Key Points
B-cell neogenesis is decreased independently by both aGVHD and cGVHD.
B cells during GVHD undergo a higher number of cell divisions related, in the chronic form, to a higher BAFF/CD19 ratio.
Abstract
Using B-cell rearrangement excision circle measurements, we analyzed B-cell reconstitution in a cohort of 243 patients who underwent allogeneic stem cell transplantation. Acute and chronic graft-versus-host disease (aGVHD and cGVHD, respectively) transiently increased B-cell replication but decreased overall B-cell neogenesis with a clear difference in terms of kinetics. Moreover, the impact of aGVHD in the absence of cGVHD was transient, recovering at month 6 similar values as in patients who did not suffer from GVHD. Conversely, impact of cGVHD at month 12 in multivariate analysis was independent of the previous aGVHD effect on B-cell output. Finally, we showed in patients affected with cGVHD a higher B-cell division rate that correlates with an elevated BAFF/CD19+ B-cell ratio, supporting a B-cell hyperactivation state in vivo.
Introduction
One of the main of concerns in allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the profound and long-lasting immunodeficiency leaving patients highly susceptible to multiple infections. Accordingly, a low B-cell recovery after allo-HSCT has been associated with high infection rate.1,2 B-cell reconstitution is a slow process characterized by a relative increase of the percentage of naïve B cells and a recovery of memory B-cell counts that lags long behind. Several factors, such as stem cell source and composition, can affect post-HSCT B-cell recovery with graft-versus-host disease (GVHD) having the strongest influence.3-5 Indeed, B-cell lymphopoiesis is highly dependent on the marrow microenvironment, susceptible to damage by the conditioning regimen and side effects of GVHD and/or its treatment.6,7 Moreover, alteration of B-cell homeostasis in chronic GVHD (cGVHD) patients associated with high BAFF levels leads to a significantly higher BAFF/B-cell ratio that could promote survival of CD27+ autoreactive B-cell clones involved in cGVHD pathogenesis.8-11
However, the direct evidence in vivo of an increased B-cell replication in patients with cGVHD is still lacking, and it remains to be clarified whether acute GVHD (aGVHD) may have a persistent impact on B-cell neogenesis. It is now possible to measure the bone marrow (BM) B-cell output using real-time polymerase chain reaction (PCR) for quantification of κ-deleting recombination excision circles (KRECs), which are generated in the BM during B-cell development.12-14 The coding joint (Cj) of this rearrangement is duplicated during each cell division, whereas the signal joint KREC (sjKREC) remains stable as episomal DNA. Measuring the ratio between Cj and sjKREC directly reflects the mean number of divisions achieved by B cells after the occurrence of the recombination event, thus providing a unique way to quantify the in vivo replicative history of mature B lymphocytes.12
Here, we analyzed the impact of aGVHD and cGVHD on B-cell reconstitution post allo-HSCT in a cohort of 243 consecutive allo-HSCT–treated patients. We showed a clear separate impact of aGVHD and cGVHD on B-cell neogenesis and, in cGVHD patients, a correlation between BAFF/CD19+ B-cell ratio and B-cell divisions in vivo.
Study design
All patients (n = 243) received a non–T-cell–depleted allo-HSCT at the Hôpital Saint-Louis (Paris, France) between January 2006 and December 2008 (supplemental Table 1, available on the Blood Web site). Patients having received cord blood graft or anti-CD20 treatments were excluded. GVHD was graded according to published criteria.15 The investigation was approved by the Medical Ethic Committee of the Hôpital Saint-Louis, and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Absolute lymphocyte count was calculated from freshly collected blood using the TruCount system (Becton Dickinson, le Pont-de-Claix, France). B-lymphocyte subpopulations were labeled with the following monoclonal antibodies (all from BD Bioscience): CD45-PercP, CD5-FITC, CD27-PE, and CD19-APC. Data were analyzed using FACSDiva (BD Biosciences).
Cells were separated on lymphocyte separation medium, lysed, and stored in TriReagent. DNA was then extracted, and Cj and sjKREC were quantified by real-time PCR (ABI PRISM7700; Applied Biosystems, Foster City, CA) as described.12
Plasma was collected from EDTA blood samples. Soluble BAFF concentration was determined using Quantikine ELISA human BAFF (R&D System). Plates were read on a Biochrom Anthos Zenyth 340s.
Results and discussion
In this study, we followed the B-cell reconstitution consecutively at 3, 6, 12, and 24 months in a large monocentric cohort of allo-HSCT adult patients. Patients who developed aGVHD had lower counts of CD19+CD27−, CD19+CD27+, and CD19+CD5+ cells than patients without aGVHD. In CD27− naïve B cells, the major subpopulation after transplantation, we observed the same effect of aGVHD as in the global CD19+ population. The impact of aGVHD was delayed in “memory” CD27+ B cells, being significant at months 6 and 12. CD19+CD5+ cell counts were markedly decreased in aGVHD patients at months 3 and 6. cGVHD reduced the recovery of all B-cell subsets at months 12 and 24 (supplemental Figure 1). Of note, the number of CD19+CD27+ or CD19+CD5+ cells was also significant lower before transplantation for GVHD patients. In agreement with previous studies,2,3,16 we observed an early impact (months 3 to 12) of aGVHD and a late impact (months 12 to 24) of cGVHD on B-cell reconstitution.
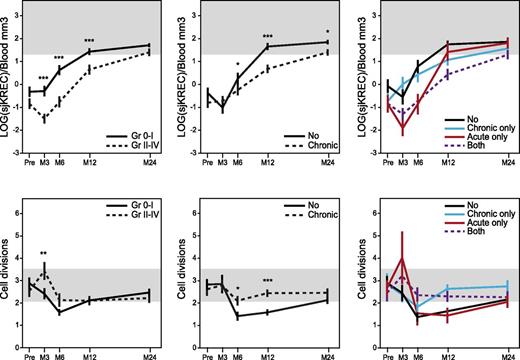
In 43 healthy donors, Cj and sjKREC mean (± standard error of the mean) normal values were 339 (±123) and 59 (±34) by blood mm3, respectively, independently from gender and age. At every time point after allo-HSCT, patients’ Cj and sjKREC correlated significantly with the number of CD19+ and CD19+CD27− B cells, respectively (supplemental Figure 2). Fewer Cj’s and sjKRECs were found in patients with severe aGVHD (grade 2 to 4) from months 3 to 12. At month 3, the number of Cj’s and sjKRECs decreased with the aGVHD grade (data not shown). From month 6 onward, Cj and sjKREC values recovered similarly in patients with 0 to 1 aGVHD. The mean number of B-cell divisions was increased in patients with severe aGVHD at month 3 (Figure 1).17 The temporal consequences of cGVHD on B-cell output were clearly separated. We observed fewer Cj’s and sjKRECs in cGVHD patients from months 6 to 24, with a more severe impact of extensive compared with limited cGVHD (data not shown). The mean number of B-cell divisions was also increased in patients with cGVHD at months 6 and 12 compared with patients without cGVHD who evidenced a lower than normal division rate, especially in the CD27+ subset, maybe reflecting a more continuous and effective supply of quiescent B cells (Figure 1 and supplemental Figure 3). In aGVHD as well as in cGVHD, there was a reduction of naïve B-cell output measured by sjKREC together with a higher division rate, consistent with a compensatory mechanism in response to GVHD-induced lymphopenia. When we looked at both forms of GVHD at months 3 and 6, only aGVHD had an impact on B-cell production regardless of the occurrence of a further cGVHD. Conversely, patients who developed cGVHD had a lower recovery at month 12 regardless of their aGVHD status (Figure 1). Factors involved in GVHD and/or lymphoid reconstitution, such as malignant vs nonmalignant diseases, myeloablative conditioning vs reduced intensity conditioning, the source of graft (BM vs mobilized peripheral blood cells), the use of antilymphocyte or antithymocyte globulins, the recipient age, HLA identical siblings vs unrelated donors, and finally, gender mismatch were also found to have a significant impact on B-cell reconstitution in univariate analysis (supplemental Table 2). So, they were included in a multivariate analysis including aGVHD (grade 0-1 vs 2-4) and cGVHD, which confirmed that aGVHD impaired B-cell reconstitution at months 3 and 6 and cGVHD at months 12 and 24 independently of other factors (supplemental Table 2).
aGVHD and cGVHD delay the B-cell reconstitution but increase B-cell divisions. Mean (± standard error) of total number of sjKREC/blood mm3 (upper panels) measured by quantitative PCR before (pre-) and 3, 6, 12, and 24 months after allo-HSCT in patients with (dotted lines, n = 116) or without (plain lines, n = 127) aGVHD (left panel) or with (dotted lines, n = 117) or without (plain lines, n = 126) cGVHD (middle panel) and number of divisions (lower panels) were represented. Values were normalized for the genomic copy number, with albumin gene quantification.17 Mean number of B-cell divisions (n) was calculated using the following formulas: number of Cj’s = 2n × number of sjKRECs, or 2n = Cj/sjKREC, or nLOG2 = LOG(Cj/sjKREC), and finally, n = LOG(Cj/sjKREC)/LOG2. In right panels, “no” represents patients who developed no GVHD (n = 79); “acute only,” patients who developed aGVHD only (n = 47); “chronic only,” patients who developed cGVHD only (n = 48); and “both,” patients who developed aGVHD followed by cGVHD (n = 69). For lower right panel: at month 3, no vs acute *; at month 12, no vs chronic ***; no vs both * and acute vs chronic **; other combinations were NS. Shaded areas indicate values in healthy controls (n = 43). *P < .05; **P < .01; ***P < .001; NS, not significant (Mann-Whitney).
aGVHD and cGVHD delay the B-cell reconstitution but increase B-cell divisions. Mean (± standard error) of total number of sjKREC/blood mm3 (upper panels) measured by quantitative PCR before (pre-) and 3, 6, 12, and 24 months after allo-HSCT in patients with (dotted lines, n = 116) or without (plain lines, n = 127) aGVHD (left panel) or with (dotted lines, n = 117) or without (plain lines, n = 126) cGVHD (middle panel) and number of divisions (lower panels) were represented. Values were normalized for the genomic copy number, with albumin gene quantification.17 Mean number of B-cell divisions (n) was calculated using the following formulas: number of Cj’s = 2n × number of sjKRECs, or 2n = Cj/sjKREC, or nLOG2 = LOG(Cj/sjKREC), and finally, n = LOG(Cj/sjKREC)/LOG2. In right panels, “no” represents patients who developed no GVHD (n = 79); “acute only,” patients who developed aGVHD only (n = 47); “chronic only,” patients who developed cGVHD only (n = 48); and “both,” patients who developed aGVHD followed by cGVHD (n = 69). For lower right panel: at month 3, no vs acute *; at month 12, no vs chronic ***; no vs both * and acute vs chronic **; other combinations were NS. Shaded areas indicate values in healthy controls (n = 43). *P < .05; **P < .01; ***P < .001; NS, not significant (Mann-Whitney).
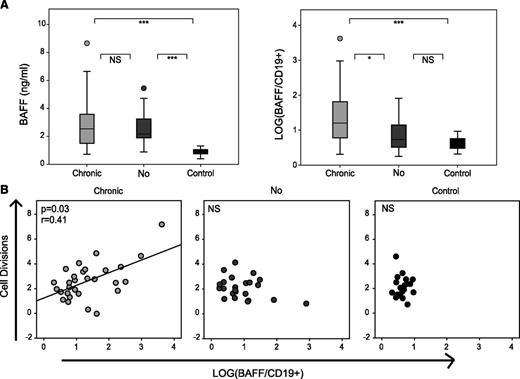
Given the importance of BAFF in cGVHD pathogenesis,8,10,18 we tested BAFF levels at 1 year after HSCT in a subgroup of 60 patients treated for a hematologic malignancy with no other difference in graft parameters (supplemental Table 1). All patients had significantly higher BAFF levels compared with healthy individuals. As patients with cGVHD had significantly lower CD19+ B-cell counts, they had an increased BAFF/CD19+ B-cell ratio compared with patients without cGVHD (Figure 2A). Notably, in patients with cGVHD, the number of B-cell divisions correlated with the level of BAFF (Figure 2B) and could correspond to the replication of cells involved in the pathogenesis of this disease.8
cGVHD high BAFF/B-cell ratio is associated with an increased in mean B-cell division. (A) Plasma concentration of BAFF and LOG(BAFF/CD19+) in healthy donors (n = 20) and BAFF cohort patients with (n = 34) or without cGVHD (n = 26) at month 12 post allo-HSCT. (B) Correlation between the number of cell divisions and BAFF concentration by CD19+ cell in healthy donors (n = 20) and patients with (n = 29) or without cGVHD (n = 23) at month 12 post allo-HSCT. *P < .05; ***P < .001; NS, not significant (Mann-Whitney).
cGVHD high BAFF/B-cell ratio is associated with an increased in mean B-cell division. (A) Plasma concentration of BAFF and LOG(BAFF/CD19+) in healthy donors (n = 20) and BAFF cohort patients with (n = 34) or without cGVHD (n = 26) at month 12 post allo-HSCT. (B) Correlation between the number of cell divisions and BAFF concentration by CD19+ cell in healthy donors (n = 20) and patients with (n = 29) or without cGVHD (n = 23) at month 12 post allo-HSCT. *P < .05; ***P < .001; NS, not significant (Mann-Whitney).
These data point out in a large cohort of patients some salient features of B-cell defects in cGVHD patients: a prolonged lymphopenia lasting more than 2 years after graft, compensatory mechanisms leading to sustained B-cell divisions up to 24 months, and elevated BAFF/CD19+ B-cell ratios. Finally, this study enabled us to analyze B-cell divisions in comparison with the elevated BAFF/CD19+ B-cell ratio, evidencing that B cells in cGVHD patients appear more responsive to BAFF than patients without cGVHD, consistently with a higher B-cell receptor activation threshold.11
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research grants from the Assistance Publique–Hôpitaux de Paris (Translational Research grant in Biology 2010, #RTB10002 and PRTS 2013), the LabEx “Milieu Intérieur” and “Transplantex,” and European Community grant ERA-NET Transcan “Haploimmune.”
Authorship
Contribution: S.G. and J.S. performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; I.F. and C.D. performed research; H.M.-T. and G.M. contributed analytical tools and collected data; R.P.d.L. and M.R. collected data; G.S. interpreted data and critically reviewed the manuscript; and E.C. and A.T. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanuel Clave, Laboratoire d'Immunologie et d'Histocompatibilité AP-HP, INSERM UMRS-1160, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, 1, avenue Claude Vellefaux, F-75475 Paris, CEDEX 10, France; e-mail: emmanuel.clave@univ-paris-diderot.fr; and Antoine Toubert, Laboratoire d'Immunologie et d'Histocompatibilité AP-HP, INSERM UMRS-1160, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, 1, avenue Claude Vellefaux, F-75475 Paris, CEDEX 10, France; e-mail: antoine.toubert@univ-paris-diderot.fr.
References
Author notes
S.G. and J.S. contributed equally to this study.
A.T. and E.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal