derived C/S paralleled their cytotoxic effects on L929 cells. Incubation of L929 cells with C/S from KM-H2, L1236, and L428 cells but not from L540 resulted in extensive L929 cell death (Figure 1C). The cytotoxic effect of KM-H2 C/S on L929 cells decreases with increasing dilution of the C/S (Figure 1D, left). However, treatment of C/S with neutralizing monoclonal antibodies (mAb) against TNF-α did not improve L929 cell survival, suggesting absence of TNF-α from the C/S. The concentration of mAb used is sufficient to neutralize 10 ng/mL of recombinant human TNF-α (Figure 1D, right). In contrast, neutralization of LTα with mAb against human LTα significantly reduced the cytotoxic effects of the different HRS cell-derived C/S on L929 cells (Figure 1E) and reduced induction of adhesion molecule expression on HUVECs (supplemental Figure 2). Quantification of LTα in the different C/S confirmed high levels of LTα present in the C/S of KM-H2, L1236, and L428 cells (Figure 1F).
CD4+ CD45RA+ naïve T-cell binding to C/S-stimulated HUVECs is mediated by CD44-hyaluronan interactions
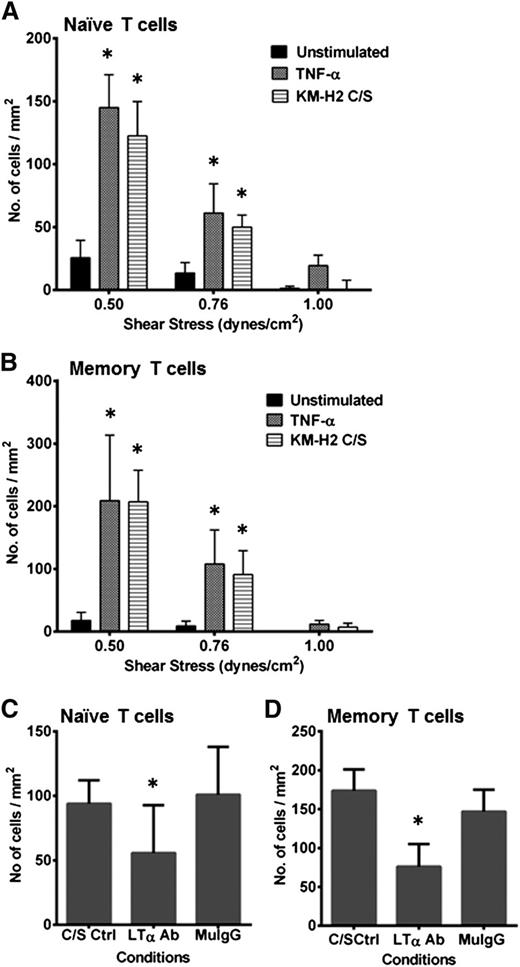
We next investigated whether C/S-stimulated HUVEC monolayers would interact with T cells under defined flow conditions. Both CD4+CD45RA+ naïve and CD45RO+ memory T cells were found to interact with C/S-stimulated HUVECs in a shear stress-dependent manner (Figure 2A-B). Both rolling and adhered phenotypes of T cells were identified (data not shown). The number of interacting CD4+ naive and memory T cells was comparable to that of TNF-α–activated HUVECs under similar conditions. Neutralization of LTα in the C/S prior to HUVEC stimulation significantly reduced the number of T-cell interactions (Figure 2C-D).
HRS cell C/S-stimulated HUVECs show enhanced T-cell interactions under flow. Both (A) CD4+ CD45RA+ naïve T cells and (B) CD4+ CD45RO+ memory T cells bind to KM-H2 C/S-stimulated HUVECs in a shear stress-dependent manner (stripe bar). The respective numbers of interacting cells is comparable to that seen with TNF-α–stimulated HUVECs (dark gray bar). Very few T cells bind to unstimulated HUVECs (black bar). Neutralization of LTα in the C/S prior to use in stimulating HUVECs resulted in a significant reduction in the numbers of interacting (C) naïve and (D) memory T cells at the shear stress of 0.76 dynes/cm2. The live time cell-cell interactions were video recorded using a CCD camera and VCR (SVT-N24P; Sony), and analysis was carried out offline. Values shown are mean ± SEM from 3-5 experiments. *P ≤ .05 compared with unstimulated HUVECs for A and B; and compared with C/S-stimulated controls for C and D.
HRS cell C/S-stimulated HUVECs show enhanced T-cell interactions under flow. Both (A) CD4+ CD45RA+ naïve T cells and (B) CD4+ CD45RO+ memory T cells bind to KM-H2 C/S-stimulated HUVECs in a shear stress-dependent manner (stripe bar). The respective numbers of interacting cells is comparable to that seen with TNF-α–stimulated HUVECs (dark gray bar). Very few T cells bind to unstimulated HUVECs (black bar). Neutralization of LTα in the C/S prior to use in stimulating HUVECs resulted in a significant reduction in the numbers of interacting (C) naïve and (D) memory T cells at the shear stress of 0.76 dynes/cm2. The live time cell-cell interactions were video recorded using a CCD camera and VCR (SVT-N24P; Sony), and analysis was carried out offline. Values shown are mean ± SEM from 3-5 experiments. *P ≤ .05 compared with unstimulated HUVECs for A and B; and compared with C/S-stimulated controls for C and D.
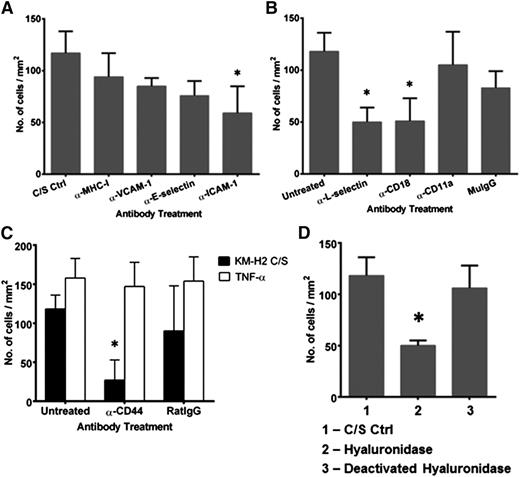
Function blocking experiments were carried out to better understand the mechanisms underlying recruitment of naïve T cells in cHL. Treatment of C/S-stimulated HUVECs with mAb against ICAM-1 partially reduced the number of interacting CD4+ naïve T cells compared with C/S-stimulated HUVEC controls or C/S-stimulated HUVECs treated with mAb against MHC class I molecules (Figure 3A). Treatment with mAb against VCAM-1 or E-selectin did not show similar reduction. Blocking of CD18, the β2-integrin subunit, but not of CD11a, on naïve T cells partially reduced their interactions with C/S-stimulated HUVECs (Figure 3B). Blocking of L-selectin also resulted in a partial reduction in the number of T-cell interactions. This suggests that a novel adhesive pathway may be involved in mediating naïve T-cell interactions with endothelial cells in the presence of HRS cell-derived factors.
Binding of naïve T cells to KM-H2 C/S-stimulated HUVECs is mediated by β2-integrin-ICAM-1 and CD44-hyaluronan interactions. C/S-stimulated HUVECs or naïve T cells were treated with function blocking mAbs for 30 and 10 minutes, respectively, prior to use in flow experiments. (A) Blocking of ICAM-1, but not VCAM-1 and E-selectin, expressed on C/S-stimulated HUVECs significantly inhibited naïve T-cell interactions. Treatment of C/S-stimulated HUVECs with mAb against MHC class I molecules was used as the binding nonblocking control. (B) Blocking of CD18 and L-selectin on the naïve T cells significantly reduced the interaction between the T cells and C/S-stimulated HUVECs. Naive T cells treated with purified mouse IgG (MuIgG) was used as the negative control. (C) Blocking of CD44 inhibited naïve T-cell interactions with C/S-stimulated HUVECs but had no effect on their interactions with TNF-α–stimulated HUVECs. Naïve T cells treated with purified rat IgG (RatIgG) was used as the negative control. (D) Treatment of C/S-stimulated HUVECs with hyaluronidase (50 µg/mL for 1 hour) resulted in a significant reduction in the number of interacting naïve T cells. Treatment with deactivated hyaluronidase was used as the vehicle control. Values shown are mean ± SEM from 3 different experiments. *P ≤ .05 compared with untreated controls. The clones of the mAb used are ICAM-1 (clone Hu5/3), VCAM-1 (clone E1/6), E-selectin (clone H18/7), CD18 (β2-integrin, clone LIA1/2), CD11a (clone HI111), CD44 (clone IM7), and L-selectin (clone DREG56).
Binding of naïve T cells to KM-H2 C/S-stimulated HUVECs is mediated by β2-integrin-ICAM-1 and CD44-hyaluronan interactions. C/S-stimulated HUVECs or naïve T cells were treated with function blocking mAbs for 30 and 10 minutes, respectively, prior to use in flow experiments. (A) Blocking of ICAM-1, but not VCAM-1 and E-selectin, expressed on C/S-stimulated HUVECs significantly inhibited naïve T-cell interactions. Treatment of C/S-stimulated HUVECs with mAb against MHC class I molecules was used as the binding nonblocking control. (B) Blocking of CD18 and L-selectin on the naïve T cells significantly reduced the interaction between the T cells and C/S-stimulated HUVECs. Naive T cells treated with purified mouse IgG (MuIgG) was used as the negative control. (C) Blocking of CD44 inhibited naïve T-cell interactions with C/S-stimulated HUVECs but had no effect on their interactions with TNF-α–stimulated HUVECs. Naïve T cells treated with purified rat IgG (RatIgG) was used as the negative control. (D) Treatment of C/S-stimulated HUVECs with hyaluronidase (50 µg/mL for 1 hour) resulted in a significant reduction in the number of interacting naïve T cells. Treatment with deactivated hyaluronidase was used as the vehicle control. Values shown are mean ± SEM from 3 different experiments. *P ≤ .05 compared with untreated controls. The clones of the mAb used are ICAM-1 (clone Hu5/3), VCAM-1 (clone E1/6), E-selectin (clone H18/7), CD18 (β2-integrin, clone LIA1/2), CD11a (clone HI111), CD44 (clone IM7), and L-selectin (clone DREG56).
CD44 is a cell surface glycoprotein expressed on many different cell types. Binding of CD44 to its ligand, hyaluronan (hyaluronic acid), could regulate T-cell adhesion to endothelial cells under physiological flow conditions and in vivo.35 Blocking of CD44 on naïve T cells inhibited the number of T-cell interactions with C/S-stimulated HUVECs by 78 ± 14%, but not with TNF-α–stimulated HUVECs (Figure 3C). In contrast, blocking of CD44 on CD4+ CD45RO+ memory T cells did not reduce the number of interactions with C/S-stimulated HUVECs, although there was a shift from arrested to the rolling phenotype (data not shown).
Pretreatment of the C/S-stimulated HUVECs with hyaluronidase, but not with deactivated (boiled) hyaluronidase, significantly reduced the number of interacting naïve T cells by 58 ± 4% compared with untreated C/S-stimulated HUVECs (Figure 3D). Because hyaluronan is a component of the endothelial glycocalyx,36 our data suggest that HRS cell C/S can augment the expression of hyaluronan on HUVECs to enhance naïve T-cell interactions in vitro.
Taken together, these in vitro data indicate that HRS cells secrete LTα that in turn activates endothelial cells to upregulate expression of adhesion molecules, including ICAM-1, VCAM-1, E-selectin, and hyaluronan, to facilitate recruitment of T cells into cHL lymph nodes.
Increased LTα expression and enhanced hyaluronan expression is detected in cHL lymph nodes
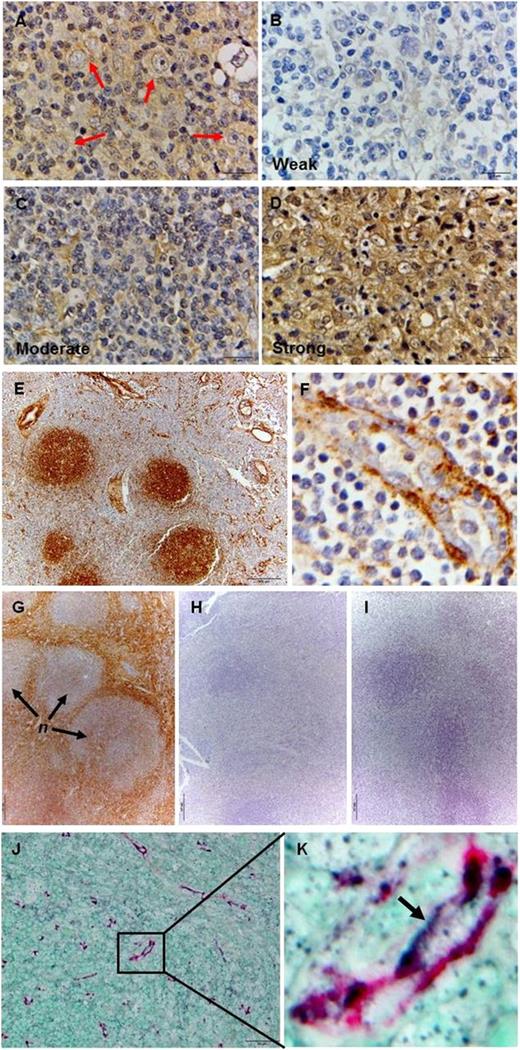
We also examined LTα and hyaluronan expression in tissue sections from 2 cohorts of cHL patients (Singapore and United Arab Emirates). LTα expression was detected in HRS cells and in the extracellular stroma (Figure 4A). Intense LTα expression was detected in 11 of the 32 cases screened, 11 other cases showed moderate LTα expression, and the remainder showed weak or negligible LTα staining (Figure 4B-D). There was, however, no difference in LTα expression between the 2 patient cohorts.
Both LTα-expressing HRS cells and enhanced hyaluronan expression are detected in cHL lymph nodes. (A) Expression of LTα could be detected in HRS cells (red arrows) and in the stroma of cHL lymph node sections by IHC. The overall LTα staining intensity was categorized as (B) weak/negligible, (C) moderate, and (D) strong as indicated. (E) Labeling by HABP shows positive staining localized to the germinal centers of B-cell follicles and vascular structures in reactive lymph nodes. (F) Strong subendothelial positivity and punctuate luminal staining are seen in the HEV. (G) Intense HABP labeling is seen in the stroma with weaker intensity detected in the nodules (n) in cHL tissue. (H) Treatment of the tissue section with hyaluronidase completely abrogated HABP staining. (I) cHL section incubated with purified mouse IgG as primary Ab serves as the negative control. (J-K) Double-staining with anti-CD31 mAb and HABP confirms the expression of hyaluronan on endothelial cells in cHL section. Images shown are representative cases from 32 cHL cases for A-D; a representative sample from 10 reactive lymph nodes screened for E-F; a representative case from 10 cHL cases for G-I; and a representative of 10 cHL cases that were doubled-stained with anti-CD31 and HABP for J-K. IHC images were acquired using an Olympus BX43 microscope equipped with the Olympus DP72 camera at the following original magnifications: ×100 objective for A-D, F, and K; ×10 objective for E and G-I; and ×40 objective for J.
Both LTα-expressing HRS cells and enhanced hyaluronan expression are detected in cHL lymph nodes. (A) Expression of LTα could be detected in HRS cells (red arrows) and in the stroma of cHL lymph node sections by IHC. The overall LTα staining intensity was categorized as (B) weak/negligible, (C) moderate, and (D) strong as indicated. (E) Labeling by HABP shows positive staining localized to the germinal centers of B-cell follicles and vascular structures in reactive lymph nodes. (F) Strong subendothelial positivity and punctuate luminal staining are seen in the HEV. (G) Intense HABP labeling is seen in the stroma with weaker intensity detected in the nodules (n) in cHL tissue. (H) Treatment of the tissue section with hyaluronidase completely abrogated HABP staining. (I) cHL section incubated with purified mouse IgG as primary Ab serves as the negative control. (J-K) Double-staining with anti-CD31 mAb and HABP confirms the expression of hyaluronan on endothelial cells in cHL section. Images shown are representative cases from 32 cHL cases for A-D; a representative sample from 10 reactive lymph nodes screened for E-F; a representative case from 10 cHL cases for G-I; and a representative of 10 cHL cases that were doubled-stained with anti-CD31 and HABP for J-K. IHC images were acquired using an Olympus BX43 microscope equipped with the Olympus DP72 camera at the following original magnifications: ×100 objective for A-D, F, and K; ×10 objective for E and G-I; and ×40 objective for J.
To detect enhanced hyaluronan expression, tissue sections were probed with hyaluronic acid binding protein (HABP), and the resultant staining profile was compared with that detected in reactive (nonmalignant) lymph nodes. Distinct labeling with HABP was seen in the germinal centers of B-cell follicles and in the subendothelial layers of large vessels in the reactive lymph nodes (Figure 4E). Punctate positivity was also detected in high endothelial venules (HEV; Figure 4F) and thin walled vessels (data not shown). Sections from cHL lymph nodes showed intense hyaluronan expression within the extracellular stroma, particularly in fibrotic areas, and membrane positivity in the HRS cells (Figure 4G and supplemental Figure 3). In contrast, staining intensity in the nodules was relatively weaker as many of the infiltrating lymphocytes exhibited low hyaluronan expression (Figure 4G and supplemental Figure 3). Treatment of the tissue sections with hyaluronidase completely abrogated such HABP labeling (Figure 4H). Two-color staining using anti-CD31 mAb and HABP showed colocalization of CD31 and HABP staining on vessel-like structures, thus confirming that high expression of hyaluronan is found on endothelial cell surfaces in cHL tissues (Figure 4J-K).
COX pathway is the dominant regulatory pathway involved in LTα production by HRS cells
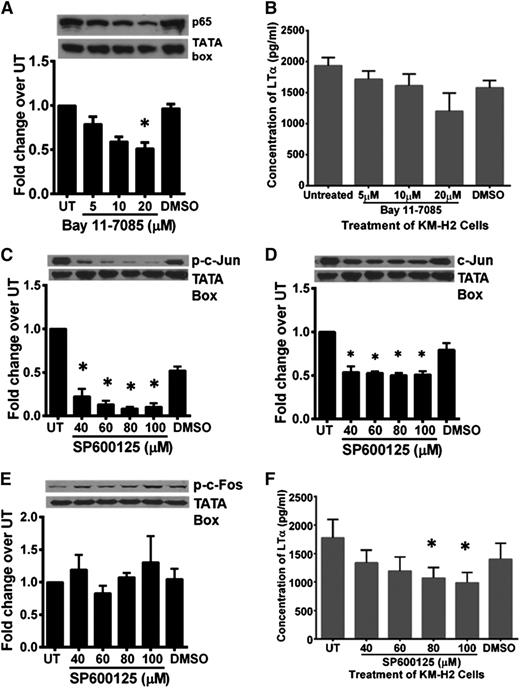
Because NF-κB, AP-1, and COX2 pathways are constitutively active in HRS cells, we next elucidated the role of these pathways in LTα synthesis in KM-H2 cells. The viability of various inhibitor-treated cells was assessed by trypan blue exclusion to ensure that any reduction in LTα concentration was not due to excessive cell death (supplemental Table 1). Treatment of KM-H2 cells with BAY11-7085 inhibited nuclear p65 translocation in a dose-dependent manner (Figure 5A). Consistent with this, LTα concentration in the C/S of BAY11-7085–treated cells was lower compared with C/S of DMSO-treated KM-H2 cells (Figure 5B).
Blocking of NF-κB and AP-1 pathways in HRS cells partially inhibited LTα production. (A) Western blot analysis and band density quantification showed reduced nuclear p65 expression in Bay11-7085–treated KM-H2 cells in a dose-dependent manner. (B) Treatment of KM-H2 cells with 3 different doses of Bay11-7085 as indicated resulted in reduced LTα production compared with untreated and DMSO-treated controls. (C-D) Western blot analysis and band density quantification showed reduction of phosphorylated and total c-Jun in SP600125-treated KMH2 cells. (E) Expression of phosphorylated c-Fos in these cells was unaffected. (F) KM-H2 cells treated with SP600125 showed reduced LTα production, with the most significant reduction observed when 80 and 100 µM of the inhibitor was used. Western blot shown is representative data of 3 independent experiments for A, C, and D and 2 independent experiments for E. The TATA box served as a nuclear protein loading control. β-Actin served as cytoplasmic protein loading control. Quantification of band density was carried out using ImageJ 1.48 software (National Institutes of Health). Density of the protein band for each condition was first normalized to respective loading control and then the fold change over untreated (UT) control cells was determined. Values shown are the mean ± standard deviation from 2 independent experiments for B and E and mean ± SEM from 3 different experiments for A, C, D, and F. *Statistical significance at P ≤ .05 compared with UT KM-H2 cells.
Blocking of NF-κB and AP-1 pathways in HRS cells partially inhibited LTα production. (A) Western blot analysis and band density quantification showed reduced nuclear p65 expression in Bay11-7085–treated KM-H2 cells in a dose-dependent manner. (B) Treatment of KM-H2 cells with 3 different doses of Bay11-7085 as indicated resulted in reduced LTα production compared with untreated and DMSO-treated controls. (C-D) Western blot analysis and band density quantification showed reduction of phosphorylated and total c-Jun in SP600125-treated KMH2 cells. (E) Expression of phosphorylated c-Fos in these cells was unaffected. (F) KM-H2 cells treated with SP600125 showed reduced LTα production, with the most significant reduction observed when 80 and 100 µM of the inhibitor was used. Western blot shown is representative data of 3 independent experiments for A, C, and D and 2 independent experiments for E. The TATA box served as a nuclear protein loading control. β-Actin served as cytoplasmic protein loading control. Quantification of band density was carried out using ImageJ 1.48 software (National Institutes of Health). Density of the protein band for each condition was first normalized to respective loading control and then the fold change over untreated (UT) control cells was determined. Values shown are the mean ± standard deviation from 2 independent experiments for B and E and mean ± SEM from 3 different experiments for A, C, D, and F. *Statistical significance at P ≤ .05 compared with UT KM-H2 cells.
KM-H2 cells treated with the JNK inhibitor, SP600125, showed dose-dependent downregulation of total and phosphorylated nuclear c-Jun expression (Figure 5C-D). This reduction in total c-Jun is consistent with previous reports on the autoregulation of c-Jun by phosphorylated c-Jun.37 The expression of phosphorylated and total c-Fos was not affected (Figure 5E; data not shown). SP600125-treated KM-H2 cells produce lower amounts of LTα (Figure 5F), with maximum inhibition (44 ± 10%) achieved at 100 μM.
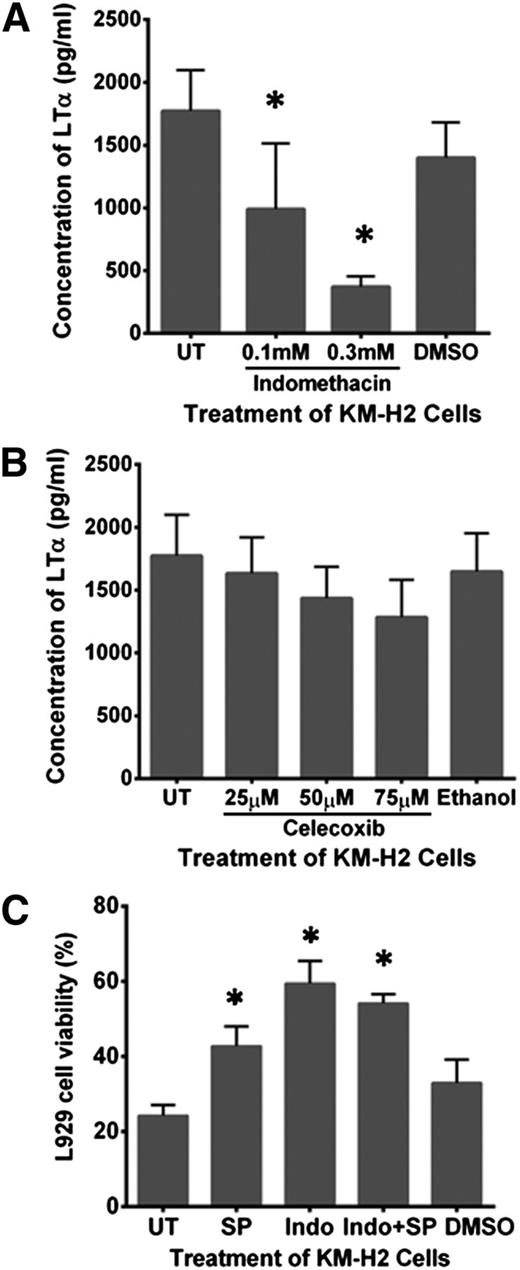
KM-H2 cells treated with 0.3 mM of the nonselective COX inhibitor, indomethacin, significantly reduced LTα production by 85 ± 3% compared with DMSO-treated or untreated KM-H2 cells (Figure 6A). In contrast, treatment of KM-H2 cells with celecoxib, a selective COX2 inhibitor, did not exhibit such an effect over the range of doses tested (Figure 6B). Indomethacin-treated L1236 cells and similarly treated L428 cells also showed the highest attenuation in LTα secretion compared with BAY11-7085–treated or SP600125-treated cells (supplemental Figure 4A-B).
Cox-1 but not Cox-2 enzyme regulates LTα production in HRS cells. (A) Indomethacin treatment at 0.1 and 0.3 mM effectively reduced LTα production by KM-H2 cells. (B) KM-H2 cells treated with Celecoxib at 25, 50, and 75 μM showed minimum reduction in LTα production. (C) Viability of L929 cells exposed to C/S derived from KM-H2 cells treated simultaneously with 60 µM SP600125 (SP) and 0.3 mM Indomethacin (Indo) was not significantly improved compared with L929 cells incubated with C/S derived from KM-H2 cells treated with indomethacin alone. Lowest L929 cell viability was seen in the presence of C/S derived from untreated KM-H2 cells and KM-H2 cells treated with DMSO. Values shown are the mean ± SEM from 3 different experiments. *Significant difference compared with untreated (UT) KMH2 cells at P ≤ .05.
Cox-1 but not Cox-2 enzyme regulates LTα production in HRS cells. (A) Indomethacin treatment at 0.1 and 0.3 mM effectively reduced LTα production by KM-H2 cells. (B) KM-H2 cells treated with Celecoxib at 25, 50, and 75 μM showed minimum reduction in LTα production. (C) Viability of L929 cells exposed to C/S derived from KM-H2 cells treated simultaneously with 60 µM SP600125 (SP) and 0.3 mM Indomethacin (Indo) was not significantly improved compared with L929 cells incubated with C/S derived from KM-H2 cells treated with indomethacin alone. Lowest L929 cell viability was seen in the presence of C/S derived from untreated KM-H2 cells and KM-H2 cells treated with DMSO. Values shown are the mean ± SEM from 3 different experiments. *Significant difference compared with untreated (UT) KMH2 cells at P ≤ .05.
This reduction of LTα in C/S correlates with a loss of stimulatory activity. HUVECs stimulated with C/S from SP600125-treated or indomethacin-treated KM-H2 cells showed attenuated inducible adhesion molecule expression and a decrease in the number of T-cell interactions (supplemental Figure 5).
To determine whether the AP-1 and COX pathways were acting synergistically, we compared the cytotoxic effect of the C/S harvested from KM-H2 cells treated with SP600125 alone, with indomethacin alone or with both inhibitors simultaneously on L929 cells. Our data showed that treatment with SP600125 or Indomethacin partially neutralized the cytotoxic effect of the C/S on L929 cells by 42.7 ± 5.2% and 59.4 ± 5.9%, respectively (Figure 6C). However, combination treatment with both inhibitors did not further improve L929 cell viability compared with indomethacin treatment alone. Thus, the COX pathway is the dominant regulator of LTα production by HRS cells.
NF-κB and COX pathways converge at the regulation of phosphorylated c-Fos expression
IHC analysis of cHL tissues showed strong cytoplasmic c-Fos expression in HRS cells in situ. Nuclear expression of c-Jun and c-Fos is also seen (supplemental Figure 6). Because SP600125 treatment downregulated c-Jun expression but did not show any effect on phosphorylated-c-Fos expression (Figure 5C,E), we next analyzed whether c-Fos expression is regulated by NF-κB or COX pathways in HRS cells.
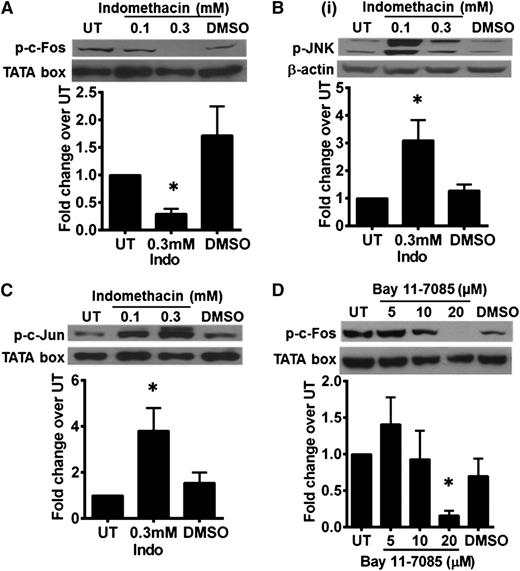
Indomethacin-treated KM-H2 cells showed a decrease in phosphorylated c-Fos with a concomitant increase in phosphorylated JNK and phosphorylated c-Jun expression (Figure 7A-C). Total JNK, total c-Jun, and nuclear p65 expression remained unchanged in these cells, thus suggesting inherent stress response (data not shown). Similar loss of phosphorylated c-Fos was also seen when the cells were treated with BAY11-7085 (Figure 7D). Hence, expression of phosphorylated c-Fos in HRS cells may be regulated concomitantly by both NF-κB and COX pathways. Our data suggest that the AP-1 c-Jun/c-Fos transcription complex in HRS cells is regulated concomitantly by JNK/AP-1, NF-κB, and COX pathways.
