Key Points
Activation of PMNs at the site of injury is required for thrombin generation.
P2X1 receptor expressed on both PMNs and platelets is crucial to initiate thrombosis.
Abstract
Adenosine triphosphate (ATP) and its metabolite, adenosine, are key regulators of polymorphonuclear neutrophil (PMN) functions. PMNs have recently been implicated in the initiation of thrombosis. We investigated the role of ATP and adenosine in PMN activation and recruitment at the site of endothelial injury. Following binding to the injured vessel wall, PMNs are activated and release elastase. The recruitment of PMNs and the subsequent fibrin generation and thrombus formation are strongly affected in mice deficient in the P2X1-ATP receptor and in wild-type (WT) mice treated with CGS 21680, an agonist of the A2A adenosine receptor or NF449, a P2X1 antagonist. Infusion of WT PMNs into P2X1-deficient mice increases fibrin generation but not thrombus formation. Restoration of thrombosis requires infusion of both platelets and PMNs from WT mice. In vitro, ATP activates PMNs, whereas CGS 21680 prevents their binding to activated endothelial cells. These data indicate that adenosine triphosphate (ATP) contributes to polymorphonuclear neutrophil (PMN) activation leading to their adhesion at the site of laser-induced endothelial injury, a necessary step leading to the generation of fibrin, and subsequent platelet-dependent thrombus formation. Altogether, our study identifies previously unknown mechanisms by which ATP and adenosine are key molecules involved in thrombosis by regulating the activation state of PMNs.
Introduction
Thrombus formation is an important mechanism required for preserving the integrity of the circulatory system and to prevent blood loss after vessel wall injury. Platelets, endothelial cells, and leukocytes, and in particular PMNs are key partners involved in thrombus formation. Recent studies have shown that PMNs are present at the site of thrombus formation.1-3 In a ferric chloride model of thrombosis, Massberg el al showed that neutrophil elastase and cathepsin G, 2 proteases released by activated PMNs, are required for thrombus formation by inactivating tissue factor (TF) pathway inhibitor, the main inhibitor of the coagulation cascade.3 von Brühl et al described in a mouse model of deep venous thrombosis, the crucial role of PMNs in the early phase of venous thrombosis.2 Recently, we demonstrated, in a laser-induced endothelial injury model, that PMNs are the first cells to accumulate at the site of injury, before platelets. PMNs recruited to the injured vessel wall express TF, the main activator leading to the initiation of the blood coagulation cascade in vivo.1 Depletion of PMNs by the use of Ly-6G2 or Gr-11 antibodies significantly decreases the size of both thrombus formation and fibrin generation. Altogether, these recent findings present PMNs as a crucial partner for both arterial and venous thrombus formation. However, molecular mechanisms involved in PMN recruitment and activation leading to thrombus formation and fibrin generation remains to be elucidated.
Under physiological conditions, ATP is constitutively released by the endothelium.4 ATP is then metabolized and successively converted in adenosine 5′-diphosphate, adenosine 5′-monophosphate (AMP), and adenosine via the action of 2 ecto-nucleotidases: CD39 and CD73.5,6 When activated, the endothelium releases high quantities of ATP.7 ATP and adenosine act as signaling molecules through their interaction with specific cell-surface purinergic receptors. The purinergic receptors mediating the action of ATP belong to 2 subclasses: the G-protein–coupled P2Y receptors and the ATP-gated P2X ion channels. ATP is thus the natural agonist of P2X1 ion channels. In vivo, previous studies showed that P2X1-deficient mice present significantly reduced thrombosis following a laser-induced injury of cremaster arterioles,8 although the platelet count was not affected by the deficiency.9 This effect was attributed to the action of ATP on platelet activation. However, the P2X1 receptor is not only present in platelets but also in PMNs where it contributes to chemotaxis.10 Adenosine acts via the P1 purinergic receptors: A1, A2A, A2B, and A3, present on PMNs surfaces. Depending on the receptor involved, adenosine exerts various effects on PMN functions.11 Of interest, the A2A receptor is known to inhibit adhesion and infiltration of PMNs, as well as to inhibit PMN activation.11
In this study, we used P2X1-deficient mice and the specific agonist of the A2A receptor, CGS 21680, to investigate the role of ATP and adenosine in PMN activation and recruitment in the in vivo laser-induced injury model. We found that the absence of P2X1 ion channels strongly inhibited PMN accumulation, and subsequently, thrombus formation at the site of injury. To restore thrombosis in the P2X1-deficient mice, infusion of both wild-type (WT) platelets and PMNs is required, since the injection of WT PMNs only increases the generation of fibrin. Last, the stimulation of the A2A receptor inhibits thrombus formation by preventing PMN activation and accumulation at the site of injury. We propose that ATP, released by activated endothelial cells, acts on the P2X1 receptor to attract and activate PMNs and is indispensable for thrombin generation and platelet accumulation at the site of injury. In contrast, adenosine, mainly produced by resting endothelial cells, maintains the PMNs in an inactivated state. We showed in this study, that ATP and its metabolite adenosine, represent key factors in regulating the initiation of thrombus formation.
Methods
Mice
WT C57BL/6J mice were from Elevage Janvier (Le Genest-Saint-Isle, France). Specific pathogen-free homozygous P2X1−/− have been described previously.12,13 All animal care and experimental procedures were performed as recommended by the European Community Guidelines (directive 2010/63/UE) and approved by the Marseille Ethical Committee #14 ( protocol number: 27-28092012).
Antibodies and reagents
The rat anti-mouse CD41 antibody was obtained from Emfret (Eibelstadt, Germany). PE rat anti-mouse Ly-6G antibody, purified rat anti-mouse Ly-6G and Ly-6C antibody, and allophycocyanin-conjugated CD11b were from BD Biosciences (Le Pont-de-Claix, France). Fluo-4-AM and BODIPY-FL–labeled DQ-elastin conjugate fragment were obtained from Life Technologies (Saint-Aubin, France). A mouse anti-human fibrin II α-chain antibody (clone NYBT2G1) was from Accurate Chemical and Scientific (Westbury, NY). Anti–Gr-1 PE labeled antibody, anti-PE microbeads, and anti-CD16 microbeads were obtained from Miltenyi Biotec (Paris, France). CGS 21680 and NF449 were purchased from Tocris (Lille, France). Prostaglandin I2 was obtained from Merck (Molsheim, France). Apyrase grade VI, dimethylsulfoxide, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France).
Isolation and culture of human umbilical vein endothelial cells (HUVECs) and culture
HUVECs were isolated from the cord vein as described14 and obtained from healthy donors in compliance with French legislation. In all experimental conditions, cells were used at passage lower than passage 4 and viability of the cells was determined by the incorporation of the Trypan Blue.
Isolation of human PMNs
Human PMNs were obtained from healthy volunteers. Peripheral blood was collected on citrated tubes. Red blood cells were lysed for 10 minutes and centrifuged 300g for 10 minutes at room temperature. The pellet was washed twice and resuspended in buffer (phosphate-buffered saline [PBS], BSA 0.5%, and EDTA 2 mM), completed with an anti-CD16 microbeads (Miltenyi Biotec) for 30 minutes in the dark at 4°C. Cells were washed and resuspended in buffer (PBS, BSA 0.5%, and EDTA 2 mM). PMNs from cells suspension were isolated using the LS column (Miltenyi Biotec).
Fluxion flow chamber
In experiments addressing PMN-endothelium interactions, HUVECs were grown in the flow chamber (Bioflux 1000 System; Fluxion Biosciences, San Francisco, CA), and precoated with gelatin overnight until they reached 100% of confluence. HUVECs were either left in their resting state or treated with 10 ng/mL interleukin-1β (IL1-β) for 4 hours at 37°C prior to the experiments. Freshly isolated human PMNs were perfused over HUVECs at a shear rate of 140 per second (1 dyn/cm2) at 37°C. The flow chamber and fluorescent-tagged cells (Calcein-AM labeled) were observed with an inverted fluorescence microscope (Nikon Eclipse TI-S) using ×10 magnification, and image acquisition was performed with BioFlux software.
Flow cytometry
Analyses of cell surface CD11b, CD18, and CD62L expression on purified human PMNs were performed with a flow cytometer (Gallios; Beckman Coulter) using appropriate antibodies. A minimum of 100 000 cells were analyzed for each condition.
Isolation of mouse PMNs
PMNs from peripheral blood were isolated as previously described15 using GR-1–coupled magnetic beads (anti–Ly-6G Microbead Kit; Miltenyi Biotec).
Calcium mobilization by PMNs in vivo
Mouse PMNs from peripheral blood were isolated as described above and were resuspended at 3 × 106 cells/mL in PBS/EDTA 2 mM. PMNs were incubated with 3 μM Fluo-4-AM (Life Technologies) for 40 minutes in the dark at room temperature, centrifuged, and resuspended in PBS at 3 × 106 PMNs/200 μl before injection into a recipient mouse.
Detection of neutrophil elastase activity in vivo
Detection of neutrophil elastase activity in vivo was performed using BODIPY-FL–labeled DQ-elastin conjugate fragment from Life Technologies. WT mice were infused with 0.3 μg/g of mouse with BODIPY-FL–labeled DQ-elastin. After laser-induced vessel wall injury, neutrophil elastase levels were observed by excitation at 488 nm, and images were recorded over time.
Depletion of mouse PMNs and platelets
Preparation of washed platelets
Blood was collected from mice in a citrate solution (anticoagulant citrate dextrose solution: 85 mM trisodium citrate, 67 mM citric acid, and 111.5 mM glucose, pH 4.5) in the presence of 0.5 mM prostacyclin (PGI2; Calbiochem-Novabiochem) and 0.02 U/mL apyrase (Sigma-Aldrich). Platelets were separated from freshly drawn blood by centrifugation and washed twice in CGS buffer (13 mM trisodium citrate, 30 mM dextrose, and 120 mM NaCl, pH 7.0) in the presence of 0.04 U/mL apyrase and 500 nM PGI2, as previously described.18 Washed platelets were resuspended at a concentration of 1 × 108 platelets/mL in Tyrode buffer (138 mM NaCl, 2.9 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 5.5 mM glucose, 1.8 mM CaCl2, and 0.4 mM MgCl2, pH 7.4) containing 0.2% BSA (Sigma-Aldrich).
Intravital optical system with spinning disk confocal microscopy
Intravital videomicroscopy of the cremaster muscle microcirculation was performed as previously described19 using an Intelligent Imaging Innovations system. Confocal imaging was obtained via a spinning disk (Intelligent Imaging Innovations, Denver, CO).
Laser-induced injury
Antibodies or exogenously labeled mouse leukocytes were infused through the jugular vein into the circulation of an anesthetized mouse. Vessel wall injury was induced with a nitrogen dye laser (MicroPoint; Photonics Instruments), focused through the microscope objective and aimed at the vessel wall on the cremaster.20 Image analysis was performed using SlideBook (Intelligent Imaging Innovations). Fluorescence data were analyzed as previously described to determine the median of fluorescent intensity signal over time.19
Statistics
Significance was determined by the Wilcoxon rank-sum test for the in vivo experiments and Student t test for the in vitro experiments. Differences were considered significant at P < .05.
Results
PMNs are activated when they bind to the injured vessel wall
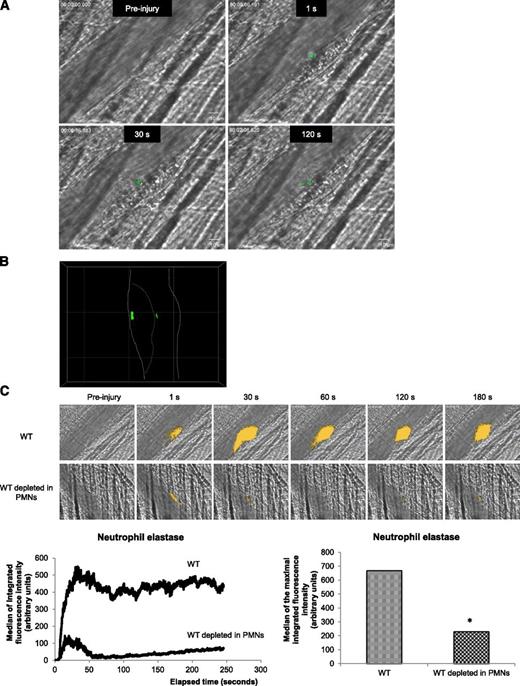
Recently, we demonstrated that PMNs are the first cells to accumulate to the vessel wall following a laser-induced injury. Interactions of PMNs with activated endothelial cells is required for the initiation of thrombus formation.1 PMN activation is accompanied by an increase in intracellular calcium mobilization.21,22 To determine whether PMNs accumulating at the site of injury are activated, exogenously Fluo-4-AM–labeled PMNs from WT mice were infused into a WT recipient mouse. Calcium mobilization (depicted in green in Figure 1A) in exogenous PMNs accumulating in the developing thrombus was detected following an excitation at 488 nm. Labeled PMNs accumulated and displayed an increase in intracellular calcium mobilization at the site of injury (Figure 1A). As expected, a confocal intravital microscopy analysis indicated that activated PMNs (depicted in green) directly interact with the vessel wall and accumulate in the growing thrombus (Figure 1B). Upon activation, PMNs release the serine protease neutrophil elastase.23 In vivo, we visualized the secretion of this enzyme using a DQ elastin fragment infused into the bloodstream. After infusion of the DQ elastin fragment, a fluorescent signal was detected immediately after the laser-induced injury, when PMNs bind to the endothelial cells (Figure 1C, right upper panel and right lower panel) and increased over time (Figure 1C, left lower panel). When 90% to 99% of circulating PMNs were depleted from the mouse using the Gr-1 antibody,16 the signal corresponding to neutrophil elastase activity (green) was reduced by more than 80% (Figure 1C, right upper panel and right lower panel). Together, these results indicate that following a laser-induced endothelial injury, PMNs accumulating at the site of injury are immediately activated and secrete the neutrophil elastase.
PMNs are activated following a laser-induced injury on the cremaster. (A) PMNs isolated from WT mice were loaded with Fluo-4-AM. Exogenous PMNs (3 × 106 cells) were infused into the circulation of a recipient WT mouse. Following a laser-induced vessel wall injury, thrombus formation was observed and images recorded over time. Representative composite images of fluorescence and brightfield data depicting thrombus formation showing calcium mobilization in Fluo-4-AM–loaded PMNs (green) in a WT mouse (representative of 30 thrombi performed in 3 mice). (B) Confocal imaging of calcium mobilization in Fluo-4-AM–loaded PMNs (green). The solid line indicates the boundary of the vessel and the dotted line indicates the boundary of the thrombus. (C) Representative pictures showing the visualization of neutrophil elastase activity in the bloodstream of a WT mouse at the site of a laser-induced injury before (upper panel) or after (lower panel) depletion of PMNs. Neutrophil elastase activity is depicted in yellow. Graphs represent the medians of neutrophil elastase-integrated fluorescence intensity (left) and the maximal-integrated fluorescence intensity of neutrophil elastase (right) in WT mice in presence (32 thrombi in 3 mice) or absence (32 thrombi in 3 mice) of PMNs. *P < .05.
PMNs are activated following a laser-induced injury on the cremaster. (A) PMNs isolated from WT mice were loaded with Fluo-4-AM. Exogenous PMNs (3 × 106 cells) were infused into the circulation of a recipient WT mouse. Following a laser-induced vessel wall injury, thrombus formation was observed and images recorded over time. Representative composite images of fluorescence and brightfield data depicting thrombus formation showing calcium mobilization in Fluo-4-AM–loaded PMNs (green) in a WT mouse (representative of 30 thrombi performed in 3 mice). (B) Confocal imaging of calcium mobilization in Fluo-4-AM–loaded PMNs (green). The solid line indicates the boundary of the vessel and the dotted line indicates the boundary of the thrombus. (C) Representative pictures showing the visualization of neutrophil elastase activity in the bloodstream of a WT mouse at the site of a laser-induced injury before (upper panel) or after (lower panel) depletion of PMNs. Neutrophil elastase activity is depicted in yellow. Graphs represent the medians of neutrophil elastase-integrated fluorescence intensity (left) and the maximal-integrated fluorescence intensity of neutrophil elastase (right) in WT mice in presence (32 thrombi in 3 mice) or absence (32 thrombi in 3 mice) of PMNs. *P < .05.
Activation of the A2A receptor prevents PMN accumulation at the site of laser-induced injury
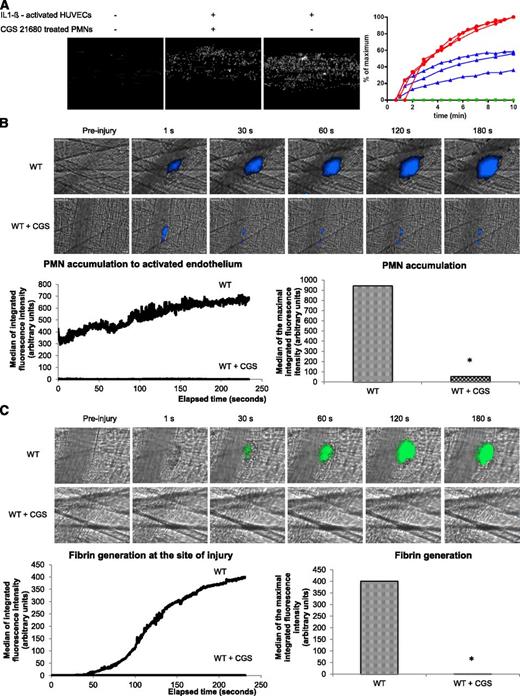
To determine if the A2A receptor was involved in the activation of PMNs, we next used an agonist for the A2A receptor, CGS 21680. At a concentration of 1 µM, this compound mimics the effect of adenosine on PMNs and inhibits PMN migration,24 efflux of Ca2+,25 as well as ROS production and degranulation.26 In vitro, in flow chambers, purified human PMNs bind on IL1-β–activated HUVEC cells over time (Figure 2A, curve red) but not on nonactivated HUVEC cells (Figure 2A; control, curve green). This binding was significantly reduced when PMNs were treated with the CGS 21680 at 1 μM (Figure 2A, curve blue).
