Abstract
The proinflammatory cytokine interferon-γ (IFN-γ) is well known for its important role in innate and adaptive immunity against intracellular infections and for tumor control. Yet, it has become clear that IFN-γ also has a strong impact on bone marrow (BM) output during inflammation, as it affects the differentiation of most hematopoietic progenitor cells. Here, we review the impact of IFN-γ on hematopoiesis, including the function of hematopoietic stem cells (HSCs) and more downstream progenitors. We discuss which hematopoietic lineages are functionally modulated by IFN-γ and through which underlying molecular mechanism(s). We propose the novel concept that IFN-γ acts through upregulation of suppressor of cytokine signaling molecules, which impairs signaling of several cytokine receptors. IFN-γ has also gained clinical interest from different angles, and we discuss how chronic IFN-γ production can lead to the development of anemia and BM failure and how it is involved in malignant hematopoiesis. Overall, this review illustrates the wide-ranging effect of IFN-γ on the (patho-)physiological processes in the BM.
Introduction
Normal tissue homeostasis and immune surveillance are highly dependent on stable production of blood cells, which is tightly orchestrated by the intricate process of hematopoiesis in the BM. Yet, infections disturb this homeostatic process and typically result in depletion of mature immune cells due to consumption of the cells fighting the invading pathogen. This requires a rapid compensatory response in hematopoiesis to provide the immune system with the appropriate type of cells during the infection and to restore cellular homeostasis in the blood system when the infection has been resolved. One of the clinically relevant consequences is that hematopoiesis is suppressed upon infection, which can lead to leucopenia and anemia. How these hematopoietic stress events are regulated is not fully understood, but it has become clear that functional crosstalk exists between the activated immune system and the hematopoietic compartment in the bone marrow (BM).1,2 An important role in this respect has been ascribed to interferon-γ (IFN-γ). This type II interferon is typically produced by T, natural killer, and natural killer T cells during an immune response to intracellular pathogens, like mycobacteria and viruses. Although IFN-γ is an important cytokine in orchestrating a vast array of immunologic responses, it is also historically known as a suppressor of hematopoiesis, and BM failure syndromes resulting from chronic inflammation are associated with IFN-γ production (reviewed in Mangan3 and Trinchieri et al4 ). Over the last decade, it has become evident which BM cell types are affected by IFN-γ (Figure 1) and through what molecular and cellular mechanisms. Here, we discuss these studies and lay out how they have contributed to our understanding of IFN-γ as a modulator of stress hematopoiesis.
Impact of IFN-γ on hematopoiesis. Overview of hematopoietic differentiation, in which is indicated whether IFN-γ has either a stimulating (thick red arrow) or inhibitory (dotted thin red arrow) effect on a particular lineage. IFN-γ receptor α (IFNγR) is expressed on all analyzed progenitors, including HSCs, MPPs, common myeloid progenitors (CMPs), GMPs, megakaryocyte-erythroid progenitors (MEPs), and common lymphoid progenitors (CLPs) (shown in green; unpublished observations; expression of IFNγR on fully differentiated cells is not indicated in this figure). LT-HSC, long-term HSC; NK, natural killer; ST-HSC, short-term HSC.
Impact of IFN-γ on hematopoiesis. Overview of hematopoietic differentiation, in which is indicated whether IFN-γ has either a stimulating (thick red arrow) or inhibitory (dotted thin red arrow) effect on a particular lineage. IFN-γ receptor α (IFNγR) is expressed on all analyzed progenitors, including HSCs, MPPs, common myeloid progenitors (CMPs), GMPs, megakaryocyte-erythroid progenitors (MEPs), and common lymphoid progenitors (CLPs) (shown in green; unpublished observations; expression of IFNγR on fully differentiated cells is not indicated in this figure). LT-HSC, long-term HSC; NK, natural killer; ST-HSC, short-term HSC.
The impact of IFN-γ on HSCs
The first experimental evidence that IFN-γ can inhibit hematopoiesis dates back to the 1980s, when IFN-γ was shown to inhibit colony formation in vitro.5-7 However, it remained obscure whether hematopoietic stem cells (HSCs) are also affected by IFN-γ, because most studies addressed the effect of IFN-γ on hematopoiesis by using total BM, lineage-depleted cells, or enriched progenitors. Instead, Snoeck et al used a 2-step culture system to study the effect of IFN-γ on purified human CD34+CD38− HSCs.8 Single HSCs were first clonally expanded in liquid cultures with or without IFN-γ for 2 weeks, followed by assessing colony-forming unit (CFU) activity in subsequent semisolid cultures, which indicated that IFN-γ strongly diminishes expansion of HSCs in the primary liquid cultures.8 Likewise, by combining long-term culture-initiating cell (LTC-IC) assays with CFU assays on purified human CD34+ BM cells, Selleri et al also showed that exogenous IFN-γ, and particularly IFN-γ produced by stromal cells, has a potent inhibiting effect on the number of LTC-ICs, indicating functional impairment of HSCs.9 Chronic exposure of IFN-γ is required for the inhibiting effect, and it has been suggested that IFN-γ negatively affects proliferation and increases apoptosis.9 Correspondingly, IFN-γ inhibits expansion of cord blood–derived CD34+CD38− HSCs in liquid cultures and impairs the maintenance of LTC-ICs, as shown by CFU assays and reconstitution of nonobese diabetic/severe combined immunodeficient mice.10
Next to these studies with human HSCs, IFN-γ also negatively affects the maintenance of murine HSCs. IFN-γ–producing stromal cells decrease CFU activity of murine BM cells and impair their long-term repopulating activity upon in vivo transfer.11 Moreover, we showed that maintenance of highly purified murine HSCs (identified as Lin−c-Kit+Sca1+CD150+CD48− BM cells) is also impaired by IFN-γ.12 IFN-γ does not affect cell-cycle entry, differentiation, or apoptosis of HSCs in these assays, but decreases the number of self-renewing cell divisions. This was attributed to the ability of IFN-γ to perturb thrombopoietin (TPO)-induced phosphorylation of signal transducer and activator of transcription 5 (STAT5), possibly through suppressor of cytokine signaling 1 (SOCS1) and thereby expression of STAT5-associated genes involved in the regulation of HSC proliferation, which provides a molecular explanation on how IFN-γ negatively affects HSC self-renewal.12 Interestingly, IFN-γ also regulates the reconstitution capacity of HSCs by modulating TPO-dependent interaction of the integrin αvβ3 with the extracellular matrix protein Nov/CCN3, indicating that TPO-mediated cellular interactions in the niche might also be modulated by IFN-γ.13
The well-established notion that in vitro exposure to IFN-γ impairs HSC maintenance is substantiated by several studies that have shown in vivo suppressive effects of IFN-γ on HSC function. Overexpression of IFN-γ in IFN-γ–transgenic mice decreases the number of CFU-GEMM colonies derived from BM, indicating a loss of functional multipotent HSCs.14 Conversely, HSCs from mice that lack IFN-γ signaling have a better reconstitution capacity during the steady-state situation compared with wild-type (WT) mice, indicating that the basal IFN-γ tone is sufficient to affect HSC function.12,15 The negative impact of IFN-γ on HSC becomes more apparent upon immune challenge, as IFN-γ production during chronic infection with Mycobacterium avium decreases HSC engraftment potential, though it would enhance HSC proliferation.15 Using an infection model with lymphocytic choriomeningitis virus (LCMV), we found no evidence for enhanced proliferation of HSCs, but we did find that IFN-γ impairs HSC self-renewal. By generating WT:IFNγR1−/− mixed BM chimeric mice, we could demonstrate this was due to a direct effect of IFNγ on HSCs and that IFN-γ modulates expression of genes (CyclinD1, p57) involved in the regulation of HSC self renewal in vivo.12 So whereas the negative effect of IFN-γ on HSC reconstitution is not disputed, discrepancies exist on the impact of IFN-γ on HSC proliferation,12,15,16 which may be due to variations in HSC characterization and the experimental models employed. The context in which HSCs are exposed to IFN-γ is indeed important, as IFN-γ production by adoptively transferred CD8 T cells in a steady-state situation is not sufficient to influence HSCs, though it does modulate more downstream progenitors17 (see below). We postulate that the impact of IFN-γ on HSCs is dependent on the status of the HSC compartment; acute viral infections, such as with LCMV, can decimate the HSC compartment through the production of type I IFNs.18 As a result, HSC self-renewal is induced to restore HSC numbers after the infection, and this compensatory proliferation is an IFN-γ–sensitive process.12 However, if HSC numbers are stable and self-renewal is not enhanced, IFN-γ may have little effect on HSCs.17
In conjunction with these context-dependent effects, it is of interest to also consider the impact of type I IFNs on HSC function. Production of interferon-α in a noninfectious setting is sufficient to activate quiescent HSCs and drive them into cell cycle,19,20 possibly through the induction of c-Myc protein expression.21 However, the impact of type I IFNs on HSCs is different between in vitro and in vivo settings and might even change depending on the time of exposure.16 The release of HSC quiescence and induction of proliferation upon initial IFN production is in fact followed by a return of HSCs to quiescence upon prolonged exposure, which protects them from IFN-induced apoptosis.16 It is thus important to consider that the impact of IFNs on HSCs is depending on the stimulatory condition. In line with this, mice deficient for adar1 or irgm1,22,23 genes induced by IFN-γ, have hyperproliferative HSCs with a decreased reconstitution capacity and elevated IFN signaling and IFN-γ serum levels, respectively. We suggest that like type I IFNs, IFN-γ can have stimulating and suppressing effects on HSC proliferation and reconstitution, and the outcome of this balance may be context dependent.
Effect of IFN-γ on multipotent progenitors and myelopoiesis
Next to its impact on HSCs, it has been suggested that IFN-γ produced upon infection or when intravenously injected expands the population of Lin−c-Kit+Sca-1+ cells, which contains both HSCs and more committed progenitor cells.24-27 However, in our opinion, these findings are difficult to interpret, as interferon-signaling induces Sca-1 expression on many hematopoietic cells2,28 and thereby leads to an overestimation of the number of Lin−c-Kit+Sca-1+ cells. IFN-γ has been shown to enhance differentiation of purified CD34+ hematopoietic stem and progenitor cells, CMPs, and granulocyte-macrophage progenitors (GMPs),12 indicating that IFN-γ can modulate lineage-specific myeloid differentiation. Indeed, a critical role for IFN-γ in infection-induced myelopoiesis has been demonstrated by infecting IFN-γ–deficient mice with Mycobacterium bovis, because these mice display a severe granulocytosis in BM, peripheral blood, and spleen.29 Similar increases in granulocytes have been reported after infection of IFN-γ–deficient mice with Mycobacterium tuberculosis30 and Toxoplasma gondii.31 Moreover, infection with either Ehrlichia muris or LCMV induces predominantly monopoiesis in WT mice, whereas neutrophil development is strongly increased in infected IFN-γ–deficient mice.32,33 This strong impact of IFN-γ on myelopoiesis is due to the fact that IFN-γ reciprocally regulates the production of neutrophils and monocytes. On one hand, IFN-γ induces expression of SOCS3 in GMPs and thereby inhibits granulocyte colony stimulating factor (G-CSF)-induced activation of STAT3,32 an essential transcription factor for emergency granulopoiesis.34 On the other hand, IFN-γ also elevates expression of monocyte-promoting transcription factors PU.1 and interferon responsive factor 8 (IRF-8) in the same myeloid progenitors.32 These findings corroborate a previous report demonstrating that IFN-γ stimulates monocytic colony formation from human progenitor cells, while G-CSF–induced granulocytic colony formation is impaired in the presence of IFN-γ.35 Interestingly, IFN-γ can also induce monocyte differentiation indirectly through production of interleukin-6 (IL-6) by BM mesenchymal stromal cells (MSCs), whereby IL-6 decreases expression of Runx1 and CEBPα in multipotent progenitors and thereby skews them toward the monocytic lineage.17 Importantly, T-cell–driven immunity and IFN-γ production can also induce the mobilization of MSCs from the BM,36 which further illustrates the complexity of the cellular dynamics in the BM during inflammation. Moreover, IFN-γ enhances the ability of MSCs to inhibit T-cell proliferation and proinflammatory cytokine production through upregulation of the immunoregulatory enzymes indoleamine 2,3-dioxygenase (IDO) and inducible nitric oxide synthase37-39 and may thus also play a role in restoring immune homeostasis in the BM.
Regarding the impact of IFN-γ on myelopoiesis, it is of interest that IFN-γ can also negatively affect eosinophil development upon viral infection or sensitization with an allergen. Mice deficient for IFN-γ signaling show accumulation of eosinophils in the lungs or brains upon infection with respiratory syncytial virus40 or Borna disease virus,41 respectively. Additionally, IL-12–mediated induction of IFN-γ signaling inhibits eosinophilia upon allergen challenge of sensitized mice42 or after parasite infection.43 Interestingly, IFN-γ has a direct inhibiting effect on the differentiation of eosinophils, as IFN-γ reduces expression of IL-5Rα and GATA-1 in progenitor cells, which are both essential factors in eosinophil development.44 Whereas IFN-γ impairs IL-5–mediated differentiation of myeloid progenitors to eosinophils, IFN-γ does not alter the response to granulocyte-macrophage colony stimulating factor–supported cultures, again demonstrating that IFN-γ has lineage-specific effects on myelopoiesis.44 Furthermore, malaria infection induces the generation of a novel type of progenitor cell, which is dependent on IFN-γ signaling. These cells express IL-7Rα, like lymphoid progenitors, but transplantation experiments revealed that these cells produce myeloid progeny in vivo, which contribute to the clearance of malaria-infected cells.45 In conclusion, IFN-γ production during immunologic challenges is very important for tightly orchestrating myelopoiesis by directly regulating growth factor responsiveness and transcription factor expression, as well as cytokine release from stromal cells in the BM
The impact of IFN-γ on erythropoiesis and thrombopoiesis
Chronic infections are notorious for inducing anemia, and a role for IFN-γ has been well recognized in this process. IFN-γ disturbs iron homeostasis and thereby the erythroid balance by inducing iron retention in macrophages.46 Furthermore, IFN-γ–activated macrophages contribute to red blood cell (RBC) loss by increased hemophagocytosis.47 However, erythroid colony formation of hematopoietic progenitors is also directly suppressed by IFN-γ,48,49 and IFN-γ primarily inhibits the earliest stages of erythroid differentiation and proliferation.50 Interestingly, it has also been suggested that IL-15 plays an important role for the IFN-γ–mediated inhibition of erythropoiesis, both in vitro and in vivo.51 Erythroid progenitors are obtained from megakaryocyte-erythroid progenitors, which, in contrast to myeloid progenitors, express low levels of PU.1 and high levels of GATA-1. Yet, exposure of erythroid progenitors to IFN-γ in vitro or in vivo is sufficient to increase PU.1 messenger RNA and protein expression.52 Using human erythroid progenitors, we established that IFN-γ–induced upregulation of PU.1 is dependent on IRF-1 and that knockdown of either IRF-1 or PU.1 expression is sufficient to overcome IFN-γ–induced reduction in erythroid colony formation.52 Because PU.1 can physically interact with GATA-1 and thereby inhibit its function in erythroid differentiation,53 we postulate that the IFN-γ–mediated increase of PU.1 alters the transcriptional profile of hematopoietic progenitors, thereby blocking their erythroid differentiation potential.52
Strikingly, although IFN-γ diminishes TPO signaling in HSCs and impairs HSC proliferation, IFN-γ does not interfere with the strongly TPO-dependent process of megakaryopoiesis (Tsuji et al54 and A.M.d.B., unpublished data). A possible explanation for this divergence is the requirement of STAT1 for megakaryopoiesis, which is a downstream target of GATA1 but also activated by IFN-γ signaling.55 Moreover, the IFN-γ target genes IRF1 and IRF2 are involved in the regulation of megakaryopoiesis by inducing expression of the integrin CD41.55,56 Whereas IFN-γ is not required for megakaryopoiesis, IFN-γ–induced factors do not reduce but instead contribute to the process of platelet formation.
The effect of IFN-γ on B lymphopoiesis
The impact of IFN-γ on hematopoiesis is not restricted to the myeloid and erythroid lineage but extends to the lymphoid lineage. IFN-γ expression is not required for B-cell development but enhanced IFN-γ signaling in vivo is sufficient to inhibit B-cell differentiation, as was shown in mice overexpressing IFN-γ14 or human IRF157 or lacking SOCS1.58 Moreover, enhanced costimulation of T cells through CD27 by overexpression of its ligand CD70 strongly increases the production of effector T cells, which inhibits B lymphopoiesis in an IFN-γ–dependent manner.59
The molecular mechanism by which IFN-γ inhibits B-cell development in these mice has not been unraveled, but there is strong evidence that this is related to IL-7 signaling, because IFN-γ inhibits IL-7–induced proliferation of primary pre-B cells in vitro.60 Whereas IFN-γ alone does not directly induce apoptosis of pre-B cells in vitro, increased IFN-γ–mediated cell death is observed in IL-7–supplemented cultures of these cells,60,61 which is rescued by overexpressing Bcl2.61 While IFN-γ does not affect IL-7 messenger RNA expression, a decreased binding of IL-7 to its receptor has been reported, which can explain the negative effect of IFN-γ on IL-7–induced survival and proliferation.60 Furthermore, IFN-γ also induces SOCS1 expression in immature B cells, thereby abrogating signaling through the IL-7 receptor and decreasing the responsiveness of these cells to IL-7.62
Although decreased IL-7 signaling may explain the negative impact of IFN-γ on B-cell differentiation, it could also be that IFN-γ changes the transcriptional profile required for B-cell differentiation. The fact that IFN-γ can induce PU.1 expression in progenitor cells52 may also be relevant in this context, because low levels of PU.1 are required for B-cell differentiation, but high PU.1 levels induce myeloid and inhibit B-cell differentiation.63 Therefore, we hypothesize that next to an IFN-γ–mediated decreased responsiveness to IL-7, IFN-γ also increases PU.1 expression in B cells and thereby contributes to the block in B-cell differentiation.
The impact of IFN-γ on cytokine signaling and transcription factor expression
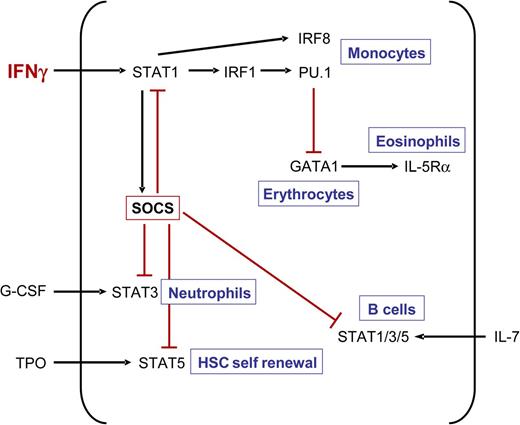
Many cytokines, including IFN-γ, induce expression of SOCS molecules, which stimulates a negative feedback to the receptor that is transmitting the signal in order to terminate the signaling process.64 We showed that IFN-γ induces SOCS1 and SOCS3 in hematopoietic progenitor cells, thereby perturbing respectively TPO12 and G-CSF responses.32 Furthermore, IFN-γ–induced SOCS1 expression was shown to abrogate IL-7 signaling in immature B cells.62 Thus, an emerging concept arises that IFN-γ negatively affects the differentiation of various hematopoietic lineages through SOCS-mediated inhibition of cytokine signaling at the level of STAT activation and thereby inhibits the proliferation and/or differentiation of the progenitors of that particular lineage (Figure 2). The question arises whether IFN-γ can in a similar manner also inhibit the signaling of other cytokines. It would be interesting to examine if responsiveness to erythropoietin (EPO) can be modulated in this fashion, because EPO acts through phosphorylation of STAT5 and induces its own negative feedback through SOCS1 and SOCS3.64,65 If so, this would imply that IFN-γ inhibits erythropoiesis at multiple levels, namely by impairing EPO signaling through SOCS and by inducing PU.1 expression through IRF1.52 Moreover, the antagonizing effect of PU.1 on GATA-153 may be relevant not only for erythropoiesis, but also for eosinophil differentiation, because GATA-1 is important in IL-5Rα+ eosinophil progenitors.66 It is therefore tempting to speculate (Figure 2) that PU.1-mediated inhibition of GATA-1 may also contribute to the IFN-γ–driven impairment of eosinophil differentiation,44 but this remains to be determined.
IFN-γ regulates function and expression of key hematopoietic transcription factors. IFN-γ impairs HSC self-renewal as well as differentiation of neutrophils and B cells by upregulating SOCS proteins, which block cytokine-induced STAT signaling. Furthermore, IFN-γ also induces expression of monocyte-inducing transcription factors IRF8 and PU.1. The latter inhibits the function of GATA1, which is important for the development of erythrocytes and eosinophilic granulocytes.
IFN-γ regulates function and expression of key hematopoietic transcription factors. IFN-γ impairs HSC self-renewal as well as differentiation of neutrophils and B cells by upregulating SOCS proteins, which block cytokine-induced STAT signaling. Furthermore, IFN-γ also induces expression of monocyte-inducing transcription factors IRF8 and PU.1. The latter inhibits the function of GATA1, which is important for the development of erythrocytes and eosinophilic granulocytes.
IFN-γ production and BM failure
Although short-term exposure of hematopoietic progenitors to IFN-γ may be beneficial during viral infection due to its monocyte-skewing effects, the coinciding suppression of other lineages should not persist, as this will negatively affect blood cell homeostasis (Figure 3). The clinical relevance of this is evident in patients with aplastic anemia (AA), which have increased levels of IFN-γ in their circulation and impaired colony-forming potential of BM cells, which improves with anti–IFN-γ antibodies.67 Although oligoclonally expanded T cells reactive to hematopoietic progenitors are present in AA patients, the antigen to which they respond is unknown.68 IFN-γ production by circulating T cells is also increased in AA patients, which may be driven by increased levels of T-bet, and the presence of these IFN-γ–producing lymphocytes correlates well with the response to immunosuppressive therapy and subsequent recovery.69,70 Moreover, a polymorphism in the gene encoding IFN-γ that results in overproduction of IFN-γ is associated with the risk of AA.71 Prolonged suppression of several hematopoietic lineages by IFN-γ is important in disease progression, but IFN-γ–dependent upregulation of Fas, resulting in increased cell death of hematopoietic progenitors, may also contribute to a decline in hematopoietic output in AA.72,73
Chronic production of IFN-γ induces BM failure, monocytosis, and AA. Chronic IFN-γ production during persistent viral infection or chronic inflammatory diseases has a dramatic impact on BM output, as it impairs HSC self-renewal, increases monopoiesis, and inhibits development of B cells, granulocytes, and RBCs (cellular identity according to Figure 1). Adapted from King et al2 with permission.
Chronic production of IFN-γ induces BM failure, monocytosis, and AA. Chronic IFN-γ production during persistent viral infection or chronic inflammatory diseases has a dramatic impact on BM output, as it impairs HSC self-renewal, increases monopoiesis, and inhibits development of B cells, granulocytes, and RBCs (cellular identity according to Figure 1). Adapted from King et al2 with permission.
Although most information regarding the role of IFN-γ in BM failure in human disease comes from studies of AA,74 chronic immune activation is a major underlying cause of IFN-γ–associated BM failure. Decreased numbers of IFN-γ–producing lymphocytes have been associated with hematologic improvement following immunosuppression in patients with hypoplastic myelodysplasia.75 IFN-γ is also associated with hematopoietic suppression observed in patients with Fanconi anemia and HIV.76,77 In a mouse model for graft-versus-host disease, it has been shown that IFN-γ produced by CD4 T cells is responsible for the development of severe anemia and BM aplasia when the recipients receive sublethal irradiation. However, when the recipients are lethally irradiated (followed by BM transplantation), IFN-γ production is rather protective.78 Using IFN-γ–reporter mice, it has also been shown that allogeneic Th1 cells generated during graft-versus-host disease can traffic to hematopoietic sites and induce BM failure via IFN-γ–mediated toxicity.79
From a therapeutic perspective it is interesting that anti–IFN-γ antibodies improve the in vitro capacity of hematopoietic progenitors derived from patients with AA.67,80 Moreover, in a mouse model of T-cell–induced BM failure, characterized by severe pancytopenia and BM hypoplasia, anti–IFN-γ can even enhance survival.81 Antibodies to IFN-γ have been clinically tested in Th1-driven diseases,82 and it would be interesting to investigate whether such a treatment is sufficient to reduce hematopoietic suppression in patients with AA or other forms of inflammation-induced BM failure.
Role of IFN-γ in malignant hematopoiesis
IFN-γ not only has a strong impact on normal hematopoiesis, but also influences malignant hematopoietic cells, though both growth limiting and promoting effects have been reported. IFN-γ inhibits colony formation and expansion of tumor cells obtained from patients with acute myeloid leukemia (AML),83 chronic myeloid leukemia (CML),84 and multiple myeloma (MM).85 Next to growth-limiting and cytotoxic effects of IFN-γ on leukemic cells, IFN-γ induces differentiation of immature myeloid blasts from AML and CML patients.86 Interestingly, these effects seem to be dependent on other cytokines, because IFN-γ enhances colony formation of IL-3–dependent AML cell lines, but inhibits the growth of IL-3–independent cell lines. Remarkably, both the inhibitory and stimulatory effects of IFN-γ can be reduced by blocking tumor necrosis factor α.87 Furthermore, IL-6 is a major cytokine for MM cells, and IFN-γ inhibits IL-6–dependent proliferation of MM cells by interfering with IL-6 signaling85 ; whether this effect is SOCS dependent is not known. Thus, the effect of IFN-γ on malignant cells seems context dependent, which is also evident from the observation that the effect of IFN-γ on AML cells depends on the cytokines added and the stromal subset that is used in cocultures.88 It could thus be that the in vivo effect of IFN-γ on growth of tumor cells depends on the local cytokine milieu created by supporting niche cells and by autocrine cytokine signaling from the malignant cells. In addition, tumor-specific genomic alteration of leukemic cells might also be involved in regulating the response to IFN-γ signaling. Activating mutations in FLT3, which occur in 20% to 25% of AML patients, enable direct phosphorylation of STAT5. Although SOCS1 is also upregulated in these cells, SOCS1 is not able to inhibit this cytokine-independent activation of STAT5. Aberrant FLT3-induced SOCS1 even prevents the inhibitory effect of IFN-γ on colony formation of transformed cells,89 demonstrating that activated oncogenic pathways can modulate the effect of IFN-γ on growth of malignant cells.
Next to regulation of cytokine responses, IFN-γ upregulates expression of Fas on MM cells, sensitizing them to Fas-mediated apoptosis.90 In contrast, IFN-γ induces PD-L1 expression in AML,91 CML,92 and MM93 cells, thereby reducing cytotoxic T-cell–mediated lysis. Using a CML mouse model, it has been shown that PD-1/PDL-1 interaction impairs the immune control of CML in vivo94 and that IFN-γ enhances leukemic outgrowth.92 Although it was claimed that IFN-γ promotes tumor progression by inducing proliferation of leukemic stem cells, it might be interesting to investigate in more detail the direct role of IFN-γ in PD-L1–dependent immune escape of transformed cells. It is also suggested that IFN-γ enables immune escape of leukemic cells by inducing IDO in AML cells, which suppresses antitumor activity of T cells.95 On the other hand, whereas IFN-γ does not affect IDO expression in MM cells, IDO levels in MSCs increase after stimulation with IFN-γ, resulting in apoptosis of cocultured MM cells.96
Altogether, IFN-γ might act as a double-edged sword in malignant hematopoiesis in which its pro- or antitumor activity is dependent on the nature of the hematologic malignancy and the interplay between a variety of molecular, cellular, and microenvironmental factors. However, as IFN-γ affects malignant cells, immune cells, and nonhematopoietic niche cells, it will be challenging to translate results of in vitro experiments to more complex in vivo situations. Therefore, in-depth studies on the in vivo effects of IFN-γ on malignant hematopoiesis are required to delineate its effect on cytokine signaling, immune escape, and the leukemic niche in the BM.
Conclusions and implications
Altogether, IFN-γ has strong and diverse effects on various levels of hematopoiesis, and these are clinically relevant. Although IFN-γ has subtle effects on steady-state hematopoiesis, it profoundly regulates blood cell production during immunologic challenges, indicating that IFN-γ is an important factor in controlling BM output during stress situations. There is also emerging evidence that MSCs are important players in mediating these effects of IFN-γ on hematopoiesis in the BM.
We postulate that the evolutionary beneficial role of IFN-γ is that it enhances the formation of the appropriate immune cells to combat an invading pathogen. Myeloid cells are the first line of defense against pathogens, and in contrast to RBCs, have a very limited lifespan. A transient decrease in erythropoiesis will therefore not directly affect oxygen transport, while decreased competition for growth factors and space in the BM may facilitate expansion of myeloid cells. IFN-γ is typically produced in immune responses against intracellular pathogens and induces monopoiesis at the cost of neutrophil and eosinophil formation, which are less relevant during such infections and more important to clear extracellular pathogens. Yet, it is interesting to speculate that this disbalance in myeloid cells may contribute to the increased susceptibility of virally infected patients to subsequent bacterial infections.97 Prolongation of IFN-γ production during chronic inflammation will lead to a disbalance in blood cell homeostasis and cause anemia. Better understanding of the underlying molecular mechanism may facilitate the development of successful strategies to tackle inflammation-induced anemia and modulate disease progression in case of malignant hematopoiesis.
Acknowledgments
The authors thank Dr Howard A. Young (Laboratory of Experimental Immunology, National Cancer Institute, Frederick, MD) and Dr Sacha Zeerleder (Department of Hematology, Academic Medical Center, Amsterdam, The Netherlands) for critical reading of the manuscript and D. Egberts for support. For this review, the authors were limited in the number of words and references they could use, and given the breadth of the topics discussed, they were obliged to make a selection of the papers that they would refer to. Hence, the authors apologize to those whose relevant work was not cited in this review.
C.V. and M.A.N. are both supported by the Landsteiner Foundation for Blood Transfusion Research (LSBR fellowships 1101 and 1014, respectively).
Authorship
Contribution: A.M.d.B., C.V., and M.A.N. jointly designed the figures and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martijn A. Nolte, Department of Hematopoiesis, Sanquin Research and Landsteiner Laboratory AMC/UvA, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: m.nolte@sanquin.nl.