Expression of phosphorylated c-Fos in HRS cells is regulated by NF-κB and COX pathways. (A) Phosphorylated c-Fos expression in KM-H2 cells was reduced by treatment with 0.3 mM indomethacin. There was a concurrent increase in (B) phosphorylated-JNK and (C) phosphorylated-c-Jun expression. (D) KM-H2 cells treated with 20 μM of BAY11-7085 showed a similar reduction in phosphorylated-c-Fos expression. The TATA box served as a nuclear protein loading control. β-Actin served as a cytoplasmic protein loading control. Western blot shown is a representative of 3 independent experiments. Band density quantification was carried out using ImageJ 1.48 software (National Institutes of Health). Density of the protein band for each condition was first normalized to respective loading control, and then fold change over untreated (UT) control cells was determined. Values shown are the mean ± SEM from 3 different experiments. *Significant difference compared with UT KMH2 cells at P ≤ .05.
Expression of phosphorylated c-Fos in HRS cells is regulated by NF-κB and COX pathways. (A) Phosphorylated c-Fos expression in KM-H2 cells was reduced by treatment with 0.3 mM indomethacin. There was a concurrent increase in (B) phosphorylated-JNK and (C) phosphorylated-c-Jun expression. (D) KM-H2 cells treated with 20 μM of BAY11-7085 showed a similar reduction in phosphorylated-c-Fos expression. The TATA box served as a nuclear protein loading control. β-Actin served as a cytoplasmic protein loading control. Western blot shown is a representative of 3 independent experiments. Band density quantification was carried out using ImageJ 1.48 software (National Institutes of Health). Density of the protein band for each condition was first normalized to respective loading control, and then fold change over untreated (UT) control cells was determined. Values shown are the mean ± SEM from 3 different experiments. *Significant difference compared with UT KMH2 cells at P ≤ .05.
Discussion
cHL is an enigma among lymphomas, as the neoplastic cells only comprise 1% of the cellular population of the lesion; the remainder are inflammatory cells recruited into the affected lymph node. Although previous studies have reported on the chemokines secreted by HRS cells to attract these inflammatory cells,20,38,39 it remains unclear how the endothelium within the lesion is modulated to enhance this inflammatory recruitment. In this study, we identify HRS cell-derived LTα as a potent endothelial cell stimulant. We show that it augments endothelial cell adhesiveness toward naïve T cells via upregulation of inducible adhesion molecules and hyaluronan. Our data also implicate the NF-κB, AP-1, and COX pathways in the production of LTα by HRS cells.
Several studies have shown that soluble tumor cell mediators, including HRS cell-derived factors, can modulate endothelial cell function.21,40 Estrada-Bernal et al reported that soluble products derived from Hodgkin lymphoma cell lines are able to induce E-selectin expression on HUVECs, as well as to enhance binding of U937 histiocytic cells.21 Although U937 adherence to stimulated HUVECs was dependent on NF-κB activation, E-selectin surprisingly did not play a role. The authors also concluded that the lymphoma-derived factor involved was neither TNF-α nor interleukin (IL)-1β. In this and other studies, the endothelial stimulatory factor(s) were not identified. In our study, we identify LTα as the endothelial stimulant in HRS cell C/S that activates HUVECs to enhance memory and naïve T-cell adhesion. In situ expression of LTα in HRS cells in cHL lymph nodes was also demonstrated in 2 cohorts of patients from Singapore and United Arab Emirates, suggesting that this is not a culture induced phenomenon.
The production of TNF-α and LTα by HRS cell lines and the cytotoxic effects of HRS cell C/S on L929 cells have been previously reported.30,41,42 Hsu et al and Krestchmer et al demonstrated that neutralization of LTα partially inhibited the cytotoxic activity in their respective HRS cell-derived C/S41,42 and that moderate to abundant levels of LTα mRNA are found in cHL lesional tissue.29,30,41,42 However, there was no correlation between LTα mRNA expression and histologic type, histological criteria for necrosis, neoangiogenesis, hyalinosis, or B symptoms.29 Consistent with these studies, our data also show that it is LTα, and not TNF-α, in C/S of the HRS cell lines that is inducing L929 cell death. We also show the presence of LTα in HRS cells in situ by IHC.
Warzocha et al also reported that plasma levels of LTα in HL patients with B symptoms were similar to those of healthy controls.31 Our data suggest that the effect of LTα is likely to be acting locally on the stromal compartment rather than systemically. LTα has been shown to act on endothelial cells to induce a morphologic change from polygonal to fibroblastoid. This is associated with an increase in expression of inducible cell surface adhesion molecules important for leukocyte recruitment.25 Consistent with this, we show that HRS cell-derived LTα can activate endothelial cells to enhance naive T-cell binding. Function blocking studies confirm these interactions are mediated in part by ICAM-1 expressed on stimulated HUVECs and by β2-integrins and l-selectin expressed on naive T cells. An unexpected finding is the involvement of hyaluronan as the predominant adhesion molecule mediating the observed naive T-cell interactions.
Ruco et al showed differential expression of ICAM-1, VCAM-1, and E-selectin in different cHL subtypes and correlated their expression with IL-1/TNF-α expression.43 Machado et al showed the presence of PNAd, in addition to ICAM-1 and VCAM-1, in cHL tissue20 and concluded that the mechanisms of T-cell recruitment in cHL resemble those involved in physiological naive T-cell recirculation. Our data suggest that recruitment of naive T cells into cHL lymph nodes involves binding of CD44 on T cells to hyaluronan expressed on the endothelial cell surface. This is further corroborated by intense HABP staining on CD31+ vessels in cHL lymph nodes. Hyaluronan, the only known nonsulfated glycosaminoglycan, is well known for involvement in wound healing,44 tumor growth,45,46 and adhesion/extravasation of activated lymphocytes.47 Induction of hyaluronan on endothelial cells by proinflammatory cytokines, such as TNF-α and IL-1β, or by lipopolysaccharides is strictly restricted to microvascular endothelial cells.48 Consistent with this, our in vitro data show that treatment of HUVECs with TNF-α does not induce an increase in hyaluronan-dependent naive T-cell binding. Hyaluronan synthesis is a rapid process regulated by 3 isoforms of hyaluronan synthases on the plasma membrane of mammalian cells.49,50 Various cytokines and growth factors can differentially regulate the transcriptional levels and/or protein activity of these enzymes and hence the biological function of hyaluronan.51-54 Currently, it is unclear how LTα in the C/S, alone or together with other factors, modulate hyaluronan synthesis in HUVECs.
A caveat in this study is the use of HUVECs to model HEV in cHL tissue. Despite this shortcoming, we believe our data elucidated an important HRS cell-derived mediator, LTα, which can regulate endothelial function. Drayton et al reported that LTαβ complex signaling could induce PNAd expression in pancreatic endothelial cells.55 Here, we propose that high concentrations of LTα in the HRS cell-derived C/S or in the cHL lymph node could enhance hyaluronan expression on endothelial cells to induce naïve T-cell recruitment.
As expected, the hyaluronan ligand expressed on the naive T cells is CD44. Interaction of CD44 and hyaluronan is well described for memory T cells, activated T-cell subsets, and neutrophils.47 Although all forms of CD44 bind hyaluronan, the affinity of CD44 as a hyaluronan ligand is greatly enhanced by posttranslational modifications.56,57 Naive T cells express low to moderate levels of CD44, and this expression is rapidly increased on activation.58 Our study shows that naive T cells bind via CD44-hyaluronan interactions to C/S-stimulated HUVECs but not to TNF-α–stimulated HUVECs. As CD44 on naive T cells is unlikely to have undergone posttranslational modification, it is probable that the enhanced CD44-hyaluronan interactions are due to high levels of hyaluronan induced on the endothelial cell surface by the HRS cell-derived factors. Alternatively, C/S stimulation might have induced the expression of a different molecular weight hyaluronan on the HUVECs. The differing functions of hyaluronan, whether proinflammatory, immune stimulatory, or immunosuppressive, are known to be dependent on molecular weight.59
Both NF-κB and AP-1 are constitutively active in HRS cells.12,60 Increased activity of COX2 enzyme in HRS cells has also been reported.61,62 All 3 factors have been implicated in the regulation of various genes for immunomodulatory molecules, including CD30, and for survival proteins that contribute to the disease process. Here we show that COX1, but not COX2, enzyme is essential for LTα synthesis in HRS cells. Our data suggest a complex interplay between the NF-ĸB, AP-1, and COX1 pathways in modulating LTα production. Although it is still unclear how the 3 pathways influence one another, previous studies have shown that AP-1 can synergize with NF-κB to enhance transcriptional activity of affected genes63 and that COX products, particularly PGE2, can modulate AP-1 protein expression.64 Currently, we are attempting to identify the exact prostaglandin or arachidonic intermediates responsible for regulating LTα production in HRS cells. Our data strongly suggest a convergence of the NF-ĸB and COX1 pathways at the regulation of c-Fos, which complexes with c-Jun to form active AP-1. AP-1, in turn, regulates LTα production. We also demonstrate that in addition to c-Jun expression,12 there is also intense cytoplasmic and nuclear c-Fos expression in HRS cells in cHL lymph nodes.
In conclusion, we show that LTα, a cytokine involved in lymph node maintenance, is an important HRS cell-derived mediator that stimulates endothelial cell activation in cHL. Inhibition of LTα activity may provide an approach to reduce inflammatory cell recruitment and disrupt signals essential for HRS cell survival in cHL.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor F. W. Luscinskas and the Vascular Research Division, Department of Pathology, Brigham and Women’s Hospital and Harvard Medical School, for the hybridoma cell lines for mAb against ICAM-1 (clone Hu5/3), VCAM-1 (clone E1/6), E-selectin (clone H18/7), and MHC class I molecules (clone W6/32).
This work was supported by funding awarded by the Ministry of Education Academic Research Fund, National University of Singapore (R179-000-034-112), National Medical Research Council, Singapore (NMRC1256/2010), and a British Council PMI2 Connect Travel Grant (RC 165).
Authorship
Contribution: C.W.F., S.M.C., and Y.-C.L. designed the experiments, analyzed the data, and prepared the manuscript; C.W.F. carried out the experiments; A.M.G. assisted in the design of the protein analysis experiments and data analysis; C.T.Y. contributed reagents and equipment and assisted in data analysis; S.M.C., S.A.-S., and A.C. provided the patient samples used in this study; and all authors critically review and edited the manuscript for intellectual content and accuracy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yaw-Chyn Lim, Department of Pathology, National University of Singapore, 5 Lower Kent Ridge Rd, 119074, Singapore; e-mail: yawchyn_lim@nuhs.edu.sg.