CGS 21680 prevents PMN accumulation and fibrin generation in WT mice. (A) Representative images of adherent PMNs pretreated with CGS 21680 (middle panel) or not (left and right panels) on nonactivated HUVECs (left panel) or IL1-β–activated HUVECs (middle and right panels). The graph represents the percentage of adherent PMNs over time on nonactivated (green curve) or IL1-β–activated HUVECs in presence (blue curve) or absence of CGS 21680 (red curve). (B-C) Thrombus formation was induced using a nitrogen dye laser on the cremaster. PMNs (B, upper panel) and fibrin (C, upper panel) were detected using the Ly-6G and fibrin-specific antibodies, respectively, before or after infusion of CGS 21680. Kinetics of PMN accumulation (B, lower panel) and fibrin generation (C, lower panel) at the site of laser-induced injury was compared before or after infusion of CGS 21680 (30 thrombi in 3 mice for each condition). The graphs depict the median of the maximal integrated fluorescence intensity of PMNs (B) and fibrin (C) in presence or absence of CGS 21680. *P < .05.
CGS 21680 prevents PMN accumulation and fibrin generation in WT mice. (A) Representative images of adherent PMNs pretreated with CGS 21680 (middle panel) or not (left and right panels) on nonactivated HUVECs (left panel) or IL1-β–activated HUVECs (middle and right panels). The graph represents the percentage of adherent PMNs over time on nonactivated (green curve) or IL1-β–activated HUVECs in presence (blue curve) or absence of CGS 21680 (red curve). (B-C) Thrombus formation was induced using a nitrogen dye laser on the cremaster. PMNs (B, upper panel) and fibrin (C, upper panel) were detected using the Ly-6G and fibrin-specific antibodies, respectively, before or after infusion of CGS 21680. Kinetics of PMN accumulation (B, lower panel) and fibrin generation (C, lower panel) at the site of laser-induced injury was compared before or after infusion of CGS 21680 (30 thrombi in 3 mice for each condition). The graphs depict the median of the maximal integrated fluorescence intensity of PMNs (B) and fibrin (C) in presence or absence of CGS 21680. *P < .05.
In vivo, PMN accumulation at the site of thrombus formation following a laser-induced injury in WT mice was compared before or after infusion of CGS 21680. PMNs were detected by the infusion of the 1A8 antibody into mice.1 In WT mice, as previously described,1 a fluorescent signal corresponding to PMNs (blue) was detected at the site of laser-induced injury and increased over time (Figure 2B, left upper panel and left lower panel). When the CGS 21680 was infused into the bloodstream, the accumulation of PMNs was strongly decreased (Figure 2B; left upper panel and left lower panel) by up to 90% (Figure 2B; right lower panel). The inhibition of PMN accumulation by CGS 21680 resulted in a significant reduction in fibrin generation (Figure 2C), thereby preventing thrombus formation (data not shown).
Stimulation of the P2X1 receptor on PMNs is important for platelet accumulation during thrombus formation in vivo
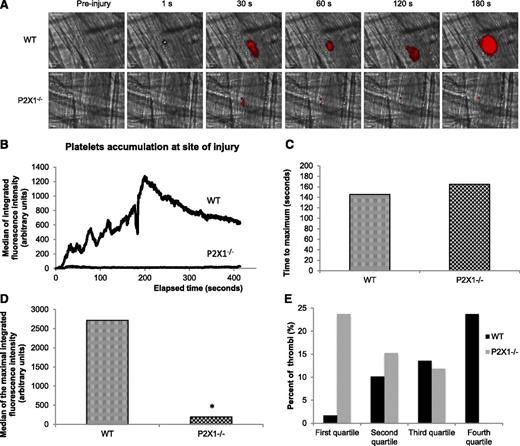
We next hypothesized that ATP could attract and activate PMNs leading to their accumulation at the site of injury and, subsequently, to platelet accumulation and fibrin generation. We first performed flow cytometry experiments where we analyzed the expression of surface activation markers on human and mouse PMNs. As illustrated in Figure 3A, the stimulation of human PMNs with phorbol myristate acetate (positive control) or ATP increases the expression levels of CD11b on PMNs. Consistent with these results, the expression levels of CD18 and CD11b at the surface of PMNs increase, whereas the expression of CD62L decreases when tumor necrosis factor (TNF)-α (positive control) or α, β-meATP, an agonist of the P2X1 receptor, is employed (Figure 3B). A previous study showed that in P2X1−/− mice, thrombus formation was decreased following a laser-induced injury model.8 Identically, we observed a strong decrease in thrombus formation in the P2X1−/− mice in comparison with WT mice (Figure 4A,B). When the time to maximum thrombus size was compared in 29 thrombi in 3 WT mice and 30 thrombi in 3 P2X1−/− mice, no significant difference was observed (Figure 4C). However, a significant reduction in the size of thrombi was observed in P2X1−/− mice in comparison with WT mice (Figure 4D). The largest thrombi observed in P2X1−/− mice were not restricted to a single P2X1−/− animal but were distributed among all of the P2X1−/− mice. This difference in thrombus size between WT and P2X1−/− mice was confirmed by plotting the quartile distribution of thrombus size (Figure 4E). Forty seven percent of the thrombi formed in P2X1−/− mice were present in the first quartile, representing the smallest thrombi, vs 3% for the WT mice. Only 3% of the thrombi formed in P2X1−/− mice were distributed in the fourth quartile corresponding to the largest thrombi, vs 45% for the WT mice. These results indicate that the maximal size of the thrombus was significantly reduced in P2X1−/− mice in comparison with WT mice, although the kinetics of thrombus formation was similar.
Characterization of P2X1 activation at the surface of PMNs. (A-B) Expression of CD11b (A), CD11b, CD18, and CD62L (B) at the surface of human PMNs (the gate of PMNs were based on their size (Side Scatter [SS]) and their granulometry (Forward Scatter [FS]) challenged with 10 μM ATP and 0.5 μM phorbol myristate acetate (PMA) (A) or 10 μM α, β-meATP, and 200 U/ml TNF-α (B), (n = 10, *P < .05).
Characterization of P2X1 activation at the surface of PMNs. (A-B) Expression of CD11b (A), CD11b, CD18, and CD62L (B) at the surface of human PMNs (the gate of PMNs were based on their size (Side Scatter [SS]) and their granulometry (Forward Scatter [FS]) challenged with 10 μM ATP and 0.5 μM phorbol myristate acetate (PMA) (A) or 10 μM α, β-meATP, and 200 U/ml TNF-α (B), (n = 10, *P < .05).
Platelet accumulation is reduced in P2X1−/− mice following a laser-induced injury on the cremaster. (A) Representative composite images of fluorescence and brightfield data depicting thrombus formation (in red) in WT (upper panel) and in P2X1−/− mice (lower panel). (B) The median of platelet-integrated fluorescence (y-axis) based on 29 thrombi was performed in 3 WT mice and 30 thrombi in 3 P2X1−/− mice and were represented over time. (C) The graph depicts the distribution of the time to acquire the maximal size for each thrombus in WT and P2X1−/− mice. NS (P < .05). (D) The graph depicts the maximal of integrated fluorescence intensity for each thrombus in WT and P2X1−/− mice. *P < .05. (E) The quartile distribution of the maximal integrated platelet fluorescence for each thrombus in WT and P2X1−/− mice. A total of 29 thrombi in WT mice and 30 thrombi in P2X1−/− mice were ranked, and the percentage of thrombi of each genotype was determined independently in each quartile of the rank order. Black bar, WT mice; gray bars, P2X1−/− mice.
Platelet accumulation is reduced in P2X1−/− mice following a laser-induced injury on the cremaster. (A) Representative composite images of fluorescence and brightfield data depicting thrombus formation (in red) in WT (upper panel) and in P2X1−/− mice (lower panel). (B) The median of platelet-integrated fluorescence (y-axis) based on 29 thrombi was performed in 3 WT mice and 30 thrombi in 3 P2X1−/− mice and were represented over time. (C) The graph depicts the distribution of the time to acquire the maximal size for each thrombus in WT and P2X1−/− mice. NS (P < .05). (D) The graph depicts the maximal of integrated fluorescence intensity for each thrombus in WT and P2X1−/− mice. *P < .05. (E) The quartile distribution of the maximal integrated platelet fluorescence for each thrombus in WT and P2X1−/− mice. A total of 29 thrombi in WT mice and 30 thrombi in P2X1−/− mice were ranked, and the percentage of thrombi of each genotype was determined independently in each quartile of the rank order. Black bar, WT mice; gray bars, P2X1−/− mice.
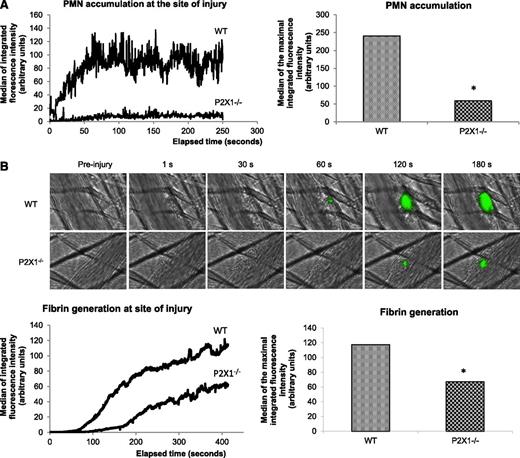
Interestingly, PMN accumulation was also strongly decreased in mice lacking the P2X1 receptor in comparison with WT mice (Figure 5A). The median of 1A8 antibody-integrated fluorescence intensity for both strains indicate a decrease of more than 70% in the P2X1−/− mice in comparison with WT mice. In addition, a significant decrease in fibrin generation was observed in the P2X1−/− mice (Figure 5B). The median of the maximal signal calculated confirmed a decrease in fibrin generation of 50% in comparison with WT mice. Altogether, these data indicate that ATP is important for PMN activation and accumulation at the site of laser-induced injury leading to fibrin generation and thrombus formation.
Accumulation of P2X1-expressing PMNs activated by ATP is required for fibrin generation. (A) The graph represents the median of Ly6-G–integrated fluorescence intensity (left) and the sum of the medians of Ly-6G–integrated fluorescence intensity (right) in WT mice (n = 21 thrombi, 4 mice) or P2X1−/− mice (n = 26 thrombi, 4 mice) (P < .05). (B) Fibrin generation (green) at the site of injury for 29 thrombi in 3 WT mice and 30 thrombi in 3 in P2X1−/− mice. Graphs represent median of fibrin-integrated fluorescence intensity (left) and the sum of the medians of fibrin-integrated fluorescence intensity in the 2 genotypes (right) (*P < .05).
Accumulation of P2X1-expressing PMNs activated by ATP is required for fibrin generation. (A) The graph represents the median of Ly6-G–integrated fluorescence intensity (left) and the sum of the medians of Ly-6G–integrated fluorescence intensity (right) in WT mice (n = 21 thrombi, 4 mice) or P2X1−/− mice (n = 26 thrombi, 4 mice) (P < .05). (B) Fibrin generation (green) at the site of injury for 29 thrombi in 3 WT mice and 30 thrombi in 3 in P2X1−/− mice. Graphs represent median of fibrin-integrated fluorescence intensity (left) and the sum of the medians of fibrin-integrated fluorescence intensity in the 2 genotypes (right) (*P < .05).
The presence of the P2X1 receptor on both PMNs and platelets is important for fibrin generation and thrombus formation in vivo
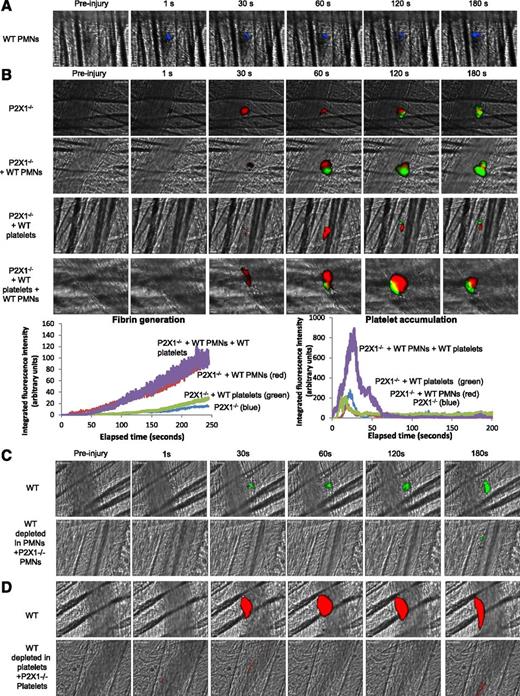
To assess respective and sequential involvement of the P2X1 receptor expressed on PMNs and on platelets in thrombus formation and fibrin generation, PMNs isolated from WT mice were first infused alone into P2X1−/− mice. Following a laser-induced injury, WT PMNs (in blue) accumulated at the site of the laser-induced injury (Figure 6A) and partially restored fibrin generation but not platelet accumulation. The median of fibrin-integrated fluorescence intensity indicates an increase of 50% in the P2X1−/− mice containing WT PMNs in comparison with the control mice and P2X1−/− mice infused with WT platelets (Figure 6B). A similar increase in fibrin generation at the site of injury was observed following infusion of both WT PMNs and WT platelets into P2X1-deficient mice (Figure 6B).
P2X1 receptor expressed on PMNs and platelets is required for fibrin generation and thrombus formation. (A) PMNs isolated from WT mice were loaded with red-orange calcein. Exogenous PMNs (3 × 106 cells) were infused into the circulation of a recipient P2X1−/− mouse. Following a laser-induced vessel wall injury on the cremaster, the PMN accumulation was observed and images recorded over time. (A) Representative composite images of fluorescence and brightfield data depicting calcein-loaded WT PMNs (blue) in a P2X1−/− mouse (representative of 31 thrombi performed in 3 mice). (B) Fibrin generation and thrombus formation at the site of the laser-injury in P2X1−/− mice before and after infusion of isolated WT PMNs, WT platelets, or WT PMNs plus WT platelets. Fibrin is depicted in green, platelets in red (representative of 30 thrombi performed in 3 mice). Graphs represent median of fibrin-integrated fluorescence intensity (left) and platelet-integrated fluorescence intensity (right) in all the conditions tested. (C-D) Fibrin generation (C) and thrombus formation (D) at the site of the laser-injury in WT mice, before and after infusion of isolated P2X1−/−, PMN, or P2X1 −/− platelets after depletion of WT PMNs and WT platelets, respectively (representative of 31 thrombi performed in 3 mice for panel C and 15 thrombi in 3 mice for panel D).
P2X1 receptor expressed on PMNs and platelets is required for fibrin generation and thrombus formation. (A) PMNs isolated from WT mice were loaded with red-orange calcein. Exogenous PMNs (3 × 106 cells) were infused into the circulation of a recipient P2X1−/− mouse. Following a laser-induced vessel wall injury on the cremaster, the PMN accumulation was observed and images recorded over time. (A) Representative composite images of fluorescence and brightfield data depicting calcein-loaded WT PMNs (blue) in a P2X1−/− mouse (representative of 31 thrombi performed in 3 mice). (B) Fibrin generation and thrombus formation at the site of the laser-injury in P2X1−/− mice before and after infusion of isolated WT PMNs, WT platelets, or WT PMNs plus WT platelets. Fibrin is depicted in green, platelets in red (representative of 30 thrombi performed in 3 mice). Graphs represent median of fibrin-integrated fluorescence intensity (left) and platelet-integrated fluorescence intensity (right) in all the conditions tested. (C-D) Fibrin generation (C) and thrombus formation (D) at the site of the laser-injury in WT mice, before and after infusion of isolated P2X1−/−, PMN, or P2X1 −/− platelets after depletion of WT PMNs and WT platelets, respectively (representative of 31 thrombi performed in 3 mice for panel C and 15 thrombi in 3 mice for panel D).
The kinetics of platelet-integrated fluorescence intensity in P2X1−/− mice was not affected by infusion of WT PMNs. Also, the infusion of WT platelets into P2X1-deficient mice did not restore thrombosis and fibrin generation (Figure 6B). However, infusion of both WT platelets and PMNs significantly increased fibrin generation and thrombus formation (Figure 6B). When the reverse experiments were performed, the generation of fibrin was not restored by the infusion of P2X1−/− PMNs into a recipient WT mouse upon PMN depletion (Figure 6C), and P2X1−/− platelets failed to form a thrombus in WT mice (Figure 6D).
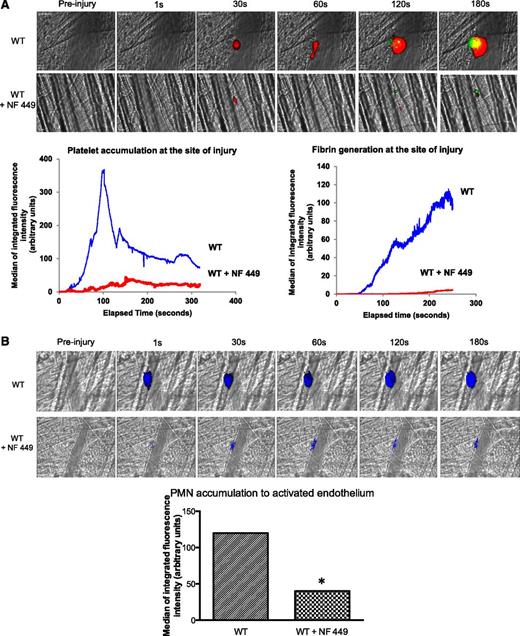
To confirm that P2X1 activation was indispensable for optimal fibrin generation and thrombus formation in the laser model of injury, we next infused a P2X1 antagonist, NF449, in WT mice, as previously described.27 The accumulation of platelets and the generation of fibrin were significantly reduced in the presence of the P2X1 antagonist (Figure 7A). Strikingly, PMN accumulation at the site of injury was almost fully inhibited by NF449 (Figure 7B). Taken together, these results indicate that sequential activation of P2X1 receptors on PMNs and platelets is needed for thrombin generation and thrombus formation, respectively.
The inhibition of P2X1 affects fibrin generation and thrombus formation in WT mice. (A) Fibrin generation and thrombus formation at the site of the laser-injury on the cremaster in WT mice in presence of absence of NF449. The median of fibrin generation and platelet accumulation during thrombus formation was calculated based on 34 thrombi for each condition performed in 3 mice. (B) PMNs were detected using the Ly-6G–specific antibody before or after infusion of NF449. The median of the integrated fluorescent signal was calculated for the 2 conditions (29 thrombi in 3 mice for each condition).
The inhibition of P2X1 affects fibrin generation and thrombus formation in WT mice. (A) Fibrin generation and thrombus formation at the site of the laser-injury on the cremaster in WT mice in presence of absence of NF449. The median of fibrin generation and platelet accumulation during thrombus formation was calculated based on 34 thrombi for each condition performed in 3 mice. (B) PMNs were detected using the Ly-6G–specific antibody before or after infusion of NF449. The median of the integrated fluorescent signal was calculated for the 2 conditions (29 thrombi in 3 mice for each condition).
Discussion
Our results demonstrate that following a laser-induced injury model, activated PMNs bind to the injured vessel wall leading to the generation of fibrin and thrombus formation. We identified the ATP-stimulated P2X1 ion channel as a crucial effector involved in PMN and platelet activation and accumulation at the site of injury, whereas stimulation of the adenosine A2A receptor prevents PMN binding, as well as fibrin generation and thrombus formation. Altogether, these data identify ATP and adenosine as key molecules involved in thrombosis by regulating the activation state of PMNs.
Consistent with our previous study,1 we observed that PMNs constitute a crucial leukocyte subset recruited at the site of injury. Although PMNs have previously been involved in initiation and propagation of thrombosis in both artery3 and vein,2 the molecular mechanisms by which they contributed to thrombus formation have remained elusive. In this study, we show that PMNs recruited to the injured vessel wall release neutrophil elastase and display an increase in intracellular calcium mobilization, which is indicative of their activation.19,22 In accordance with these observations, Massberg et al demonstrated that in response to injury leading to collagen exposure, neutrophil elastase promoted coagulation in vivo by activating the TF pathway inhibitor incorporated into the growing thrombus.3 Several studies have shown that ATP can activate PMNs leading to the reorganization of integrin and selectin surface expression and oxidative burst.23,28 ATP acts through P2 purinergic receptors, in particular through the P2X1 receptor. This receptor is expressed on PMNs10 and is the only receptor for ATP on platelets.29 In vivo studies showed that overexpression of the P2X1 ion-channel on platelets of transgenic mice induce a prothrombotic phenotype by enhancing platelet activation and aggregation,30 whereas in vitro inhibition of this receptor prevents platelet shape change and aggregation in response to low concentrations of collagen.31 In vivo studies in P2X1-deficient mice demonstrated that following laser-induced endothelial injury of mouse cremaster or mesenteric arterioles, thrombosis is inhibited in these genetically modified mice in comparison with WT mice.8,30 Here, we confirm that ATP (and its stable analog, α,βMeATP) activates PMN in vitro. In addition, in vivo, we showed that the lack of the P2X1 receptor induces a strong decrease in PMN accumulation and thrombus formation at the site of laser-induced injury. P2X1 present on PMNs is involved in thrombin generation, whereas its expression at the surface of platelets is needed for thrombus formation. Together, this study provides direct in vivo evidence that stimulation of the P2X1 receptor by ATP represents an important mechanism leading to thrombosis by a sequential activation of PMNs and platelets.
In contrast to ATP, adenosine is an important mediator of platelet inhibition.32,33 Adenosine acts via the P1 purinergic receptors: A1, A2A, A2B, and A3. These receptors modulate intracellular cyclic adenosine monophosphate levels.34,35 Activation of the A2A receptor in platelets induces an inhibition of platelet aggregation through the elevation of cyclic adenosine monophosphate production.34,35 Linden et al showed that administration of CGS 21680, a specific agonist of the A2A receptor, leads to the inhibition of platelet aggregation and to the reduction of platelet activation markers like P-selectin.32 However, in addition to its role in platelet function, adenosine also regulates PMN function.11 Depending on the concentration of adenosine, the different receptors may be differentially activated. The A1 and A3 receptors have a high affinity for adenosine, whereas A2A and A2B have a lower affinity. Stimulation of these receptors in PMNs can have various effects. Stimulation of the A1 receptor promotes chemotaxis and adhesion, as well as superoxide production but inhibits TNF release.11 In contrast, stimulation of the A2A receptor promotes COX-2 and PGE-2 production and inhibits adhesion and infiltration, as well as degranulation or oxidative burst and release of inflammatory cytokines11 accompanying PMN activation. Here, we showed that the use of the CGS 21680 in vivo prevents PMN accumulation at the site of injury, and subsequently, inhibits fibrin generation and thrombus formation. Together, these results indicate that adenosine, by acting on A2AR, downregulates the activation of PMNs and prevents fibrin generation and thrombus formation at the site of injury.
The cellular sources of ATP leading to PMN activation and accumulation triggering thrombus formation in vivo remain to be identified. Gödecke et al showed that thrombin- stimulated HUVECs release ATP,7 suggesting that endothelial cells may be the source of ATP in vivo. In the dynamic model of flow chamber we used, in which only endothelial cells and purified PMNs were present, binding of PMNs to the endothelial cells was observed after stimulation with IL1-β but not in resting conditions. This suggests that endothelial cells may represent the unique source of ATP, leading to the activation and accumulation of PMNs. ATP is released from cells and rapidly metabolized via 2 major ecto-nucleotidases: CD39 that converts ATP in adenosine 5′-diphosphate, and AMP and CD73 that eventually converts AMP in adenosine.6 These molecules are expressed in endothelial cells and leukocytes, including monocytes and PMNs.36 In the case of inflammation, two processes would occur chronologically. First, a strong release of ATP occurs, leading to PMN activation and recruitment. Then, CD39 and CD73 expression is increased, leading to ATP conversion into adenosine, which limits the inflammatory response.36,37 In contrast to the venular inflammation process, in our model of arterial thrombosis, PMNs bind in a second, without rolling, to the site of injury. This process would depend on local ATP concentration, and thrombus formation might thus be governed by the ratio of ATP to adenosine. Under physiological conditions, ATP is rapidly metabolized in adenosine maintaining an antithrombotic state. In contrast, following an injury induced by the use of a nitrogen dye laser, the endothelium is activated, and in turn, may release high levels of ATP leading to PMN accumulation, and subsequently, promoting thrombus formation.
Altogether, these data contribute to the understanding of the molecular mechanisms involved in thrombus formation following a laser-induced injury. In this model, the crosstalk between the innate immune actors represented by PMNs, platelets, and the endothelium is crucial to assure an immediate and appropriate response to the injury. These cascades of molecular and cellular events may clinically be relevant in different situations, including thrombus formation associated with myeloproliferative disorders such as polycythemia vera. Indeed, thrombotic events occurring in arteries or veins represent a typical complication associated with polycythemia vera,38 and the physiopathology of the disease is associated with the activation of PMNs and endothelial cells. The activation of the endothelium leads to an increase of endothelial adhesion receptors favoring the recruitment of activated PMNs and platelets leading to the formation of a thrombus.39 Similarly, the activation of leukocytes, including PMNs, is also important in the formation of a thrombus following cerebral injuries.40 Thus, the understanding of the mechanisms leading to thrombus formation may help to develop efficient alternative strategies to prevent thrombo-inflammation. Our results indicate that preventing the activation of both PMNs and platelets, by targeting the P2X1 receptor, could potentially represent a therapeutic strategy to prevent arterial thrombosis.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stephane Robert and Stéphanie Simoncini (INSERM Unité Mixte de Recherche-S1076) for technical assistance and Beckman Coulter for the kind technical support.
This study was supported by grants from the Fondation pour la Recherche Médicale) (C.D.), institutional funding from INSERM, the Aix-Marseille University, the Agence Nationale pour la Recherche (ANR-09-JCJC-0053) (C.D.), and the Agence pour la Recherche sur le Cancer (C.D.). C.L. was a postdoctoral researcher at the Fond pour la Recherche Scientifique, C.D. was supported by a “Fond pour la recherche industrielle et agricole” fellowship, and C.O. is a research associate at the Fond pour la Recherche Scientifique.
Authorship
Contribution: R.D., C.D., S.M., A.H., C.L., and I.G. contributed to the design and performance of the research, analysis of data, and writing of the manuscript; and R.D., M.A.R., R.J.E., F.D.-G., C.O., L.P.-D., and C.D. contributed to the experimental design, analysis of data, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christophe Dubois, AMU, VRCM, INSERM UMR-S1076, Faculty of Pharmacy, 27 Blvd Jean Moulin, 13385 Marseille, France; e-mail: christophe.dubois@univ-amu.fr.

![Figure 3. Characterization of P2X1 activation at the surface of PMNs. (A-B) Expression of CD11b (A), CD11b, CD18, and CD62L (B) at the surface of human PMNs (the gate of PMNs were based on their size (Side Scatter [SS]) and their granulometry (Forward Scatter [FS]) challenged with 10 μM ATP and 0.5 μM phorbol myristate acetate (PMA) (A) or 10 μM α, β-meATP, and 200 U/ml TNF-α (B), (n = 10, *P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/16/10.1182_blood-2014-04-571679/4/m_2575f3.jpeg?Expires=1769220259&Signature=Ql9TE2SY~wmeGdWhLFaqSUUncS5BvF2oNjvrM4AQxz5q2jacge~HErU~P76mkHtvvhSOsUNc4xbVu4DfbZFkIFWJlL7tiEDToKV4QvFlztrPDrYoT4Wu4UZRZFDXd6biGsu~cQ4G5jimC3M5kiH6gSujWOfKnlOyjc9L0FSDSW0Am1tHTT0Ycj9gmOUrZcqwR83PWg4R1oPMpzoFzuAEXchGEU-QkCPZqy6zhPygz0pVY3w~ITDFndshFedpCYv3XTk0EQd0kyfVwwiybJewEscIMhm2X-3lXa4IbIgciNTKGUytpuGdmLhwuMUo1-W1QhYZf95fCAW3LThETPdYyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal