Key Points
A novel NFKB2 mutation confers a severe B-cell deficiency, but antibody production is partially preserved.
Unprocessed p100 results in an IκB-like action on the canonical nuclear factor-κB pathway.
Abstract
Most genetic defects that arrest B-cell development in the bone marrow present early in life with agammaglobulinemia, whereas incomplete antibody deficiency is usually associated with circulating B cells. We report 3 related individuals with a novel form of severe B-cell deficiency associated with partial persistence of serum immunoglobulin arising from a missense mutation in NFKB2. Significantly, this point mutation results in a D865G substitution and causes a failure of p100 phosphorylation that blocks processing to p52. Severe B-cell deficiency affects mature and transitional cells, mimicking the action of rituximab. This phenotype appears to be due to disruption of canonical and noncanonical nuclear factor κB pathways by the mutant p100 molecule. These findings could be informative for therapeutics as well as immunodeficiency.
Introduction
Primary antibody deficiency diseases can be divided broadly into those that present in early infancy and those that present later in life.1 The former are usually characterized by agammaglobulinemia and absence of circulating B cells resulting from a complete arrest in B-cell ontogeny before expression of the mature surface immunoglobulin (also known as B-cell receptor [BCR]).2 The second category, which includes the syndromes of common variable immune deficiency (CVID) defined by a reduction or absence of IgG plus either immunoglobulin A (IgA) or IgM, is more heterogeneous with regard to age of onset, severity and spectrum of immunoglobulin deficiency, nature of B-cell deficiency, and other immune cell phenotypes.3 In CVID, the total number of circulating B cells is often normal, although there may be defects in mature B-cell differentiation.3 Primary antibody deficiency provides unique insights into B-cell maintenance and survival, relevant not only to immune deficiency itself, but also to potential therapeutic targets for autoimmune disease and lymphoproliferative diseases.
Both BCR and B-cell activating factor receptor (BAFFR or tumor necrosis factor receptor superfamily member 13C [TNFRSF13C]) are important for development and maintenance of B cells, although significant differences exist between man and mouse with regard to the actions of these receptors on B-cell survival and function. This is reflected in phenotypes arising from downstream signaling defects. Approximately 85% of cases of human agammaglobulinemia arise from mutations in BTK, a tyrosine kinase activated by BCR ligation (X-linked agammaglobulinemia [XLA], Online Mendelian Inheritance in Man [OMIM] 600755).4 XLA is characterized by early arrest of B-cell development in the bone marrow.5 In mice, mature B cells form in the complete absence of Btk even though BCR expression is necessary for B-cell survival.6,7 By contrast, human B-cell development is independent of the interleukin-7 (IL-7)–cytokine receptor common γ chain-JAK3 axis, whereas defects in this pathway cause severe B-cell deficiency in mice.8-11
Both BCR and BAFFR activate nuclear factor κB (NF-κB), which comprises 5 related proteins (RelA/p65, NF-κB1/p50, NF-κB2/p52, RelB, c-Rel). We have an incomplete understanding of the actions of this crucial transcription family in human B cells. BCR ligation activates the canonical NF-κB via the Card11-MALT1-Bcl10 complex.12 Human immune deficiencies arising from null mutations of CARD11 and MALT1 indicate that the canonical pathway is crucial for B- and T-cell activation, but these defects alone do not affect numbers of cells in the periphery.13,14 BAFFR ligation activates the noncanonical NF-κB pathway, and deletion of Nfkb2 in mouse models results in disorganization of secondary lymphoid architecture and defective germinal center B-cell formation and differentiation.15 By contrast, deletion of BAFFR itself in mice (Tnfrsf13c−/−) results in severe deficiency of late transitional and follicular B-cell numbers and almost complete absence of marginal zone B cells.16 Human defects in TNFRSF13C17 result in B-cell developmental arrest at the transitional B-cell stage, IgG deficiency, and preservation of IgA production.
Activating somatic mutations in NF-κB family members have been reported in B-cell lymphoma.18,19 Recently, 4 individuals were described with loss-of-function mutations (C-terminal truncation of NF-κB), which resulted in antibody deficiency, normal or slight reduction of circulating B cells, and alopecia areata.20 Here, we report an autosomal-dominant missense mutation of NFKB2. This mutation interferes with processing of p100 to p52. Contrary to findings in humans and mice with truncation variants, the D865G missense mutation results in partial antibody deficiency but absence of mature circulating B cells as well as alopecia areata. The severity of the B-cell phenotype appears to result from a failure to phosphorylate the C-terminal serine residues of p100 and the dominant negative IκB-like action of p100.
Methods
Patients and family
The family described is part of a larger cohort of primary antibody-deficiency kindreds enrolled and recruited through the Australian and New Zealand antibody deficiency allele study. This study has been approved by human research ethics committees at each institution and was conducted in accordance with the Declaration of Helsinki.
Antibodies and flow cytometry
Peripheral blood mononucleated cells (PBMCs) were isolated by density gradient centrifugation on Ficoll, and maintained in complete RPMI containing 10% fetal bovine serum, 2 mM l-glutamine (Sigma-Aldrich), and 100 U/mL penicillin and streptomycin (Sigma-Aldrich). For flow cytometry, PBMCs were washed with fluorescence-activated cell sorter buffer containing 2% fetal bovine serum and 1% of NaN3 in phosphate-buffered saline and maintained at 4°C.
The following antibodies were used: CD19 (SJ25C1), CD27 (L128), CD38 (HB7), CD3 (HIT3A), CD4 (SK3), CD8 (SK1), CCR7 (3D12) (all from BD Biosciences), CD45RA (HI100; Biolegend), CXCR5 (RF8B2; BD Biosciences), PD-1 (J105; eBioscience), and CD31 antibodies (WM59; BD Biosciences). Effector subsets were enumerated by intracellular staining according to standard protocols after 4 hours of stimulation with a leukocyte activation cocktail (BD Biosciences) using the following antibodies: IL-4 (7A3-3; Miltenyi Biotec), IL-10 (JES3-19F1; BD Biosciences), IL-17 (SCPL1362; BD Biosciences), and interferon-γ (25723.11, BD). Regulatory T cells were identified using CD4, CD25 (2A3; BD Biosciences), CD127 (A7R34; eBioscience), and Foxp3 (259D/C7; BD Biosciences). For immunoblot, we used NFKB2 (C5; Santa Cruz Biotechnology), TBP antibody (1TBP18, Abcam), and goat anti-mouse IgG horseradish peroxide antibody (Santa Cruz Biotechnology).
Immunoblotting
Cell lysates were prepared in RIPA buffer with a protease inhibitor cocktail (Roche) and a Halt phosphatase inhibitor (Pierce Net); the lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. DC protein assay (Bio-Rad) was performed according to manufacturer’s instructions; equal amounts of proteins were loaded in each well. Blots were performed with antibodies to: p100/p52 (Santa Cruz Biotechnology), phospho-NF-κB2 p100 serine 866/870 (Cell Signaling), NIK (Cell Signaling), and TATA box (Abcam). Horseradish peroxide–conjugated secondary antibodies were detected with Amersham ELC western blotting system (GE Health Care); band intensity was quantitated with Fujifilm multigauge software and normalized to a loading control.
Immunofluorescence
HEK293 cells were transfected with indicated constructs and treated with TNF or left unstimulated. Cells were cytospun, fixed with 4% paraformaldehyde in phosphate-buffered saline, permeabilized with 1% of triton X, and stained with anti-p65 (Cell Signaling) followed by staining with Alexa Flour 555–conjugated anti-mouse antibody (Cell Signaling). Slides were mounted with anti-fade mounting media containing 4,6 diamidino-2-phenylindole. Images were acquired by Leica SP5 confocal microscope with a ×63 oil immersion objective.
Proliferation assay
Naive CD4+ T cells were bead-sorted (Miltenyi Biotec). Enriched naive CD4+ cells or PBMCs were cultured with anti-CD3/CD28 expansion beads (Miltenyi Biotec) for 4 or 6 days. Proliferation of cells was then analyzed using CellTrace Violet Cell Proliferation Kit (Invitrogen) according to the manufacturer’s instructions.
Monocyte-derived human dendritic cell culture
CD14+ cells were isolated from PBMCs by magnetic anti-human CD14 microbeads according to the manufacturer’s instructions (Miltenyi Biotech). CD14+ cells (1 × 106 cells/mL) were cultured with granulocyte macrophage–colony-stimulating factor (100 ng/mL), IL-4 (30 ng/mL), and 2-mercaptoethanol (50 μM) in complete RPMI1640 for 7 days. CD14+ cells were cultured with or without CD40L (1 μg/mL) for an additional 3 days.
Genotyping and Sanger sequencing
Genomic DNA was isolated from saliva using Oragene-DNA OG-500 kit (DNAgenoTek) according to the manufacturer’s instructions. Genomic DNA was then amplified with primers via a thermocycler. The presence of candidate mutations was then confirmed by Sanger sequencing.
Whole exome capture sequencing
The Illumina paired-end genomic DNA sample preparation kit (PE-102-1001, Illumina) was used for preparing the libraries including end repair, A-tailing, and ligation of the Illumina adaptors. Each sample was prepared with an index using the Illumina multiplexing sample preparation oligonucleotide kit (PE-400-1001, Illumina) and then pooled in batches of 6 in equimolar amounts before exome enrichment. The Illumina TruSeq exome kit (FC-121-1008, Illumina) was used to capture the human exome for each sample pool. Each 6-plex exome-enriched library was sequenced in 2 lanes of an Illumina HiSequation 2000 with version 2 chemistry as 100-bp paired-end reads.
Sequence reads were mapped to the GRCh37 assembly of the reference human genome using the default parameters of the Burrows-Wheeler Aligner (bio-bwa.sourceforge.net).21 Untrimmed reads were aligned by allowing a maximum of 2 sequence mismatches; reads with multiple mappings to the reference genome were discarded along with polymerase chain reaction duplicates. Sequence variants were identified with SAMtools (samtools.sourceforge.net)22 and annotated using Annovar (www.openbioinformatics.org).23 A version of PolyPhen-2 (genetics.bwh.harvard.edu/pph2)24 was used for the calculation of variant effect.
Novel alleles were filtered according to the sum of scores for mouse mutant phenotype in the homologous mouse gene, Mendelian disease associations (OMIM), pathway analysis (gene ontology [GO]), immune system expression (Immgen), and PolyPhen-2 score according to the following scheme: mouse mutant phenotype: nonimmune = 0, nil = 1, immune = 2; OMIM, nonimmune disease = −1, nil = 0, immune disease = 1; GO, nonimmune tissue-specific function = 0, general cellular process = 1, immune-related = 2, proven T- or B-cell function = 4; Immgen, high = 3, medium = 2, low = 1, nil = 0; PolyPhen-2 score.
Gene expression
Mutant and wild-type NFKB2 constructs were obtained from Biomatik, which were then cloned into mammalian expression vector, pcDNA 3.1(−)/myc-His (Invitrogen). The wild-type and mutant vectors were transfected into HEK293 with lipofectamine (Invitrogen). pDONR223 vector containing NIK was obtained from Addgene. NIK was amplified and cloned into mammalian expression vector, pcDNA 3.1(−)/myc-His (Invitrogen). HEK293 cells were co-transfected with NIK vector and either wild-type or mutant NFKB2 vectors, and then incubated for 36 to 48 hours at 37°C with 5% CO2. Transfectants were treated with 100 ng/mL of lymphotoxin α1β2 (Sigma-Aldrich) for 24 hours after 36 to 48 hours of incubation.
Results
We identified an individual with complete B-cell deficiency from within a larger cohort of patients with primary immune deficiencies. She had been diagnosed with CVID at the age of 40 but had a long history of chronic sinusitis, bacterial pneumonia, recurrent intestinal giardiasis, and periodontitis. She also had alopecia areata at age 14 years (see the supplemental information on the Blood Web site). At the time of investigation, she was receiving intravenous immunoglobulin (IVIG) replacement.
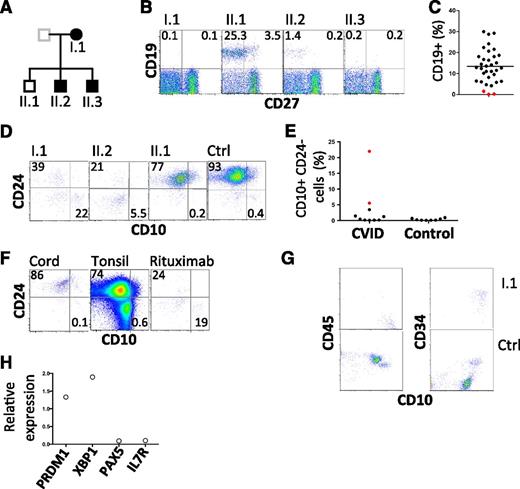
Further investigation of the kindred revealed 2 of 3 offspring with hypogammaglobulinemia, 1 diagnosed at age 20 years and the other in infancy (supplemental information; Figure 1A). Both sons have a history of chronic sinusitis from childhood and remarkably, both have a history of childhood alopecia totalis. One is receiving IVIG, and the other remains healthy despite refusing immunoglobulin replacement therapy. Analysis of peripheral blood samples from the proband and all 3 offspring revealed severe B-cell deficiency in those with hypogammaglobulinemia (Figure 1B-C, Table 1). We failed to detect any transitional B cells in the peripheral blood (supplemental Figure 1). Indeed, careful analysis of the few remaining CD19+ cells in affected individuals revealed an absence of mature and transitional cells and a relative expansion in CD10+ CD24− cells (Figure 1D-E). This population is typically rare in cord blood and tonsil as well as adult peripheral blood, but is relatively prominent in patients who have received rituximab (anti-CD20) (Figure 1F). Consistent with these findings, analysis of a bone marrow biopsy sample obtained from the proband revealed an arrest in early B-cell ontogeny (pro-B cells) (Figure 1G-H). Despite profound B-cell deficiency, serum Ig was measurable (Table 1). Furthermore, antibodies to specific antigens were also detected (tetanus toxoid and 7/14 pneumococcal polysaccharides, Table 1). Consistent with this finding, analysis of transcripts prepared from the aspirate are consistent with the presence of plasma cells (Figure 1H).
Flow cytometric analysis of B lymphocytes. (A) Pedigree with those affected shown in closed symbols. (B) Analysis of circulating B cells. CD19+ CD27− cells represent naive B cells; CD19+ CD27+ cells indicate memory B cells. (C) Summary of B-cell numbers relative to other CVID-affected patients. (D) Analysis of transitional B cells in family members. (E) Summary of CD10hi CD24lo cells in CVID patients and normal controls. I.1 and II.2 are indicated by red symbols. (F) Analysis of CD10 and CD24 expression by CD19+ cells obtained from cord blood, tonsil, and peripheral blood of a patient after treatment with rituximab. Plots are representative of 5 similar samples. (G) Bone marrow leukocytes of proband and control, gated on CD19+ cells to show CD10, CD45, and CD34. CD45+ CD10+ CD34+ cells are pro-B cells and CD45+ CD10+ CD34− cells are pre-B cells. (H) Expression of indicated transcripts in bone marrow aspirate from proband relative to their expression in 2 normal control marrows. Ctrl, control.
Flow cytometric analysis of B lymphocytes. (A) Pedigree with those affected shown in closed symbols. (B) Analysis of circulating B cells. CD19+ CD27− cells represent naive B cells; CD19+ CD27+ cells indicate memory B cells. (C) Summary of B-cell numbers relative to other CVID-affected patients. (D) Analysis of transitional B cells in family members. (E) Summary of CD10hi CD24lo cells in CVID patients and normal controls. I.1 and II.2 are indicated by red symbols. (F) Analysis of CD10 and CD24 expression by CD19+ cells obtained from cord blood, tonsil, and peripheral blood of a patient after treatment with rituximab. Plots are representative of 5 similar samples. (G) Bone marrow leukocytes of proband and control, gated on CD19+ cells to show CD10, CD45, and CD34. CD45+ CD10+ CD34+ cells are pro-B cells and CD45+ CD10+ CD34− cells are pre-B cells. (H) Expression of indicated transcripts in bone marrow aspirate from proband relative to their expression in 2 normal control marrows. Ctrl, control.
Summary of laboratory findings in the members of the kindred
| . | I.1 . | II.1 . | II.2 . | II.3 . |
|---|---|---|---|---|
| IgG (g/L) | 5.53 | 11.4 | 2.7 | 1.64 |
| IgA (g/L) | 0.18 | 1.86 | 0.1 | 0 |
| IgM (g/L) | 0.09 | 0.66 | 0.4 | 0.05 |
| Specific antibodies | On IVIG | Tetanus toxoid 0.16 IU/mL PNab detected for 7/14 serotypes* | On IVIG | |
| Lymphocytes (×109/L) | 2.7 | 1.8 | 1.9 | 2.0 |
| IgE (kU/L) | 6 | 175 | <2 | <2 |
| CD4 (×109/L) | 1.46 | NA | 0.92 | 0.74 |
| CD8 (×109/L) | 0.99 | BA | 0.74 | 1.75 |
| B cells (×109/L) | 0 | 23.1 | 0.02 | 0 |
| . | I.1 . | II.1 . | II.2 . | II.3 . |
|---|---|---|---|---|
| IgG (g/L) | 5.53 | 11.4 | 2.7 | 1.64 |
| IgA (g/L) | 0.18 | 1.86 | 0.1 | 0 |
| IgM (g/L) | 0.09 | 0.66 | 0.4 | 0.05 |
| Specific antibodies | On IVIG | Tetanus toxoid 0.16 IU/mL PNab detected for 7/14 serotypes* | On IVIG | |
| Lymphocytes (×109/L) | 2.7 | 1.8 | 1.9 | 2.0 |
| IgE (kU/L) | 6 | 175 | <2 | <2 |
| CD4 (×109/L) | 1.46 | NA | 0.92 | 0.74 |
| CD8 (×109/L) | 0.99 | BA | 0.74 | 1.75 |
| B cells (×109/L) | 0 | 23.1 | 0.02 | 0 |
NA, not applicable; PNab, antibodies to pneumococcal polysaccharides.
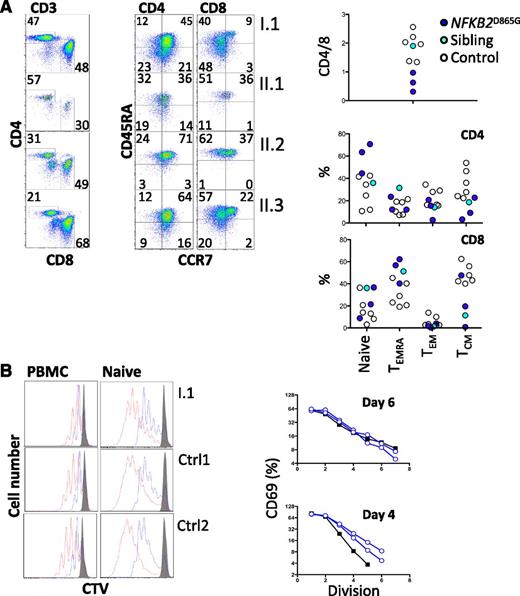
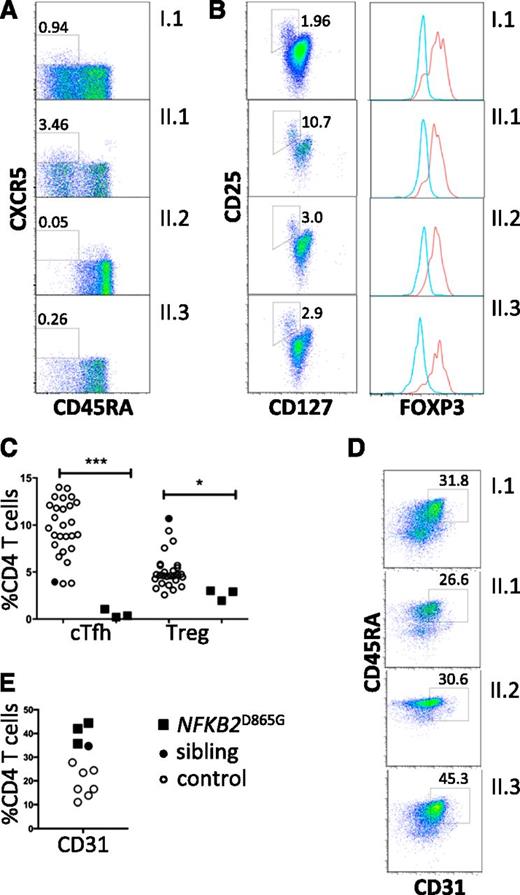
All family members had normal numbers of circulating T cells (Table 1). Analysis of the T-cell compartment revealed an inversion of the normal CD4/8 ratio in patients but no substantial abnormality of T-cell differentiation to memory cells (Figure 2A) or CD4 effector cells (not shown). To test for abnormalities of T-cell activation, PBMCs and purified naive T cells were isolated from both proband and controls and activated with CD3 and CD28. Activation according to CD69 and CD25 induction and proliferation appeared similar in patients and controls (Figure 2B). We did, however, identify a consistent reduction by approximately 20% in circulating CXCR5+ CD45RA− CD4+ T cells (P < .0001; Figure 3A,C). This population is related to follicular helper T cells (Tfh), which are largely confined to secondary lymphoid organs where they provide crucial helper signals for B cells and antibody production.25 The mean frequency of natural regulatory T cells (FoxP3+) among CD4 cells was decreased to approximately 30% of normal in those affected (P = .03; Figure 3B-C). We observed an increase in recent thymic emigrants, as determined by CD31+ and CD45RA+ cell expression (Figure 3D), which raises the possibility of an abnormality in thymic function.26
T-cell differentiation and activation. (A) Enumeration of memory and effector T cells. Representative profiles (left) and summary data (right). (B) Analysis of proliferation of either naive CD4+ T cells (right) separated by negative selection with magnetic beads or CD4+ T cells within PBMCs (left) by dilution of cell trace violet after stimulation with CD3 and CD28. Cells were harvested and analyzed by flow cytometry on day 4 (blue) and day 6 (red). Histograms were gated on CD3 and CD4. Unstimulated control cells are shown (gray). In the same experiment, naive CD4+ T cells were analyzed for activation based on induction of CD69. The percentage of CD69+ cells at each division was determined (day 6, upper panel; day 4, lower panel) and plotted (controls, open blue; I.1, black filled). CTV, cell trace violet; TCM, central memory T cells; TEM, effector memory T cells; TEMRA, CD45RA+ effector memory T cells.
T-cell differentiation and activation. (A) Enumeration of memory and effector T cells. Representative profiles (left) and summary data (right). (B) Analysis of proliferation of either naive CD4+ T cells (right) separated by negative selection with magnetic beads or CD4+ T cells within PBMCs (left) by dilution of cell trace violet after stimulation with CD3 and CD28. Cells were harvested and analyzed by flow cytometry on day 4 (blue) and day 6 (red). Histograms were gated on CD3 and CD4. Unstimulated control cells are shown (gray). In the same experiment, naive CD4+ T cells were analyzed for activation based on induction of CD69. The percentage of CD69+ cells at each division was determined (day 6, upper panel; day 4, lower panel) and plotted (controls, open blue; I.1, black filled). CTV, cell trace violet; TCM, central memory T cells; TEM, effector memory T cells; TEMRA, CD45RA+ effector memory T cells.
Regulatory T cells and recent thymic emigrants. (A) Peripheral blood flow cytometric analyses, gated on CD4+ T cells and analyzed for Tfh-like cells (boxed as CXCR5+ CD45RA−). (B) Regulatory T cells (boxed CD127low, CD25high). Percentage of CD4 T cells within the gated subsets is shown. Histograms show intracellular staining for Foxp3 expression in cells within the CD127low, CD25high gate (red histogram) and within the cells excluded from this gate (blue histogram). (C) Frequencies of circulating Tfh-like cells (cTfh) and Tregs (*P = .03; ***P < .0001). (D) Frequencies of CD31+ CD4+ recent thymic emigrants (E) among CD4 T cells from healthy controls (open circles); healthy sibling (black-filled circles); NFKB2 mutant siblings and parent (black-filled squares). (E) Flow cytometric analysis of peripheral blood mononuclear cells for recent thymic emigrants (CD31+ CD4+).
Regulatory T cells and recent thymic emigrants. (A) Peripheral blood flow cytometric analyses, gated on CD4+ T cells and analyzed for Tfh-like cells (boxed as CXCR5+ CD45RA−). (B) Regulatory T cells (boxed CD127low, CD25high). Percentage of CD4 T cells within the gated subsets is shown. Histograms show intracellular staining for Foxp3 expression in cells within the CD127low, CD25high gate (red histogram) and within the cells excluded from this gate (blue histogram). (C) Frequencies of circulating Tfh-like cells (cTfh) and Tregs (*P = .03; ***P < .0001). (D) Frequencies of CD31+ CD4+ recent thymic emigrants (E) among CD4 T cells from healthy controls (open circles); healthy sibling (black-filled circles); NFKB2 mutant siblings and parent (black-filled squares). (E) Flow cytometric analysis of peripheral blood mononuclear cells for recent thymic emigrants (CD31+ CD4+).
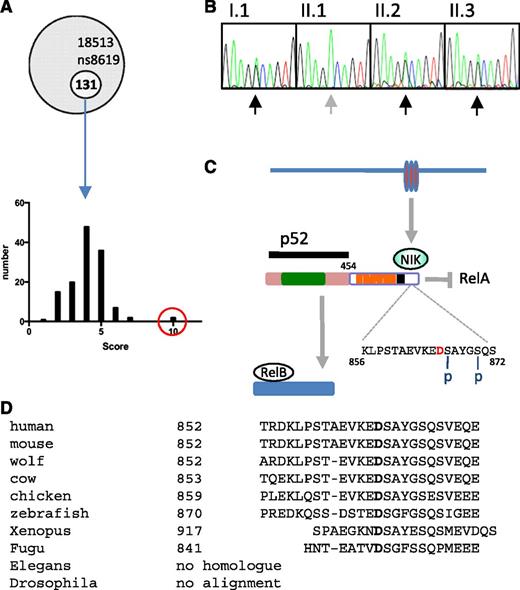
Whole exome sequencing of the proband identified 131 novel mutations, which were then filtered according to pattern of tissue expression, GO pathways, phenotypes of mice harboring genetic mutations in orthologs, disease association (OMIM), and PolyPhen-2 score (see “Methods”) (Figure 4A). From this analysis, 3 clear candidates emerged: TNFRSF10A, TNFRSF1A, and NFKB2. All 3 mutations were confirmed by Sanger sequencing, but only the NFKB2 mutation segregated with the B-cell phenotype. Furthermore, during the course of the project, 2 other kindreds with dominant NFKB2 mutations that resulted in C-terminal truncation and antibody deficiency were described.20
NFKB2 mutation. (A) Frequency histogram of novel alleles according to filter based on tissue expression, phenotype of mice with mutations in orthologs, disease association, GO, and PolyPhen-2 scores (NFKB2 in red circle). (B) Sanger sequencing of NFKB2 (according to pedigree in Figure 1). (C) Summary of p100 processing. Amino acid D in red indicates the location of D865G mutation, which is adjacent to one of the N-terminal phosphorylation sites (S866). Rel homology domain (green), ankyrin repeat domains (orange), death domain (black). (D) Conservation of mutated residue of NF-κB2.
NFKB2 mutation. (A) Frequency histogram of novel alleles according to filter based on tissue expression, phenotype of mice with mutations in orthologs, disease association, GO, and PolyPhen-2 scores (NFKB2 in red circle). (B) Sanger sequencing of NFKB2 (according to pedigree in Figure 1). (C) Summary of p100 processing. Amino acid D in red indicates the location of D865G mutation, which is adjacent to one of the N-terminal phosphorylation sites (S866). Rel homology domain (green), ankyrin repeat domains (orange), death domain (black). (D) Conservation of mutated residue of NF-κB2.
Members of the kindred described here have a heterozygous missense mutation encoding an amino acid substitution of aspartate to glycine at position 865 (NFKB2 D865G) (Figure 4B, Table 2). Based on interrogation of dbSNP, 1000 Genomes Project, Human Genome Mutation Database, and ClinVar databases as well as other CVID kindreds within our cohort, this appears to be a novel mutation. Aspartate 865 is located in the NF-κB–inducing kinase (NIK)-responsive domain of the p100 protein product of NFKB227 and is absolutely conserved in vertebrates from humans to fish (Figure 4C). The substitution with glycine is predicted to be damaging by 3 different in silico tests (Table 2).
Summary of mutation
| . | NFKB2 . |
|---|---|
| Variant | chr10:104162024A>G |
| g.2594 A>G; c.2594A>G; | |
| Protein | D865G |
| Mutation taster | Disease causing P = 0.999979076300663 |
| SIFT | Damaging (0.04) |
| PolyPhen-2 | Probably damaging 1.0 (sensitivity 0, specificity 1.0) |
| . | NFKB2 . |
|---|---|
| Variant | chr10:104162024A>G |
| g.2594 A>G; c.2594A>G; | |
| Protein | D865G |
| Mutation taster | Disease causing P = 0.999979076300663 |
| SIFT | Damaging (0.04) |
| PolyPhen-2 | Probably damaging 1.0 (sensitivity 0, specificity 1.0) |
Signaling via the noncanonical NF-κB pathway depends on accumulation of NIK (MAP3K14), a serine/threonine protein kinase that becomes stabilized after engagement of several TNF superfamily receptors including lymphotoxin α1β2, BAFF, and CD40.28-31 NIK cooperates with another serine kinase, IκB kinase α (IKKα), to bind the full-length NF-κB2 (p100) protein and phosphorylate it on 2 critical serines, S866 and S870, in the C-terminal processing inhibitory domain (death domain; Figure 4C).27,32 Phosphorylation of these sites allows binding of the ubiquitin ligase SCFβTrCP and polyubiquitination of lysine 855, tagging the p100 protein for limited proteasomal processing to yield the transcriptionally active p52 subunit of NF-κB2.33 The D865G mutation is located immediately adjacent to the critical S866 phosphorylation site.
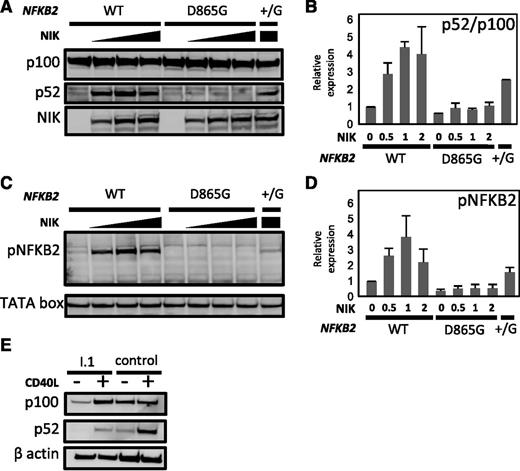
We investigated the effect of the D865G amino acid substitution on p100 processing. First, we transfected HEK293 cells with vectors expressing either wild-type or D865G mutant NFKB2 alleles. Analysis of cell lysates demonstrated dose-dependent processing of normal p100 stimulated by co-transfected NIK. By contrast, NFKB2D865G exhibited near-absence of p100 processing after NIK co-transfection (Figure 5A-B). Only a small residual p52 band was detected, which is probably accounted for by endogenous NF-κB2 in the cell line. Co-transfection of mutant and normal alleles (NFKB2+/D865G) resulted in approximately 50% of normal processing to p52. The defect in NFKB2D865G p100 processing was not corrected by stimulation with lymphotoxin-α. Processing of wild-type p100 was blocked by MG132, a proteasome inhibitor, confirming its dependence on the proteasome (not shown). Mutation of conserved S866 or S870 abolishes p100 processing.27 Next, we demonstrated that the D865G substitution affects p100 phosphorylation (Figure 5C-D), even though this mutation does not involve a serine residue. This would appear to account for the loss of p100 processing.
Effect of D865 > G mutation on NIK-induced p100 processing and serine phosphorylation. (A-B) HEK293 cells were co-transfected with expression vectors encoding wild-type (WT) or D865 > G mutant NFKB2 together with varying amounts of MAP3K14 expression vector encoding NIK. (B) Summary of relative expression of p100 and p52 determined as determined in (A). Results were normalized to the ratio in the absence of NIK. (C-D) Phosphorylation of serines 867 and 870 in response to increasing dose of NIK. (D) Summary of phospho-NFKB2 as determined in (C). TATA box was used as a loading control. (E) p100 processing in response to CD40L in dendritic cells generated in vitro from PBMCs from I.1 or a healthy control.
Effect of D865 > G mutation on NIK-induced p100 processing and serine phosphorylation. (A-B) HEK293 cells were co-transfected with expression vectors encoding wild-type (WT) or D865 > G mutant NFKB2 together with varying amounts of MAP3K14 expression vector encoding NIK. (B) Summary of relative expression of p100 and p52 determined as determined in (A). Results were normalized to the ratio in the absence of NIK. (C-D) Phosphorylation of serines 867 and 870 in response to increasing dose of NIK. (D) Summary of phospho-NFKB2 as determined in (C). TATA box was used as a loading control. (E) p100 processing in response to CD40L in dendritic cells generated in vitro from PBMCs from I.1 or a healthy control.
Finally, we examined p100 processing in patient cells. B cells are too few in affected patients to examine for a biochemical defect in response to CD40L. Instead, we generated dendritic cells in vitro from donor PBMCs. These cells were then stimulated with CD40L; this revealed a similar defect in p100 processing as we had observed in transfectants (Figure 5E).
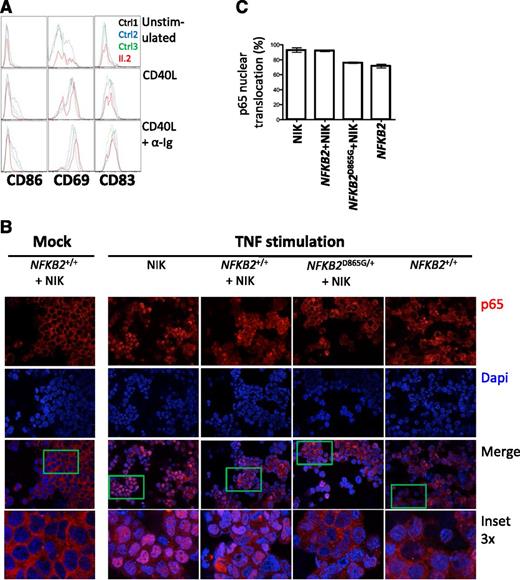
p100 is thought to exert an IκB-like action on the canonical pathway,34 which prompted us to investigate whether the accumulation of p100 exerts a dominant negative action that contributes to the severity of the B-cell phenotype in this syndrome. To directly test the effect of the mutation on both canonical and noncanonical pathways, we compared the actions of CD40L, which is considered a noncanonical stimulus, but also activates the canonical pathway, and anti-Ig (a canonical stimulus) for their abilities to activate rare peripheral B cells from an affected individual (Figure 6A), according to expression of surface antigens generally considered to be NF-κB–responsive (CD86, CD69, and CD83). B cells from patient II.2 exhibit much less CD86 induction but preserved CD69 expression with CD40L compared with healthy controls; the difference was more pronounced with CD40L and anti-Ig stimulation. These findings are consistent with defects in both canonical and noncanonical pathways.
Defective canonical pathway activation in the presence of nonprocessable p100. (A) Analysis of B cells from II.2 relative to controls for expression of CD86, CD69, and CD83 after 24 hours of treatment with indicated stimuli. (B) Nuclear translocation of p65 (red) in HEK293 cells transfected with the indicated constructs after stimulation with TNF. A 4,6 diamidino-2-phenylindole counterstain identifies cell nuclei. (C) Summary of p65 translocation of immunofluorescence confocal.
Defective canonical pathway activation in the presence of nonprocessable p100. (A) Analysis of B cells from II.2 relative to controls for expression of CD86, CD69, and CD83 after 24 hours of treatment with indicated stimuli. (B) Nuclear translocation of p65 (red) in HEK293 cells transfected with the indicated constructs after stimulation with TNF. A 4,6 diamidino-2-phenylindole counterstain identifies cell nuclei. (C) Summary of p65 translocation of immunofluorescence confocal.
Activation of the canonical pathway results in translocation of p65 to the nucleus, where it regulates gene transcription. To further explore the possible action of p100 on canonical pathway signaling, we examined the location of p65 in cells transfected with NIK plus either wild-type or wild-type and D865G NFKB2 (Figure 6B-C). NIK alone results in activation of the noncanonical pathway, processing of p100 and abundant nuclear translocation of p65 in response to a canonical stimulus (TNF). A similar response is observed in the presence of NF-κB2, whereas D865G NF-κB2 results in cytoplasmic retention of p65.
Discussion
Our findings reveal the importance of D865 for NF-κB2 (p100) phosphorylation and processing to p52 for maintaining both integrity of noncanonical NF-κB signaling and efficiency of canonical NF-κB signaling for maintaining normal numbers of human B cells. Several previous lines of evidence have identified the importance of the noncanonical NF-κB pathway for human B-cell survival. NF-κB2 is known to be activated by signals from TNFRSF members lymphotoxin-β receptor, BAFFR, RANKL (receptor activator of NF-κB), and CD40.29,30,35-37 Of these, CD40 and BAFFR are thought to provide B-cell survival signals.38,39
Mutations in CD40 and CD40LG both confer a phenotype of hyper-IgM, with absent germinal centers and absence of class switch recombination.40,41 BAFF acts relatively selectively on the noncanonical NF-κB pathway.42 BAFFR deficiency has been reported to cause a primary antibody deficiency syndrome, based on the phenotypes of 2 siblings, both of whom presented relatively late in life (CVID4, OMIM 606269).17 In one of these individuals, there was an arrest of peripheral B-cell development at the transitional stage of B-cell ontogeny, and a significant deficiency of IgG. In the other sibling, the defect appeared to be milder with detectable mature B cells. Similarly, antibody-mediated blockade of BAFF in humans is an approved therapy but induces a surprisingly gradual decrease in naive circulating B cells.43 Finally, truncation mutations of NFKB2 have been reported to cause antibody deficiency but normal levels of circulating B cells.20
The phenotype of NFKB2D865G/+ is significantly different from that described for human mutations in CD40, CD40LG, and TNFRSF13C (BAFFR), and indeed for truncation mutations of NFKB2.20 NFKB2D865G missense mutation results in unprocessable p100 and consistent and severe B-cell deficiency. Chen et al reported small reductions in B cells in 2 of 4 patients with truncation mutations (855X or 853X), but the mean reduction in total B-cell count is 30-fold greater in patients with the missense mutation described here (5.23% vs 0.16% of PBMCs). We demonstrate defects in B-cell activation via the canonical pathway and reduced nuclear translocation of p65 (Figure 6); we propose that the profound reduction in B cells conferred by NFKB2D865G mutation could be accounted for by the combined effects of reduced noncanonical signaling plus inhibition of the canonical pathway by unprocessed p100.
p100 exhibits IκB-like activity, which serves to inhibit assembly of the p50/RelA complex of the canonical NF-κB pathway as well as RelB/p52 itself and sequester the complexes in the cytoplasm.34,44 In other words, unprocessable p100 acts in a dominant negative manner to impair canonical signaling, while simultaneously compromising noncanonical signaling by haploinsufficiency. Consistent with this possibility, mice deficient for both NFKB1 and NFKB2 have more severe B-cell deficiency and profound maturation arrest than mice with either single deficiency,31,45 although not as severe as the human deficiency observed here. By contrast, Nfkb2−/− mice exhibit subtle reductions in B-cell numbers, normal Ig apart from IgA, but abnormal splenic white pulp architecture with failure of germinal center formation.46 Consistent with the dominant negative action proposed for the human mutation reported here, mice with an Nfkb2 truncating mutation 2 residues distal to D865 exhibit failure of p100 processing but have a relatively mild decrease in mature B cells even in homozygous state, although they have a deficit in lymph node development.44,47
Defects in B-cell survival and differentiation have been reported in patients with mutations affecting BAFFR, NEMO, CD40, and CD40L as well as truncation mutations of NFKB2, but none of these cause B-cell deficiency as severe as we report here.17,20,40,41,48 This is likely because NFKB2D865G disrupts noncanonical signaling normally activated by several ligands, and because the mutation results in impaired canonical NF-κB signaling as well. The noncanonical defect in p52 production is a defect in response to the combined actions of CD40L, CD27, and BAFF—all ligands that normally signal via the noncanonical pathway to maintain B-cell homeostasis. p100 not only functions as a precursor of p52, but also as the fourth IκB protein (along with IκBα, β, and γ [NEMO]), which inhibits nuclear translocation of RelA/p50 by the canonical pathway.34 Thus, noncanonical stimuli result in p100 processing to reduce the restraint on canonical signaling as well. Accumulation of p100 conferred by NFKB2D865G appears to result in enhanced IκB activity, based on dampened response to canonical stimuli (anti-Ig) and reduced nuclear translocation of p65. As in mice, in which combined Nfkb1 and Nfkb2 defects cause much more severe defects than either defect alone, the combined effects on both canonical and noncanonical pathways are likely to explain the comparative severity of the B-cell phenotype.
Two possible explanations emerge to account for differences in B-cell phenotype between truncation and missense mutations. First, D865G results in more profound inhibition of the noncanonical pathway than the truncation mutant. For example, p100 protein translated from the missense mutation fails to undergo either phosphorylation or processing and might sequester IKKα, thereby reducing phosphorylation and processing of the protein product of the normal allele. Second, the full-length nonprocessable D865G p100 inhibits canonical signaling more effectively than the truncated protein. Our data favor the latter possibility. Co-transfection of normal and mutant alleles into HEK293 cells resulted in approximately 50% reduction in p52, arguing against a dominant negative action on the noncanonical pathway (Figure 5).
Although the most obvious phenotype of the NFKB2 mutation described here is B-cell deficiency, all 3 affected individuals exhibited alopecia areata, with no other evidence of other autoimmunity. Taken together with findings reported by Chen et al, we confirm the autosomal-dominant syndrome of antibody deficiency and alopecia arising from NFKB2 mutation. We found no evidence for other autoimmunity (including no ACTH deficiency). This independent confirmation would appear to substantiate an autosomal-dominant syndrome of antibody deficiency and alopecia areata as a consequence of NFKB2 mutation.
Further studies will be necessary to delineate the cause for alopecia. Mouse models have established that NF-κB2 is important for central T-cell tolerance mediated by negative selection and Treg induction.49-51 Noncanonical NF-κB signaling downstream of RANK and LTβR is necessary for normal maturation of the thymic medullary epithelial cells that express AIRE. We observed a significant reduction in natural Tregs. Consistent with this postulate, mutations in AIRE are the most penetrant genetic defects associated with alopecia so far.52 Either way, abnormalities in the noncanonical signaling provide a new mechanism to account for the curious and well-established coincidence of autoimmunity and primary antibody deficiency, and the occurrence of Treg deficiency in some cases of CVID.
NF-κB2 is also important for cell migration by transactivating genes encoding chemokines and their ligands, including CCL19, and CCL21, which are important for thymic retention of T cells. This pathway could account for the increase in recent thymic emigrants in the peripheral blood. We also observed a deficiency of CXCR5+ T cells in the periphery. Although the precise ontogeny of this T-cell subset remains uncertain, there is evidence that they have an enhanced capacity to provide help to B cells when compared with CXCR5- memory T-cell counterparts,53 and mouse studies have shown their dependence on Nfkb2. It is also plausible, however, that this Tfh defect reflects the absence of peripheral blood B cells because B cells are known to provide important trophic signals for this T-cell compartment.54
Finally, this phenotype of the patients reported here is remarkable for the discordance between B-cell deficiency and antibody deficiency. Other examples of late-onset B-cell deficiency have been described in which the phenotype is more consistent with XLA. In XLA, however, there is usually a good correlation between the B-cell deficiency and the severity of the antibody deficiency. By contrast, the D865G NFKB2 mutation appears to cause profound B-cell deficiency, in which Ig levels including antigen-specific antibodies, remain detectable. The persistence of specific antibody in the absence of B cells raises the possibility that medium to long-lived plasma cells can form independently of NF-κB2. It is also possible that mimicking the effect of this mutation to obtain a partial reduction in NF-κB2 might offer therapeutic approaches for B-cell– and antibody-mediated disease, in which the objective is to eliminate ongoing aberrant immune responses while preserving protective immunity already established.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the genomics team at the Australian Phenomics Facility for exome library preparation and the Biomolecular Research Facility at the John Curtin School of Medical Research for performing the sequencing. The authors acknowledge and appreciate the generosity of our patients and their relatives who participated in the study.
This work was supported by National Health and Medical Research Council Project Grants (585464 and 1049760) (D.A.F., M.C.C.) and (1016953) (C.C.G., M.C.C.) and the Bev and Alan Harvey Bequest.
Authorship
Contribution: C.E.L. performed experiments and drafted the manuscript; D.A.F. helped phenotype the patients and draft the manuscript; B.W., M.F., and D.A. undertook the DNA sequencing and bio-informatics analysis; R.C. and N.F. performed experiments; C.C.G. helped draft the manuscript and design experiments; and M.C.C. conceived the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew Cook, Department of Immunology and Translational Research, Level 6 Building 10, Canberra Hospital, Yamba Dr, Woden, ACT 2606, Australia; e-mail: matthew.cook@anu.edu.au.