Key Points
TSOACs are associated with less major bleeding, fatal bleeding, clinically relevant nonmajor bleeding, and total bleeding.
The meta-analysis does not show increased risk of major gastrointestinal bleeding in patients who received TSOACs compared with warfarin.
Abstract
Vitamin K antagonists (VKAs) have been the standard of care for treatment of thromboembolic diseases. Target-specific oral anticoagulants (TSOACs) have been developed and found to be at least noninferior to VKAs with regard to efficacy, but the risk of bleeding with TSOACs remains controversial. We performed a systematic review and meta-analysis of phase-3 randomized controlled trials (RCTs) to assess the bleeding side effects of TSOACs compared with VKAs in patients with venous thromboembolism or atrial fibrillation. We searched MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials; conference abstracts; and www.clinicaltrials.gov with no language restriction. Two reviewers independently performed study selection, data extraction, and study quality assessment. Twelve RCTs involving 102 607 patients were retrieved. TSOACs significantly reduced the risk of overall major bleeding (relative risk [RR] 0.72, P < .01), fatal bleeding (RR 0.53, P < .01), intracranial bleeding (RR 0.43, P < .01), clinically relevant nonmajor bleeding (RR 0.78, P < .01), and total bleeding (RR 0.76, P < .01). There was no significant difference in major gastrointestinal bleeding between TSOACs and VKAs (RR 0.94, P = .62). When compared with VKAs, TSOACs are associated with less major bleeding, fatal bleeding, intracranial bleeding, clinically relevant nonmajor bleeding, and total bleeding. Additionally, TSOACs do not increase the risk of gastrointestinal bleeding.
Introduction
Vitamin K antagonists (VKAs) have been the standard of care for thromboembolic diseases including venous thromboembolism (VTE) and stroke from systemic embolism attributable to atrial fibrillation (AF). VKAs provide an estimated 95% relative risk (RR) reduction in recurrent VTE compared with the placebo.1 In nonvalvular AF (NVAF), VKAs are highly effective for the prevention of stroke with a relative reduction of 65% compared with placebo.2 Although effective, the major obstacle to the use of VKAs is bleeding complications. The rate of major bleeding among long-term users of VKAs is 1.5% to 5.2% per year. The mortality rate from major bleeding events exceeds 13%.3,4 Intracranial bleeding is the most devastating complication of VKA use, comprising ∼8.7% of all major bleeding episodes and resulting in a 46% to 55% mortality rate.5,6
Apart from hemorrhage, VKAs have several limitations including the need for laboratory monitoring, dietary and drug interactions, a slow onset of action, and a narrow therapeutic window. Target-specific oral anticoagulants (TSOACs), which directly inhibit coagulation factor Xa (rivaroxaban, apixaban, edoxaban, betrixaban, and darexaban) or thrombin (dabigatran) have been developed to overcome these limitations.
Recent clinical trials demonstrated that TSOACs were noninferior to VKAs for the treatment of acute VTE7-9 and extended use of TSOACs reduced the risk of recurrent VTE when compared with placebo.10,11 Furthermore, TSOACs demonstrated comparable or better efficacy to VKAs with respect to the prevention of stroke or systemic embolism in patients with AF.12-15
Bleeding still remains a major concern of TSOACs. The risk of bleeding from TSOACs is uncertain, and reported rates are conflicting and heterogeneous. Despite some clinical trials reporting that TSOACs are associated with lower risks of major bleeding,8,13,15 other studies suggest that the bleeding profiles are similar to that of VKAs.14,16 Notably, the real-world data suggest the observed bleeding risk is lower than that experienced using warfarin.17 Although systematic reviews on the efficacy and safety of TSOACs have been published,18-20 there are no systematic reviews examining the bleeding complications across various indications of TSOACs. We therefore performed a systematic review and meta-analysis to examine the impact of bleeding complications of TSOACs compared with the VKAs in patients with VTE or AF.
Methods
Selection criteria
Studies were included if they were phase-3 randomized controlled trials (RCTs) of adult patients at least 18 years old who received a TSOAC (dabigatran, rivaroxaban, apixaban, edoxaban, betrixaban, or darexaban) for the treatment of VTE (deep vein thrombosis [DVT] or pulmonary embolism [PE]) or stroke and systemic embolism prevention from AF compared with VKAs and reported the rate of bleeding between the groups. Studies that used heparin or low-molecular-weight heparin (LMWH) followed by VKAs were also included. There were no limitations based on blinding, language, or publication status. We included unpublished trials if the methodology and data met our eligibility criteria. We excluded studies of TSOACs used for primary VTE prophylaxis or other indications (eg, mechanical heart valves, acute coronary syndrome, and treatment of thrombus in left atrial appendage). We excluded ximelagatran as this drug has been withdrawn from the market. We excluded studies that used non-VKAs as the comparator (eg, aspirin, heparin, and placebo). Cointervention with antiplatelet agent (aspirin or clopidogrel) was allowed. The primary outcome of the review was major bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH)21 or as defined by the studies. The secondary outcomes included fatal bleeding, intracranial bleeding, clinically relevant nonmajor bleeding, total bleeding, and gastrointestinal (GI) bleeding (as defined by the studies).
Data sources and searches
The electronic searches were performed in MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials databases. The search strategy for MEDLINE is available in supplemental Table 1 (available on the Blood Web site). The search strategy was slightly modified for the other databases. The articles published from inception to January 2014 were eligible for inclusion in this review.
A search for unpublished studies was performed in January 2014 using www.clinicaltrials.gov. We also manually searched abstract books (January 2006 to January 2014) from the congresses of the American Society of Hematology, European Hematology Association, ISTH, American College of Cardiology, European Society of Cardiology, and American Heart Association. Reference lists of relevant articles were manually reviewed.
Study selection
Two reviewers (C.C.-A. and T.I.) performed the study selection independently based on the defined inclusion and exclusion criteria. Disagreements were resolved through discussion or through a third reviewer (W.L.). The κ statistic was used to assess the agreement between reviewers for study selection. A κ value of 0.75 or more indicates excellent agreement.22 For trials that reported results in more than 1 publication, we extracted data from the most complete publication and used the other publications to clarify the data. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for reporting of systematic reviews and meta-analyses of randomized clinical trials was followed.23
Data extraction
Two reviewers (C.C.-A. and T.I.) performed data extraction independently using standardized data extraction sheets. Discrepancies between the reviewers were resolved by consensus or through a third reviewer (W.L). The following data were extracted from the included trials: study design, year of publication, source of funding, population characteristics (number of patients, mean or median age, and sex), therapeutic indication (VTE or AF), interventions (type of TSOAC and duration of treatment), treatment in the control arm, cointerventions, mean time in therapeutic range (TTR) during VKA therapy, and relevant information related to bleeding (major bleeding [as per ISTH or defined by the study], fatal bleeding, intracranial hemorrhage, clinically relevant nonmajor bleeding, total bleeding, and GI bleeding).
Quality assessment
In order to ascertain the validity of eligible randomized trails, 2 reviewers (C.C.-A. and T.I.) independently assessed study quality using the methods specified in the Cochrane Handbook for Systematic Reviews of Interventions.22 This tool evaluates the following domains: allocation sequence generation, allocation concealment, methods of blinding, completeness of outcome data, selective outcome reporting, and other risks of bias. A judgment of “Yes” indicates a low risk of bias, “No” indicates a high risk of bias, and “Unclear” indicates an unclear risk of bias. Disagreement was resolved by discussion or through a third reviewer (W.L). Rating of the overall quality of evidence was performed using the Grading of Recommendations Assessment, Development, and Evaluation system for systematic reviews.24
Data synthesis and analysis
Primary analyses.
Baseline characteristics of the included studies were summarized using descriptive statistics. Results from the different TSOACs were pooled to perform an overall comparison with VKAs. We calculated a pooled RR and corresponding 95% confidence interval (CI) for the outcomes of major bleeding and other secondary outcomes using the Mantel-Haneszel random-effects model. A P value <.05 was considered statistically significant. The rationale for the use of a random-effects model was based on the assumption that there was heterogeneity in the individual studies as a result of variation in indication for treatment, types of TSOAC, duration of treatment, and individual patients’ characteristics.25 Forest plots were created for each outcome. Absolute risk differences with 95% CIs and the number needed to treat (NNT) were reported. All analyses were performed using Review Manager (RevMan, version 5.2; the Nordic Cochrane Centre, the Cochrane Collaboration, 2012, Copenhagen, Denmark).
Heterogeneity between individual studies was formally assessed using the I2 statistic ([Q − degrees of freedom]/Q × 100).22 An I2 of 0 to 40% was considered unimportant heterogeneity; 30% to 60%, moderate heterogeneity; and 50% to 90%, substantial heterogeneity. An I2 of 75% to 100% indicates that variability in the effect estimate is attributable to considerable heterogeneity.22 In order to assess for publication bias, we investigated the funnel plots of effect size vs standard error of the effect estimate. Potential publication bias was considered if the visual inspection of the funnel plots revealed substantial asymmetry.26
Subgroup analyses.
We performed 2 prespecified a priori subgroup analyses, namely the indication for anticoagulation (AF vs VTE) and types of TSOAC (dabigatran, rivaroxaban, apixaban, and edoxaban).
Sensitivity analyses.
We performed 3 sensitivity analyses. The first was based on the quality of the studies to demonstrate the robustness of the effect estimates when studies with a high risk of bias were excluded. Studies were considered low quality if there was a lack of blinding or if there was a “No” response in the Risk of Bias Assessment table (supplemental Figure 2). We also repeated our analyses based on the duration of treatment (≤12 months and >12 months). Finally, because we used the random-effects model in the primary analyses, we performed an analysis using a fixed-effects model.
Results
Study identification and selection
Using electronic searches in MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials, 7937 citations were obtained. An additional 28 studies were identified from hand searching conference proceedings (supplemental Figure 1). After removal of 2675 duplicates, we screened 5290 references through title and abstract review. Of these, 77 studies underwent full-text review. After full-text review, 65 studies were excluded. Reasons for exclusion were duplicate or multiple publications, nonphase-3 RCTs, did not use VKAs as the comparator, and unpublished data. A total of 12 studies were included (4 evaluating dabigatran7,11,12,27 ; 4, rivaroxaban14,16,28,29 ; 2, apixaban8,13 ; and 2, edoxaban9,27 ) enrolling 102 607 patients. Agreement between reviewers was excellent, with a κ agreement of 0.96. Among 12 RCTs, 57 850 patients were assigned to receive TSOACs, and 44 757 to receive VKAs. The quality of the evidence was moderate to high for all outcomes.
Baseline characteristics
The main characteristics of the included studies are summarized in Table 1, and the baseline characteristics of the patients are shown in Table 2. Indications for anticoagulation were VTE (7 trials) and for stroke and systemic embolism prevention from AF (5 trials). The patients were treated for 1.6 to 2.0 years in most of the AF trials, whereas patients in the VTE trials were treated for 3 to 12 months. All of the 12 studies were sponsored by pharmaceutical companies. The mean (or median) age of participants ranged from 70 to 73 years (AF) and 54 to 57 years (VTE). TTR in patients receiving VKAs ranged from 55% to 65%.
Characteristics of the included studies
| Study . | Population . | Duration of treatment . | Patients randomized (n) . | Funding . | |
|---|---|---|---|---|---|
| Intervention . | Control . | ||||
| 1. RE-COVER,7 2009 | Proximal DVT of PE | 6 mo | UFH or LMWH for 5 d, followed by dabigatran 150 mg bid (1273) | UFH or LMWH for at least 5 d with warfarin, INR 2-3 (1266) | Boehringer Ingelheim |
| 2. RE-LY,12 2009 | AF, ≥1 risk factors (previous stroke or TIA, LVEF <40%, NYHA class II or higher, CHF and age ≥75 y or an age of 65-74 y plus DM, HTN, or CAD) | Median 2 y | Dabigatran 100 mg bid (6015) or 150 mg bid (6076) | Warfarin, INR 2-3 (6022) | Boehringer Ingelheim |
| 3. EINSTEIN-DVT,16 2010 | Proximal DVT without symptomatic PE | 3/6/12 mo | Rivaroxaban 15 mg bid for 3 wk, followed by 20 mg OD (1731) | Subcutaneous enoxaparin for at least 5 d with warfarin, INR 2-3 (1718) | Bayer Schering Pharma and Ortho-McNeil |
| 4. ARISTOTLE,13 2011 | AF, ≥1 risk factors (age ≥75, previous stroke/TIA, or systemic embolism, CHF within the previous 3 mo or LVEF ≤40%, DM, HTN) | Median 1.8 y | Apixaban 5 mg bid (9120) | Warfarin, INR 2-3 (9081) | Bristol-Myers Squibb and Pfizer |
| 5. ROCKET AF,14 2012 | NVAF, CHADS2 score ≥2 | Median 590 d | Rivaroxaban 20 mg OD (7131) | Warfarin, INR 2-3 (7133) | Johnson & Johnson and Bayer |
| 6. EINSTEIN-PE,29 2012 | Acute PE | 3/6/12 mo | Rivaroxaban 15 mg bid for 3 wk, followed by 20 mg OD (1731) | Subcutaneous enoxaparin at least 5 d with warfarin, INR 2-3 (1718) | Bayer HealthCare and Janssen Pharmaceuticals |
| 7. J-ROCKET AF,28 2012 | NVAF, ≥2 risk factors (CHF and/or LVEF ≤35%, HTN, DM) | Median: 71 wk (rivaroxaban), 69 wk (warfarin) | Rivaroxaban 15 mg OD (639) | Warfarin, INR 2-3 (639) | Bayer Yakuhin Ltd. |
| 8. AMPLIFY,8 2013 | Proximal DVT or PE | 6 mo | Apixaban 10 mg bid for 7 d, followed by 5 mg bid (2691) | Subcutaneous enoxaparin for at least 5 d with warfarin, INR 2-3 (2704) | Pfizer and Bristol-Myers Squibb |
| 9. ENGAGE-AF-TIMI- 48,15 2013 | AF, CHADS2 score ≥2 | Median 907 d | Edoxaban 30 mg OD (7034) or edoxaban 60 mg OD (7035) | Warfarin, INR 2-3 (7036) | Daiichi Sankyo Pharma Development |
| 10. RE-MEDY,11 2013 | Proximal DVT or PE | 6-36 mo | Dabigatran 150 mg bid (1430) | Warfarin, INR 2-3 (1426) | Boehringer Ingelheim |
| 11. HOKUSAI-VTE,9 2013 | Proximal DVT or PE | 3-12 mo | UFH or LMWH for at least 5 d, followed by edoxaban 60 mg OD (4122) | UFH or LMWH for at least 5 d, followed by warfarin, INR 2-3 (4122) | Daiichi Sankyo Pharma Development |
| 12. RE-COVER II,27 2014 | Proximal DVT or PE | 6 mo | UFH or LMWH for 5 d, followed by dabigatran 150 mg bid (1273) | UFH or LMWH for at least 5 d with warfarin, INR 2-3 (1266) | Boehringer Ingelheim |
| Study . | Population . | Duration of treatment . | Patients randomized (n) . | Funding . | |
|---|---|---|---|---|---|
| Intervention . | Control . | ||||
| 1. RE-COVER,7 2009 | Proximal DVT of PE | 6 mo | UFH or LMWH for 5 d, followed by dabigatran 150 mg bid (1273) | UFH or LMWH for at least 5 d with warfarin, INR 2-3 (1266) | Boehringer Ingelheim |
| 2. RE-LY,12 2009 | AF, ≥1 risk factors (previous stroke or TIA, LVEF <40%, NYHA class II or higher, CHF and age ≥75 y or an age of 65-74 y plus DM, HTN, or CAD) | Median 2 y | Dabigatran 100 mg bid (6015) or 150 mg bid (6076) | Warfarin, INR 2-3 (6022) | Boehringer Ingelheim |
| 3. EINSTEIN-DVT,16 2010 | Proximal DVT without symptomatic PE | 3/6/12 mo | Rivaroxaban 15 mg bid for 3 wk, followed by 20 mg OD (1731) | Subcutaneous enoxaparin for at least 5 d with warfarin, INR 2-3 (1718) | Bayer Schering Pharma and Ortho-McNeil |
| 4. ARISTOTLE,13 2011 | AF, ≥1 risk factors (age ≥75, previous stroke/TIA, or systemic embolism, CHF within the previous 3 mo or LVEF ≤40%, DM, HTN) | Median 1.8 y | Apixaban 5 mg bid (9120) | Warfarin, INR 2-3 (9081) | Bristol-Myers Squibb and Pfizer |
| 5. ROCKET AF,14 2012 | NVAF, CHADS2 score ≥2 | Median 590 d | Rivaroxaban 20 mg OD (7131) | Warfarin, INR 2-3 (7133) | Johnson & Johnson and Bayer |
| 6. EINSTEIN-PE,29 2012 | Acute PE | 3/6/12 mo | Rivaroxaban 15 mg bid for 3 wk, followed by 20 mg OD (1731) | Subcutaneous enoxaparin at least 5 d with warfarin, INR 2-3 (1718) | Bayer HealthCare and Janssen Pharmaceuticals |
| 7. J-ROCKET AF,28 2012 | NVAF, ≥2 risk factors (CHF and/or LVEF ≤35%, HTN, DM) | Median: 71 wk (rivaroxaban), 69 wk (warfarin) | Rivaroxaban 15 mg OD (639) | Warfarin, INR 2-3 (639) | Bayer Yakuhin Ltd. |
| 8. AMPLIFY,8 2013 | Proximal DVT or PE | 6 mo | Apixaban 10 mg bid for 7 d, followed by 5 mg bid (2691) | Subcutaneous enoxaparin for at least 5 d with warfarin, INR 2-3 (2704) | Pfizer and Bristol-Myers Squibb |
| 9. ENGAGE-AF-TIMI- 48,15 2013 | AF, CHADS2 score ≥2 | Median 907 d | Edoxaban 30 mg OD (7034) or edoxaban 60 mg OD (7035) | Warfarin, INR 2-3 (7036) | Daiichi Sankyo Pharma Development |
| 10. RE-MEDY,11 2013 | Proximal DVT or PE | 6-36 mo | Dabigatran 150 mg bid (1430) | Warfarin, INR 2-3 (1426) | Boehringer Ingelheim |
| 11. HOKUSAI-VTE,9 2013 | Proximal DVT or PE | 3-12 mo | UFH or LMWH for at least 5 d, followed by edoxaban 60 mg OD (4122) | UFH or LMWH for at least 5 d, followed by warfarin, INR 2-3 (4122) | Daiichi Sankyo Pharma Development |
| 12. RE-COVER II,27 2014 | Proximal DVT or PE | 6 mo | UFH or LMWH for 5 d, followed by dabigatran 150 mg bid (1273) | UFH or LMWH for at least 5 d with warfarin, INR 2-3 (1266) | Boehringer Ingelheim |
CHADS2 score, 1 is given for point for CHF, HTN, age ≥75 y, and DM; 2 points are given for previous stroke or TIA, and systemic embolism.
AMPLIFY, Apixaban for the initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy; ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; bid, twice daily; CAD, coronary artery disease; CHADS2 score, a HYPERLINK “http://en.wikipedia.org/wiki/Clinical_prediction_rule” \o “Clinical prediction rule” clinical prediction rule for estimating the risk of HYPERLINK “http://en.wikipedia.org/wiki/Stroke” \o “Stroke” stroke in patients with HYPERLINK “http://en.wikipedia.org/wiki/Rheumatic_fever” \o “Rheumatic fever” nonrheumatic AF; CHF, congestive heart failure; d, day; DM, diabetic mellitus (2 points are given for previous stroke or TIA); EINSTEIN-DVT, Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis; ENGAGE-AF TIMI 48, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48; EINSTEIN-PE, Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Pulmonary Embolism; HOKUSAI-VTE, Edoxaban vs Warfarin for the Treatment of Symptomatic Venous Thromboembolism; HTN, hypertension (age ≥75 y); INR, international normalized ratio; J-ROCKET-AF, An Efficacy and Safety Study of Rivaroxaban With Warfarin for the Prevention of Stroke and Non-Central Nervous System Systemic Embolism in Patients With NonValvular Atrial Fibrillation in Japan; LVEF, left ventricular ejection fraction; mo, month; NVAF, nonvalvular AF; NYHA, New York Heart Association; OD, once daily; RE-COVER I, Efficacy and Safety of Dabigatran Compared to Warfarin for 6 Month Treatment of Acute Symptomatic Venous Thromboembolism I; RE-COVER II, Efficacy and Safety of Dabigatran Compared to Warfarin for 6-Month Treatment of Acute Symptomatic Venous Thromboembolism II; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy study; RE-MEDY, A phase III, randomised, multi-center, double-blind, parallel-group, active controlled study to evaluate the efficacy and safety of oral dabigatran etexilate compared to warfarin (INR 2.0-3.0) for the secondary prevention of venous thromboembolism; ROCKET-AF; Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; TIA, transient ischemic attack; UFH, unfractionated heparin; y, year.
Baseline patient characteristics
| Study . | Age . | TTR . | Male (%) . | Mean CHADS2 . | Concomitant ASA (%) . | Isolated DVT (%) . | PE or PE with DVT (%) . | |
|---|---|---|---|---|---|---|---|---|
| TSOACs . | VKAs . | |||||||
| 1. RE-COVER,7 2009 | 55.0 ± 15.8* | 54.4 ± 16.2* | 59.9 | 58.4 | NA | NA | 1749 (69) | 786 (31) |
| 2. RE-LY,12 2009 | 71.4 ± 8.6* (dabigatran 110), 71.5 ± 8.8* (dabigatran 150) | 71.6 ± 8.6* | 64 | 63.6 | 2.1 | 39.8% | NA | NA |
| 3. EINSTEIN-DVT,16 2010 | 55.8 ± 16.4* | 56.4 ± 16.3* | 57.7 | 56.8 | NA | NA | 3405 (99) | 23 (1) |
| 4. ARISTOTLE,13 2011 | 70 (63-76)† | 70 (63-76)† | 62.2 | 64.7 | 2.1 | 5632 (30.9) | NA | NA |
| 5. ROCKET AF,14 2012 | 73 (65-78)† | 73 (65-78)† | 55 | 60.3 | 3.48 (rivaroxaban), 3.46 (warfarin) | 5205 (36.5) | NA | NA |
| 6. EINSTEIN-PE,29 2012 | 57.9 ± 7.3* | 57.5 ± 7.2* | 62.7 | 52.9 | NA | NA | 0 (0) | 4832 (100) |
| 7. J-ROCKET AF,28 2012 | 71.0 (34-89)† | 71.2 (43-90)† | 65 | 80.6 | 3.25 | NA | NA | NA |
| 8. AMPLIFY,8 2013 | 57.2 ± 16* | 56.7 ± 16* | 61 | 58.7 | NA | NA | 3532 (65) | 1836 (34) |
| 9. ENGAGE-AF-TIMI- 48,15 2013 | 72 (64-78)† | 72 (64-78)† | 64.9 | 61.9 | 2.8 | 29.3% | NA | NA |
| 10. RE-MEDY,11 2013 | 55.4 ± 15.0* | 53.9 ± 15.3* | 65.3 | 61 | NA | NA | 1860 (65.1) | 994 (34.8) |
| 11. HOKUSAI-VTE,9 2013 | 55.7 ± 16.3* | 55.9 ± 16.2* | 63.5 | 57.2 | NA | NA | 4921 (59.7) | 3319 (40.3) |
| 12. RE-COVER II,27 2014 | 54.7 ± 16.2* | 55.1 ± 16.3* | 57 | 60.6 | NA | NA | 1750 (68.1) | 816 (31.8) |
| Study . | Age . | TTR . | Male (%) . | Mean CHADS2 . | Concomitant ASA (%) . | Isolated DVT (%) . | PE or PE with DVT (%) . | |
|---|---|---|---|---|---|---|---|---|
| TSOACs . | VKAs . | |||||||
| 1. RE-COVER,7 2009 | 55.0 ± 15.8* | 54.4 ± 16.2* | 59.9 | 58.4 | NA | NA | 1749 (69) | 786 (31) |
| 2. RE-LY,12 2009 | 71.4 ± 8.6* (dabigatran 110), 71.5 ± 8.8* (dabigatran 150) | 71.6 ± 8.6* | 64 | 63.6 | 2.1 | 39.8% | NA | NA |
| 3. EINSTEIN-DVT,16 2010 | 55.8 ± 16.4* | 56.4 ± 16.3* | 57.7 | 56.8 | NA | NA | 3405 (99) | 23 (1) |
| 4. ARISTOTLE,13 2011 | 70 (63-76)† | 70 (63-76)† | 62.2 | 64.7 | 2.1 | 5632 (30.9) | NA | NA |
| 5. ROCKET AF,14 2012 | 73 (65-78)† | 73 (65-78)† | 55 | 60.3 | 3.48 (rivaroxaban), 3.46 (warfarin) | 5205 (36.5) | NA | NA |
| 6. EINSTEIN-PE,29 2012 | 57.9 ± 7.3* | 57.5 ± 7.2* | 62.7 | 52.9 | NA | NA | 0 (0) | 4832 (100) |
| 7. J-ROCKET AF,28 2012 | 71.0 (34-89)† | 71.2 (43-90)† | 65 | 80.6 | 3.25 | NA | NA | NA |
| 8. AMPLIFY,8 2013 | 57.2 ± 16* | 56.7 ± 16* | 61 | 58.7 | NA | NA | 3532 (65) | 1836 (34) |
| 9. ENGAGE-AF-TIMI- 48,15 2013 | 72 (64-78)† | 72 (64-78)† | 64.9 | 61.9 | 2.8 | 29.3% | NA | NA |
| 10. RE-MEDY,11 2013 | 55.4 ± 15.0* | 53.9 ± 15.3* | 65.3 | 61 | NA | NA | 1860 (65.1) | 994 (34.8) |
| 11. HOKUSAI-VTE,9 2013 | 55.7 ± 16.3* | 55.9 ± 16.2* | 63.5 | 57.2 | NA | NA | 4921 (59.7) | 3319 (40.3) |
| 12. RE-COVER II,27 2014 | 54.7 ± 16.2* | 55.1 ± 16.3* | 57 | 60.6 | NA | NA | 1750 (68.1) | 816 (31.8) |
CHADS2 score, 1 is given for point for CHF, HTN, age ≥75 y, and DM; 2 points are given for previous stroke or TIA, and systemic embolism.
ASA, acetylsalicylic acid; NA, not applicable.
Mean ± standard deviation.
Median (minimum-maximum).
Study quality
The risk of bias assessment is demonstrated in supplemental Figure 2. The method used to generate the random sequence and allocation concealment was inadequately reported in 1 study.28 The EINSTEIN DVT,16 EINSTEIN PE,29 and RE-LY12 trials were not blinded. TSOACs are typically dose reduced in patients with renal impairment, which may also have contributed to bias in the bleeding outcomes. Visual inspection of funnel plots for all outcomes suggested no evidence of publication bias supplemental Figure 3.
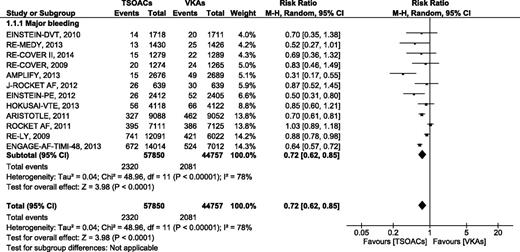
Major bleeding
In the 12 RCTs comparing TSOACs with VKAs with a target INR of 2 to 3, major bleeding as defined by the studies or using ISTH criteria21 occurred in 2320 of 57 850 (4%) of the patients treated with TSOACs and in 2081 of 44 757 (4.64%) of the patients treated with VKAs. The pooled RR for major bleeding was 0.72 (95% CI, 0.62-0.85), P < .01, I2 = 78%. (Figure 1). The absolute risk difference for major bleeding was −0.64%, with an NNT of 156 using TSOACs compared with VKAs. The sensitivity analysis using a fixed-effects model had no effect on our results supplemental Figure 4.
Major bleeding events comparing target-specific anticoagulants with VAKs.
Subgroup analysis by indication for anticoagulation (VTE vs AF) provided the same results as the primary analysis. In contrast, subgroup analysis by type of TSOACs demonstrated a significant reduction in major bleeding for the trials evaluating dabigatran (RR 0.86 [95% CI, 0.77-0.96], P = .006, I2 = 0%) and edoxaban (RR 0.70 [95% CI, 0.54-0.90], P = .006, I2 = 55%), but not for rivaroxaban (RR 0.78 [95% CI, 0.54-1.12], P = .18, I2 = 68%) or apixaban (RR 0.49 [95% CI, 0.22-1.10], P = .08, I2 = 87%) (supplemental Figure 5). Analysis using a fixed-effects model resulted in all TSOACs except rivaroxaban demonstrating statistically significant reductions in major bleeding (data not shown).
Fatal bleeding
Fatal bleeding occurred in 173 of 57 850 (0.30%) patients treated with TSOACs and in 234 of 44 757 (0.52%) patients treated with VKAs in the 12 studies reporting this outcome (Figure 2). TSOACs were associated with a statistically significant reduction in fatal bleeding (RR 0.53 [95% CI, 0.43-0.64], P < .01, I2 = 0%). The pooled absolute risk reduction was −0.22%, resulting in an NNT of 454. Analysis with a fixed-effects model did not change the results. Subgroup analyses based on indication for anticoagulation and type of TSOACs provided similar results as the primary analysis.
Intracranial bleeding
Intracranial bleeding occurred in 297 of 57 850 (0.51%) patients treated with TSOACs and in 485 of 44 757 (1.08%) patients treated with VKAs in the 12 studies reporting this outcome (Figure 3). TSOACs were associated with a significant reduction in intracranial bleeding (RR 0.43 [95% CI, 0.37-0.50], P < .01, I2 = 2%). The pooled absolute risk reduction was −0.57%, resulting in an NNT of 185. Analysis with a fixed-effects model did not change the results. Subgroup analyses based on indication for anticoagulation and type of TSOACs provided similar results as the primary analysis.
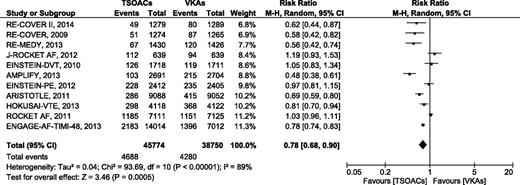
Clinically relevant nonmajor bleeding
A total of 11 studies provided information for this outcome. Clinically relevant nonmajor bleeding occurred in 4688 of 45 774 (10.24%) patients treated with TSOACs and in 4280 of 38 750 (11.05%) patients treated with VKAs (Figure 4). TSOACs were associated with a significant reduction in clinically relevant nonmajor bleeding (RR 0.78 [95% CI, 0.68-0.90], P < .01, I2 = 89%). The pooled absolute risk reduction was −1.01%, resulting in an NNT of 99. Analysis with a fixed-effects model did not change the results. The subgroup analysis according to type of TSOACs revealed a significant reduction in clinically relevant nonmajor bleeding in patients treated with dabigatran, apixaban, and edoxaban, but not in patients treated with rivaroxaban (supplemental Figure 6).
Clinically relevant nonmajor bleeding events comparing TSOACs with VAKs.
Total bleeding
Data on total bleeding were reported in 8 studies. Total bleeding occurred in 11 429 of 45 970 (24.86%) patients treated with TSOACs and in 10 002 of 32 877 (30.42%) patients treated with VKAs (Figure 5). TSOACs were associated with a significant reduction in total bleeding (RR 0.76 [95% CI, 0.71-0.82], P < .01, I2 = 86%). The pooled absolute risk reduction was −5.56%, resulting in an NNT of 18. Reanalysis with a fixed-effects model did not change the results. Subgroup analyses according to indication of anticoagulation and type of TSOACs provided similar results.
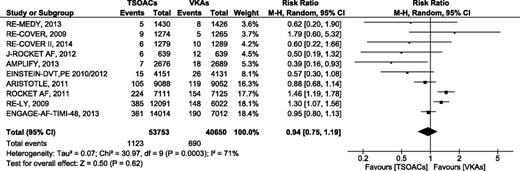
Major GI bleeding
Data on major GI bleeding were available from 11 studies. Of these, major GI bleeding occurred in 1123 of 53 753 (2.09%) patients treated with TSOACs and in 690 of 40 650 (1.70%) patients treated with VKAs (Figure 6). There was no difference in the risk of GI bleeding between TSOACs and VKAs (RR 0.94 [95% CI, 0.75-1.99], P = .62, I2 = 71%). Analysis with a fixed-effects model did not change the results. Subgroup analysis according to indication of anticoagulation demonstrated that TSOACs were associated with a significant reduction in the risk of major GI bleeding in patients with VTE (RR 0.64 [95% CI, 0.41-0.99], P = .04, I2 = 16%), but not in patients with AF (supplemental Figure 7). The subgroup analysis according to type of TSOACs provided similar results to the primary analysis.
Sensitivity analyses
Sensitivity analyses evaluating only the high-quality studies (excluding studies with lack of blinding), did not change the findings from the primary analysis for all outcomes (supplemental Figures 8-11). Similarly, sensitivity analyses according to duration of treatment confirmed the results of the primary analysis except for the patients who were treated with TSOACs ≤12 months, where a marginally lower RR of GI bleeding was observed (supplemental Figure 12).
Discussion
This systematic review and meta-analysis compared the risk of bleeding associated with TSOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) with that of VKAs administered to a target INR of 2.0 to 3.0 in patients with VTE or AF. Although there were several previous systematic reviews investigating the efficacy and safety of TSOACs compared with conventional treatment, most of these reviews focused on a particular AF20,30-37 or VTE18,19,38,39 population. To our knowledge, this is the first comprehensive analysis of bleeding outcomes from 12 phase-3 RCTs across 2 major indications for anticoagulation that incorporated >100 000 patients.
The results of the meta-analysis indicate that the use of TSOACs is associated with significant reductions in the risk of major bleeding (RR 0.72; NNT 156), fatal bleeding (RR 0.53; NNT 454), intracranial bleeding (RR 0.43; NNT 185), clinically relevant nonmajor bleeding (RR 0.78; NNT 99), and total bleeding (RR 0.76; NNT 18), but not in major GI bleeding.
Our finding is consistent with the previous systematic reviews19,34,38 that TSOACs are associated with lower major bleeding. We observed statistically significant heterogeneity for major bleeding (I2 = 78%) and clinically relevant nonmajor bleeding (I2 = 89%). The observed heterogeneity can potentially be explained by the different TSOACs included in this review. The subgroup analyses demonstrated that the risk reduction of bleeding from trials evaluating rivaroxaban was not significantly different compared with VKAs. One potential explanation is that the results of trials evaluating rivaroxaban are mainly driven by ROCKET AF14 and J-ROCKET AF,28 and these 2 trials enrolled AF patients with relatively high CHADS2 scores that are known to place such patients at high risk of bleeding.40 Other heterogeneity might be explained by differences in baseline patient characteristics in the AF and VTE trials that we included in this review. Therefore, the pooled estimates of these outcomes should be interpreted as a whole for all TSOACs and not for any 1 individual TSOAC.
Despite the low incidence of fatal and intracranial bleeding with warfarin (0.52% and 1.08%, respectively), our meta-analysis demonstrated that TSOACs are associated with a further reduction of these events. This finding was similar to the previous systematic review.18,20,30,31,35 Moreover, the observed outcomes are consistent for all prespecified subgroup and sensitivity analyses. The mechanism for the lower rate of intracranial bleeding with the TSOACs compared with VKAs is unclear. One postulated mechanism is that warfarin is associated with greater thrombin suppression in the brain and pathological thrombosis at sites of atherosclerotic plaque disruption.41 This might support the selection of a TSOACs compared with VKAs in patients considered to be at high risk of intracranial bleeding who require anticoagulant treatment.
An increased rate of GI bleeding has been identified with TSOACs. Two early RCTs evaluating dabigatran in AF patients reported that dabigatran 150 mg twice daily increased risk of major GI bleeding compared with warfarin (RR 1.50 [95% CI, 1.19-1.89], P < .001); this was not seen with dabigatran 110 mg twice daily.12 Likewise, rivaroxaban 20 mg once daily was associated with increased rate of major GI bleeding compared with warfarin (3.2% vs 2.2%, P < .001).14 One of the mechanism of TSOAC-associated GI bleeding might be explained from the active drugs remaining in the GI tract and precipitating bleeding from vulnerable lesions.42 The previous systematic reviews reported that TSOACs were associated with significantly increased risk of GI bleeding compared with standard care (warfarin, LMWH, and LMWH followed by warfarin or placebo).30,43 However, our meta-analysis did not demonstrate that TSOACs increase the rate of major GI bleeding (RR 0.94 [95% CI, 0.88-1.34], P = .62). The discrepancy of this finding might be explained from the difference of the population and the comparators included in the reviews. Again, the observed heterogeneity is likely attributable to the different TSOACs and baseline characteristics of the patients in the review. When trials were grouped according to indication for treatment, we found that the risk of GI bleeding in patients with VTE was significant lower with TSOACs (RR 0.64 [95% CI, 0.41-0.99], P = .04). In contrast, there was no difference in the rates of GI bleeding between TSOACs and VKAs among AF patients. Patients with AF are typically older and have more comorbidities compared with VTE patients, which may make them vulnerable to GI bleeding from TSOACs.
The strengths of this review include the rigorous methodologic approach and large number of included patients. We included 12 large RCTs evaluating 4 TSOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) across 2 major indications. We included >100 000 patients in the meta-analysis and consequently have the statistical power to detect differences in uncommon outcomes including fatal bleeding and intracranial bleeding. In contrast to previous studies,18,34,35,38 we only included studies using VKAs as the comparator to generate more precise estimates of risk.
There are several limitations to this study. First, there were differences in the study population, type of TSOACs evaluated, and duration of treatment that may have contributed to the heterogeneity observed in the results. However, our sensitivity analyses did not demonstrate different findings for most of the outcomes. Second, because this was a study-level meta-analysis, we were unable to compare the outcome in patient subgroups by use of antiplatelet agents. In an analysis of the RE-LY data, the concomitant use of single or dual antiplatelet agents was associated with an increased risk of major bleeding.44 Data from the ARISTOTLE trial found that concomitant aspirin therapy increased bleeding, but the combination of apixaban and aspirin was associated with less major bleeding compared with warfarin and aspirin (hazard ratio [HR] 0.75 [95% CI, 0.59-0.94]).45 Moreover, we could not test for interaction for particular subgroups, for example, the elderly or patients with renal impairment. Third, we included 3 studies (RELY, EINSTEIN DVT, and EINSTEIN PE) that did not have concealed treatment allocation. We addressed this concern in the sensitivity analyses that included only studies with a low risk of bias and did not observe any differences in outcomes. Fourth, we did not perform a network meta-analysis. Therefore, we could not compare the bleeding complications between various types of TSOACs. Fifth, we only investigated for the safety profile, particularly bleeding side effects, but not for efficacy of the anticoagulants. Finally, the results of this meta-analysis should not be generalized to patients taking TSOACs for indications other than VTE or stroke prevention from AF (eg, VTE prophylaxis following orthopedic surgery or in medically ill patients).
In conclusion, when compared with VKAs administered to a target INR of 2.0 to 3.0, TSOACs are associated with less major bleeding, fatal bleeding, intracranial bleeding, clinically relevant nonmajor bleeding, and total bleeding. Additionally, TSOACs do not appear to increase the risk of GI hemorrhage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.C.-A., W.L., and M.C. designed the methods; C.C.-A. and T.I. performed study selection, data extraction, study quality assessment, and analysis; C.C.-A. drafted the manuscript; and W.L. and M.C. critically revised the manuscript.
Conflict-of-interest disclosure: M.C. sat on advisory boards for Janssen, Leo Pharma, Portola, and AKP America; his institution has received funding for research projects from Leo Pharma; and he received funding for presentations from Leo Pharma, Bayer, Celgene, Shire, and CSL Behring. The remaining authors declare no competing financial interests.
Correspondence: Mark Crowther, Room L208, 50 Charlton Ave East, St. Joseph’s Hospital, Hamilton, ON L8N 4A6, Canada; e-mail: crowthrm@mcmaster.ca.