In this issue of Blood, Fhu et al report that Reed-Sternberg cell-derived lymphotoxin-α activates endothelial cells to enhance T-cell recruitment in classical Hodgkin lymphoma (cHL), a process that is regulated by cyclooxygenase/nuclear factor-κB/activator protein 1 signaling pathways.1
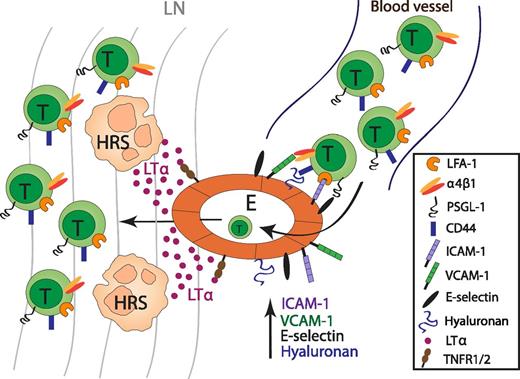
The role of LTα in the recruitment of T cells in cHL. HRS-cell derived LTα acts on endothelial cells to upregulate the expression of the adhesion molecules ICAM-1, VCAM-1, E-selection, and hyaluronan, which are important for T-cell recruitment into lesional lymph nodes in cHL. E, endothelium; LN, lymph node.
The role of LTα in the recruitment of T cells in cHL. HRS-cell derived LTα acts on endothelial cells to upregulate the expression of the adhesion molecules ICAM-1, VCAM-1, E-selection, and hyaluronan, which are important for T-cell recruitment into lesional lymph nodes in cHL. E, endothelium; LN, lymph node.
Hodgkin lymphoma (HL) is one of the most frequent lymphomas occurring in the Western world and can be subdivided into 2 entities: cHL and nodular lymphocyte-predominant HL. cHL is characterized by the presence of a minority of neoplastic cells, the mononucleated Hodgkin (H) and the multinucleated Reed-Sternberg (RS) cells, surrounded by a prominent inflammatory cellular infiltrate.2 Specific localization and confinement of HRS cells within target organs, the lesional lymph nodes, is dependent on a differential chemokine receptor expression profile and the secretion of their cognate ligands within the distinct lymphoid compartments. The chemokine/chemokine receptor system in concert with adhesion molecules mediates migration and lodging behavior of lymphoma cells to and within secondary lymphoid organs.3,4
The cellular microenvironment that surrounds HRS cells is unique and comprises CD4+ and CD8+ T cells, B cells, macrophages, granulocytes, and fibroblasts. There is ample evidence that HRS cells actively recruit these cellular infiltrates by secretion of numerous cytokines and chemokines. In return, HRS survival is dependent on growth- and survival-promoting signals received from infiltrating immune cells.2
However, there remains a lack of knowledge regarding the mechanisms of how HRS cells modulate endothelial cell function to enhance lymphocyte recruitment in affected lymph nodes.
In this study, Fhu et al demonstrate that HRS cell-derived lymphotoxin-α (LTα) acts as a mediator that induces endothelial cell activation to enhance interaction with CD4+ T cells. LTα expression in human HRS cells was visualized by immunohistological staining.
In carefully conducted in vitro experiments, the authors show that LTα acts on endothelial cells to upregulate the expression of adhesion molecules, such as intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and E-selectin. Using a sophisticated flow chamber system, the authors studied T-cell binding to LTα-stimulated human endothelial cell monolayers under defined physiological flow conditions. Naïve and memory T cells were found to interact with the LTα-activated endothelial monolayer. Blocking experiments revealed that not only does the adhesion molecule ICAM-1 regulate T-cell adhesion to endothelial cells, but unexpectedly, it also regulates CD44-hyaluronan interactions (see figure).
In addition to the in vitro experiments, they validated their data by detecting LTα expression in HRS cells as well as tissue stroma. Hyaluronan overexpression was seen in endothelial cells within cHL lymph node biopsies. LTα production in HRS cells is regulated by a complex interplay between nuclear factor-κB (NF-κB), c-Jun N-terminal kinase/AP1, and COX1 signaling pathways. Importantly, using pharmacological inhibitors, the authors discover that cyclooxygenase 1 (COX1) is the dominant regulator of LTα production in HRS cells. Finally, immunohistological staining of cHL tissues identified a strong cytoplasmic c-Fos expression, and inhibitory studies suggest a concomitantly regulated expression of phosphorylated c-Fos induced by both the NF-κB and COX pathways. From these results, the authors conclude that HRS cell-derived LTα activates the endothelium to enhance T-cell recruitment and suggest that therapeutic inhibition of LTα activity could reduce inflammatory cell recruitment and disrupt survival signals for HRS cells in cHL.
The cytokine LTα together with LTβ and tumor necrosis factor (TNF) are structurally related members of the TNF ligand family. LTα and LTβ are expressed on activated lymphocytes, natural killer cells, and lymphoid tissue inducer cells involved in lymphoid organogenesis. All 3 members are indispensable for the formation of B-cell follicles, germinal centers, and follicular dendritic cells. LTα can either be secreted as a homotrimer or it can be retained on the cell surface in heterotrimeric complexes with LTβ (LTα1LTβ2). LTα shares its receptors with TNF, the TNFR1 and TNFR2 receptors, whereas LTα1LTβ2 signals through a distinct receptor, the LTβR. LTα, but not LTβ, plays a crucial role in lymphatic vessel function and lymphangiogenesis.5,6
The relevance of LTα-mediated activation of lymphoid microenvironmental endothelium goes beyond the recruitment of T cells in cHL as described in the work of Fhu et al.
Various studies have provided compelling evidence that LT signaling is involved in inflammation-induced carcinogenesis, in primary tumor development, and in the host’s adaptive antitumor immune response.7 In lymphoid neoplasia, it was recently shown that a reciprocal cross-talk between stromal and lymphoma cells was facilitated by cell-presented LTα1LTβ2, which signaled via LTβR-bearing stromal cells. In a preclinical therapeutic approach, inhibition of this cross-talk with LTβR-immunoglobulin successfully impaired aggressive Myc-driven lymphoma development in mice.8
The study from Fhu et al expands the importance of LTα as a mediator of endothelial cell activation to enhance cellular infiltration in cHL. An unforeseen result of this study was that high concentrations of LTα in HRS cell-derived supernatant and in cHL lymph node tissues enhance hyaluronan expression on endothelial cells. Hyaluronan is a major component of the extracellular matrix and supports hematopoetic but also nonhematopoetic cell migration and proliferation by binding to its ligand CD44. An impact of a hyaluronan-rich tumor microenvironment on the recruitment of inflammatory tumor-associated immune cells and on tumor angiogenesis has been described.9
The data of Fhu et al provide an intriguing link between HRS cell-secreted LTα as an important potent stimulant of endothelial activation and upregulated endothelial expression of adhesion molecules, and hyaluronan, which refines endothelial adhesiveness to naïve T cells.
In this regard, it will be exciting to validate the mechanistic concept arising from the work by Fhu et al using primary endothelial cell culture systems and/or suitable transgenic mouse models for cHL. Overall, this work substantially improves our understanding of how HRS cells interact with the microenvironmental endothelium to support recruitment of inflammatory infiltrates in cHL and may lead to future therapeutic approaches.
Conflict-of-interest-disclosure: The author declares no competing financial interests.