To the editor:
The risk for a hepatitis C virus (HCV) RNA–positive donor to transmit the virus to the recipient through a hematopoietic stem cell transplantation (HSCT) is constant.1 Although this situation is not an absolute contraindication to donation from an HLA-identical sibling,2,3 the main long-term risk is developing cirrhosis, faster and more often than in nontransplanted, HCV-positive patients.4 An undetectable viral load at the time of donation should reduce the risk of hepatitis C in the recipient. Attempts to avoid HCV transmission from a viremic donor have been reported with α-interferon (IFN) given for 3 months5 or with pegylated (peg) IFN-α-2b combined with ribavirin for 5 weeks.6 However, IFN given to the donor might increase the risk of graft-versus-host disease (GVHD).7 Additionally, the delay to get a virological response may be inconsistent with the emergency of the transplant procedure in high-risk leukemias.
Sofosbuvir is a nucleotide analog HCV NS5B polymerase inhibitor administered orally once daily with a potent antiviral activity, broad genotypic coverage, and high genetic barrier to resistance.8 Among patients with genotype 1 infection who received sofosbuvir combined with ribavirin for 24 weeks, the viral decay was observed within the very first days of treatment, and 68% of naive patients had sustained virological response (SVR) 12 weeks after the end of therapy.8
We report here the use of sofosbuvir in an HCV-RNA-positive identical sibling in order to transplant the patient with an HCV-free graft.
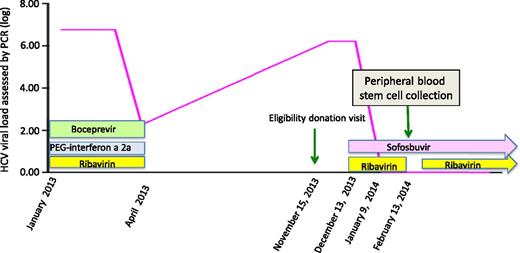
An HCV-antibody- and HCV-RNA-negative, 37-year-old woman presented in August 2013 with acute myeloid leukemia with a complex karyotype. After a daunorubicin-aracytine induction, she achieved a complete cytologic and cytogenetic remission on October 4, then received a consolidation course. Because of the poor prognosis of the leukemia, we searched for a familial donor. Among 6 siblings, the only HLA-identical brother, aged 49, revealed a recent history of chronic HCV infection with genotype 1a. From January 2013, he received triple therapy including peg-IFN-α-2a, ribavirin, and boceprevir. The treatment failed (HCV-RNA: 2.27 log IU/mL) and was stopped at week 12, in April 2013. At the eligibility donation visit in November, HCV-RNA was 6.22 log IU/mL; the alanine and aspartate aminotransferase levels were 2 and 3 times the normal upper limits, respectively. A quick search for an alternative donor failed. The transplant, initially planned for December 12, was delayed, and the patient received an additional consolidation course to maintain remission. The brother was treated with sofosbuvir 400 mg/d in a compassionate use, combined with ribavirin (1200 mg/d) from December 13. The viral load decreased from day 6 of treatment (Figure 1) and was undetectable on January 9, 2014, and the liver tests normalized. The treatment was well tolerated, with only a slight decrease of hemoglobin level from 14.8 to 12.9 g/dL, likely attributable to ribavirin. We finally proceeded to a peripheral blood stem cell transplantation conditioned with fludarabine, Busilvex, and antithymocyte globulin on February 13, while the patient was still in first remission. The stem cell collection was performed under sofosbuvir and after ribavirin had been transitorily stopped 10 days before. Two cytaphereses collected 3.12 × 106 CD34+ cells, and the graft tested negative for HCV-RNA. The recipient’s HCV-RNA was constantly negative, and transaminases did not increase. At day 120 after transplant, the patient is at home, with normal liver tests and no GVHD, and is HCV-RNA negative.
Evolution of the HCV-RNA viral load (log) over time in a stem cell transplantation donor treated with sofosbuvir and ribavirin before stem cell collection.
Evolution of the HCV-RNA viral load (log) over time in a stem cell transplantation donor treated with sofosbuvir and ribavirin before stem cell collection.
This case illustrates the remarkable efficacy of sofosbuvir, combined with ribavirin, for quickly clearing HCV load and the feasibility of using it for HSCT donation. This combination has several advantages: (1) the short time required to get an undetectable viral load (27 days in this case); (2) the safety and tolerability of the drugs, as well as the absence of hematopoietic toxicity of sofosbuvir; and (3) the avoidance of IFN and its risk of stimulating GVHD. The donor has now received 24 weeks of sofosbuvir and ribavirin,8 and SVR will be assessed in 12 weeks. Because of the availability of sofosbuvir in December 2013, we chose it as the optimal IFN-free regimen, combined with ribavirin.8 However, more effective antiviral treatment combining at least 2 direct antiviral agents for 12 weeks should be optimal in the future with a similar early effect on the viral load in such situations.9,10
Authorship
Contribution: F.B., C.H., and C.C. designed the study and wrote the manuscript; and C.H., C.R., J.-L.B., E.G., and S.M. collected the data and provided study patients and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Cordonnier, Hematology Department, Henri Mondor University Hospital, 94000 Créteil, France; e-mail: carlcord@club-internet.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal