Key Points
RUNX1/RUNX1T1-based MRD status at 1, 2, and 3 months after HSCT could discriminate patients at high risk of post-HSCT relapse.
Rather than c-KIT mutations, MRD monitoring allows further rapid identification of patients at high risk of relapse after allo-HSCT.
Abstract
We asked whether minimal residual disease (MRD) determined by RUNX1/RUNX1T1 transcript levels could identify allogeneic hematopoietic stem cell transplantation (allo-HSCT) t(8;21) (q22;q22) acute myeloid leukemia patients who are at high risk for relapse, together with the impact of c-KIT mutations. Ninety-two consecutive adult t(8;21) patients who received allo-HSCT in complete remission were enrolled. MRD status at 1, 2, and 3 months after HSCT identified relapse patients (P = .05, P < .001, P = .0001, respectively). The 2-year cumulative incidence of relapse (CIR) and leukemia-free survival (LFS) was 32% vs 9% (P = .01) and 55% vs 70% (P = .12) for patients with and without c-KIT mutations, respectively. In multivariate analysis, MRD at the first 3 months after HSCT, rather than c-KIT mutations, was an independent factor for CIR (P = .001) and LFS (P = .001). In addition, 17 patients received donor lymphocyte infusion (DLI) as interventional therapy for MRD, and the 2-year CIR and LFS for patients with or without DLI was 24% vs 87% (P = .001) and 64% vs 0% (P < .001), respectively. In conclusion, MRD monitoring early after transplant allows further rapid identification of t(8;21) patients at high risk of relapse and was more predictive of relapse risk than c-KIT mutations.
Introduction
Acute myeloid leukemia (AML) with t(8;21)(q22;q22) is a heterogeneous disease entailing different prognoses.1-5 Monitoring of minimal residual disease (MRD) during chemotherapy can identify high-risk patients with t(8;21).6-10 Additionally, c-KIT mutations are associated with a worse outcome.11 Recently, our prospective multicenter study demonstrated that allogeneic hematopoietic stem cell transplantation (allo-HSCT) significantly improved clinical outcomes of high-risk patients,12 so there remains a relevant role of allo-HSCT for the management of the high-risk t(8;21) population.
Studies have revealed the predictive role of MRD status after transplant.13-15 For example, recent results from our center showed that the presence of MRD after HSCT as determined by flow cytometry and Wilm’s tumor (WT1) gene expression by real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was associated with higher risk of relapse compared with patients without MRD (46% vs 18%).16 More importantly, risk stratification–directed donor lymphocyte infusion (DLI) could reduce relapse and improve survival for those acute leukemia patients without t(8;21), t(15;17), inv(16), t(16;16), or t(9;22) at the time of molecular relapse.16
RUNX1/RUNX1T1 quantification in the period of chemotherapy can predict the risk for relapse.17,18 Whether RUNX1/RUNX1T1-based MRD continues to serve as an efficient tool for further risk stratification after HSCT is not known, and if this were the case, the optimal time for risk-directed strategies after HSCT would need to be addressed. In addition, the relative predictive value of MRD and c-KIT mutations after HSCT has not yet been assessed. The present study addresses the potential of RUNX1/RUNX1T1-based MRD early after HSCT and the presence of c-KIT mutations to identify t(8;21) patients at high risk of posttransplant relapse. We found that serial early MRD monitoring of t(8;21) AML by qRT-PCR after allo-HSCT could allow further risk stratification, and MRD seemed to be more predictive than c-KIT mutations for subsequent relapse.
Patients and methods
Eligibility criteria
One hundred and eleven consecutive AML patients with t(8;21) who received allo-HSCT at 4 transplant centers in China between January 2006 and July 2013 were assessed for inclusion criteria including the following: (1) aged 18 to 60 years old; (2) had AML with t(8;21) and/or RUNX1/RUNX1T1 transcripts and had achieved and maintained complete remission (CR; first or second) before HSCT; and (3) had no contraindications to HSCT. We excluded 8 patients under 18 years and 11 patients transplanted with refractory/relapsed disease. The remaining 92 subjects were enrolled; 49 patients transplanted in Peking University have previously been reported12 and followed further in this study. Before transplant, 75 of the 92 patients met the high-risk criteria we recently published and received HSCT as recommended.12 Reasons for the other 17 patients receiving HSCT were loss of Y or X chromosome (n = 9), c-KIT mutations (n = 5), extramedullary disease (n = 1), and patient’s persistence (n = 2). The study protocol was approved by the institutional review boards at each center. Written informed consent was obtained from all patients and donors in accordance with the Declaration of Helsinki. Patient and donor characteristics are shown in Table 1.
Characteristics of patients and donors
| Characteristics . | n = 92 . |
|---|---|
| Age in y, median (range) of the recipient | 36 (18-54) |
| Gender, no. | |
| Male | 50 |
| Female | 42 |
| WBCs at diagnosis ×109/L, median (range) | 9.6 (0.8-87) |
| c-KIT mutations, no. | |
| Positive | 33 |
| Negative | 48 |
| Unknown | 11 |
| Karotype | |
| Sole t(8;21) | 51 |
| Additional abnormalities other than t(8;21) | 35 |
| Complex karotype | 6 |
| Disease status | |
| First CR (CR1) | 86 |
| Second CR (CR2) | 6 |
| Courses required to achieve CR | |
| 1 | 57 |
| >1 (including CR2) | 35 |
| Time from diagnosis to transplant in mo, median (range) | 6 (2.4-25) |
| Donor source | |
| Haploidentical | 44 |
| HLA-matched sibling | 43 |
| Unrelated donor | 5 |
| Stem cell source | |
| G-CSF mobilized BM + peripheral blood | 75 |
| G-CSF mobilized peripheral blood | 17 |
| Conditioning regimen | |
| Chemotherapy based | 88 |
| TBI based | 4 |
| Median CD34+ count, ×106/kg (range) | 2.2 (0.4-5.5) |
| Median CD3+ count, ×108/kg (range) | 1.7 (0.3-8.4) |
| Characteristics . | n = 92 . |
|---|---|
| Age in y, median (range) of the recipient | 36 (18-54) |
| Gender, no. | |
| Male | 50 |
| Female | 42 |
| WBCs at diagnosis ×109/L, median (range) | 9.6 (0.8-87) |
| c-KIT mutations, no. | |
| Positive | 33 |
| Negative | 48 |
| Unknown | 11 |
| Karotype | |
| Sole t(8;21) | 51 |
| Additional abnormalities other than t(8;21) | 35 |
| Complex karotype | 6 |
| Disease status | |
| First CR (CR1) | 86 |
| Second CR (CR2) | 6 |
| Courses required to achieve CR | |
| 1 | 57 |
| >1 (including CR2) | 35 |
| Time from diagnosis to transplant in mo, median (range) | 6 (2.4-25) |
| Donor source | |
| Haploidentical | 44 |
| HLA-matched sibling | 43 |
| Unrelated donor | 5 |
| Stem cell source | |
| G-CSF mobilized BM + peripheral blood | 75 |
| G-CSF mobilized peripheral blood | 17 |
| Conditioning regimen | |
| Chemotherapy based | 88 |
| TBI based | 4 |
| Median CD34+ count, ×106/kg (range) | 2.2 (0.4-5.5) |
| Median CD3+ count, ×108/kg (range) | 1.7 (0.3-8.4) |
BM, bone marrow; G-CSF, granulocyte colony-stimulating factor; TBI, total body irradiation; WBC, white blood cell.
MRD monitoring, c-KIT mutations screening, and chimerism analysis
BM samples were collected as part of the treatment protocol. BM samples were requested directly before transplant, as well as serially at 1, 2, 3, 6, 9, 12, 24, 36, and 60 months after HSCT and at relapse; however, in patients with >1-log rising levels of RUNX1/RUNX1T1 transcripts, monitoring was performed every 2 weeks. MRD was monitored using qRT-PCR to quantify the level of RUNX1/RUNX1T1 transcripts in 3 central laboratories (Beijing People, Suzhou, and Guangzhou) according to recommendations of the Europe Against Cancer Program.19 Results were expressed as a [fusion gene/Abelson gene (ABL1)] × 100 transcript ratio. c-KIT mutations in exons 17 and 8 were screened at diagnosis in the previously mentioned 3 central laboratories using the direct sequencing method. We did not include the c-KIT mutations screening in the initial protocol. After the prognostic value of the c-KIT mutations was confirmed in 2006, consecutive patients were screened for c-KIT mutations at diagnosis from the year 2007. In addition, c-KIT mutations were sought in patients with fusion gene level >10% (based on the baseline level of 388% at diagnosis12 and the widely recognized sensitivity of 10%∼20% with direct-sequencing for c-KIT mutations) directly before transplant and in patients at relapse.
At Peking University, a high proportion (>90%) of full donor chimerism was found by DNA fingerprinting of short tandem repeat on blood samples when positive MRD was detected, as previously reported.16 To improve sensitivity and linearity, from the year 2010, quantitative chimerism analysis was performed on BM samples using real-time PCR based on 29 sequence polymorphism system markers, including short deletions or insertions according to Alizadeh et al20 and single nucleotide polymorphisms according to Maas et al.21,22 The procedure was previously described in detail and detects recipient signals >0.1%.23,24 The time points of chimerism evaluation were the same as for MRD assessment.
Treatment procedure
All patients were similarly treated during induction and/or consolidation. Induction chemotherapy was composed of 1 to 2 cycles of induction (not double induction; a second induction was only given to patients who did not achieve CR after the first induction course) with an anthracycline in combination with cytarabine. The first and second consolidation therapies included intermediate-dose cytarabine with or without an anthracycline. More details were described previously.12 High-risk patients as defined subsequently were recommended for allo-HSCT. Eighty-eight patients received an intensive chemotherapy-based conditioning regimen composed of busulfan and cyclophosphamide, and the other 4 patients received a conditioning regimen based on TBI, as described previously in detail.16
Protocol of intervention for MRD
Based on the MRD status post-HSCT and clinical conditions at the time of presence of MRD, modified DLI would be given before hematologic relapse as the intervention therapy after 3 months post-HSCT following a trial of immunosuppressant withdrawal. The detailed criteria for DLI administration included the following: (1) patients not achieving major molecular remission (MMR) at the time of 3 months or losing MMR after 3 months post-HSCT; (2) no uncontrolled graft-versus-host disease (GVHD) or life-threatening infection; and (3) donor availability and willingness. Patients with GVHD first received GVHD therapy; after GVHD was controlled, MRD testing was repeated, and those patients not achieving MMR received modified DLI. The modified DLI regimen was previously described.16
Definition
MMR was defined as >3-log reduction in RUNX1/RUNX1T1 transcripts when compared with the pretreatment baseline level as previously described.12 Before transplant, the high-risk criteria from our recent report12 were defined as those patients not achieving MMR after the second consolidation therapy or those exhibiting the loss of MMR within 6 months of achieving MMR.
End points and statistical analysis
The primary end point studied was relapse rate; the secondary end points were overall survival (OS) and leukemia-free survival (LFS). Cumulative incidences were estimated for nonrelapse-mortality and relapse (CIR) to accommodate competing risks. The probabilities of OS and LFS were estimated by the Kaplan-Meier method. Cox regression full model was used to identify indicative variables for the patients, which included c-KIT status at diagnosis (mutation or no mutation), WBC count at diagnosis (<30 or ≥30 × 109/L), additional chromosome abnormalities (yes or no), the number of courses required to achieve CR (1 or >1 course), time from diagnosis to transplant (<6 months or >6 months), achieving MMR directly before HSCT (yes or no), donor source (HLA-identical sibling donor or alternative donor), stem cell source (peripheral blood or BM plus peripheral blood), achieving MMR at all of the first 3 months after HSCT (yes or no), GVHD occurrence (yes or no), and interventional DLI (yes or no). Conditioning regimen was not included in the factor analysis because only 4 patients received TBI-based regimen. Surviving patients were censored at January 31, 2014.
Results
MRD in the first 3 months after transplantation predicts relapse
In univariate analysis, MRD early after transplantation can predict relapse at 1, 2, and 3 months and can predict survival at the second and third months (Table 2; Figures 1-3), respectively.
Univariate analysis of transplant outcomes
| Risk factors . | Two-year CIR . | P . | Two-year LFS . | P . |
|---|---|---|---|---|
| WBCs at diagnosis | ||||
| <30 × 109/L | 18 | .94 | 64 | .48 |
| >30 × 109/L | 18 | 74 | ||
| c-KIT mutations | ||||
| Positive | 9 | .01 | 70 | .12 |
| Negative | 32 | 55 | ||
| Karotype | ||||
| Sole t(8;21) | 18 | .76 | 65 | .85 |
| Additional abnormalities | 20 | 59 | ||
| Courses required to achieve CR | ||||
| 1 | 11 | .02 | 71 | .07 |
| >1 | 29 | 51 | ||
| Time to transplant | ||||
| <6 mo | 34 | .11 | 65 | .53 |
| >6 mo | 16 | 62 | ||
| Donor source | ||||
| HLA-matched sibling | 21 | .42 | 53 | .15 |
| Alternative donor | 16 | 73 | ||
| Stem cell source | ||||
| G-BM + G-PB | 19 | .60 | 63 | .23 |
| G-PB | 26 | 60 | ||
| MRD directly before transplant | ||||
| Reaching MMR | 6 | .08 | 76 | .17 |
| Not reaching MMR | 24 | 60 | ||
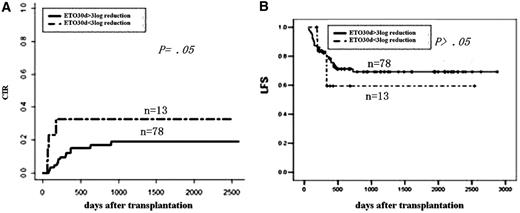
| MRD at 1 mo after transplant | ||||
| Reaching MMR | 17 | .05 | 71 | .51 |
| No MMR | 33 | 59 | ||
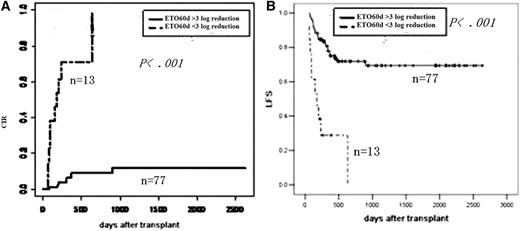
| MRD at 2 mo after transplant | ||||
| Reaching MMR | 9 | <.001 | 73 | <.001 |
| No MMR | 100 | 0 | ||
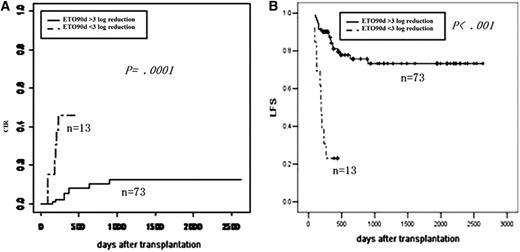
| MRD at 3 mo after transplant | ||||
| Reaching MMR | 11 | .0001 | 76 | <.001 |
| No MMR | 46 | 23 | ||
| MRD at the first 3 mo post-HSCT | ||||
| All achieving MMR | 8 | <.001 | 75 | <.001 |
| Not achieving MMR at least once | 56 | 28 | ||
| Acute GVHD | ||||
| With | 15 | .42 | 67 | .75 |
| Without | 21 | 64 | ||
| Transplant center | ||||
| Peking University | 18 | .69 | 65 | .84 |
| Others | 17 | 39 |
| Risk factors . | Two-year CIR . | P . | Two-year LFS . | P . |
|---|---|---|---|---|
| WBCs at diagnosis | ||||
| <30 × 109/L | 18 | .94 | 64 | .48 |
| >30 × 109/L | 18 | 74 | ||
| c-KIT mutations | ||||
| Positive | 9 | .01 | 70 | .12 |
| Negative | 32 | 55 | ||
| Karotype | ||||
| Sole t(8;21) | 18 | .76 | 65 | .85 |
| Additional abnormalities | 20 | 59 | ||
| Courses required to achieve CR | ||||
| 1 | 11 | .02 | 71 | .07 |
| >1 | 29 | 51 | ||
| Time to transplant | ||||
| <6 mo | 34 | .11 | 65 | .53 |
| >6 mo | 16 | 62 | ||
| Donor source | ||||
| HLA-matched sibling | 21 | .42 | 53 | .15 |
| Alternative donor | 16 | 73 | ||
| Stem cell source | ||||
| G-BM + G-PB | 19 | .60 | 63 | .23 |
| G-PB | 26 | 60 | ||
| MRD directly before transplant | ||||
| Reaching MMR | 6 | .08 | 76 | .17 |
| Not reaching MMR | 24 | 60 | ||
| MRD at 1 mo after transplant | ||||
| Reaching MMR | 17 | .05 | 71 | .51 |
| No MMR | 33 | 59 | ||
| MRD at 2 mo after transplant | ||||
| Reaching MMR | 9 | <.001 | 73 | <.001 |
| No MMR | 100 | 0 | ||
| MRD at 3 mo after transplant | ||||
| Reaching MMR | 11 | .0001 | 76 | <.001 |
| No MMR | 46 | 23 | ||
| MRD at the first 3 mo post-HSCT | ||||
| All achieving MMR | 8 | <.001 | 75 | <.001 |
| Not achieving MMR at least once | 56 | 28 | ||
| Acute GVHD | ||||
| With | 15 | .42 | 67 | .75 |
| Without | 21 | 64 | ||
| Transplant center | ||||
| Peking University | 18 | .69 | 65 | .84 |
| Others | 17 | 39 |
G-BM, G-CSF mobilized bone marrow; G-PB, G-CSF mobilized peripheral blood.
Impact of MRD at 1 month after transplantation on outcomes. (A) CIR by log reduction. (B) LFS by log reduction.
Impact of MRD at 1 month after transplantation on outcomes. (A) CIR by log reduction. (B) LFS by log reduction.
Impact of MRD at 2 months after transplantation on outcomes. (A) CIR by log reduction. (B) LFS by log reduction.
Impact of MRD at 2 months after transplantation on outcomes. (A) CIR by log reduction. (B) LFS by log reduction.
Impact of MRD at 3 months after transplantation on outcomes. (A) CIR by log reduction. (B) LFS by log reduction.
Impact of MRD at 3 months after transplantation on outcomes. (A) CIR by log reduction. (B) LFS by log reduction.
Impact of serial MRD monitoring after transplantation on outcomes
MRD status at 1, 2, and 3 months after HSCT could identify patients at high risk of post-HSCT relapse. To assess the value of serial monitoring, we compared patients achieving MMR at each of the first 3 months after transplantation (n = 67) with patients not in MMR at least once during the first 3 months after HSCT (n = 25). The MMR status is predictive both for CIR and LFS (Table 2).
The role of serial MRD in terms of DLI
For the 25 patients not in MMR at least once during the first 3 months, 18 (76%) were not in MMR at the 3-month time point (including 5 never achieving MMR within 3 months), among whom 9 (9/25, 36%) received interventional DLI; while for the 67 patients achieving MMR at each of the first 3 months after transplantation, 12 (18%) subsequently lost MMR, among whom 8 (8/67, 12%) received interventional DLI.
In total, 17 patients relapsed during the follow-up period. Fourteen (accounting for 82% of the 17 relapsed patients) of the 30 patients not in MMR at the 3-month time point (n = 18) or losing MMR after 3 months post-HSCT (n = 12) eventually relapsed, occurring at a median of 90 days (range, 30-570 days) after a <3-log reduction in transcript level to morphologic relapse. Among these patients, 4 of the 17 patients (23%) with interventional DLI and 10 of the other 13 patients (77%) without DLI relapsed (P = .009, Fisher’s test). The basic characteristics (listed in Table 1) were comparable between patients who did or did not receive DLI. The 2-year CIR and LFS for patients with or without DLI was 24% vs 87% (P = .001) and 64% vs 0% (P < .001), respectively.
MRD status directly before transplant does not seem to predict relapse robustly
The 2-year CIR was 12%, 33%, 23%, and 6% for the patients achieving <1 (n = 8), >1 and <2 (n = 18), >2 and <3 (n = 49), and ≥3-log (n = 17) reduced transcript levels directly before transplant, respectively (P = .17). The 2-year CIR was 6% and 24% for the patients achieving or not achieving MMR directly before transplant (P = .08, Table 2), respectively.
Subgroup analysis regarding chimerism evaluation
From year 2010 to 2012, chimerism by sequence polymorphism–based assay in 18 patients was serially evaluated. During this time period, the quantitative chimerism levels of 129 acute leukemia patients (including patients with t[8;21]) were measured by this method, and the receiver operating characteristic curve indicated that the optimal cutoff point of the recipient chimerism level to predict relapse was 1.0% (Q-X-Y, BMT-2014-199R, accepted). By applying this cutoff point, 2 of 13 patients with chimerism <1.0% at each of the first 3 months after HSCT and 4 of 5 patients with chimerism >1.0% at least once during the first 3 months after HSCT eventually relapsed (P = .02, Fisher’s test); whereas 2 of 12 patients achieving MMR at each of the first 3 months after transplantation and 4 of 6 patients not in MMR at least once during the first 3 months after HSCT finally relapsed (P = .10, Fisher’s test). The application of these standards resulted in 67% sensitivity and 92% specificity for chimerism evaluation as well as 67% sensitivity and 83% specificity for MRD assessment among this small population. One of 11 patients with both chimerism <1.0% and achieving MMR at each of the first 3 months after transplantation and 4 of 6 patients with either chimerism >1.0% or not in MMR at least once during the first 3 months after HSCT eventually relapsed (P = .01, Fisher’s test). The combined use of these 2 markers resulted in 83% sensitivity and 83% specificity to predict relapse.
Subgroup analysis regarding c-KIT mutations
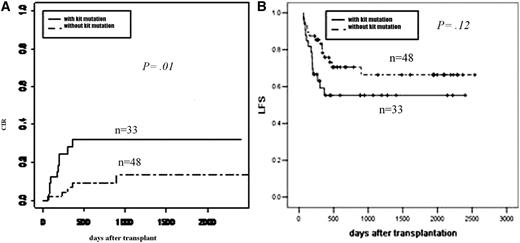
After year 2007 (as mentioned in “Patients and methods”), among the 81 consecutive patients screened for c-KIT mutations at diagnosis, 40 of the 48 patients (83%) without c-KIT mutations and 22 of the 33 patients (67%) with c-KIT mutations achieved >3-log reduction at all of the first 3 months after transplantation (P = .08, Pearson χ2). Five patients (10%) without c-KIT mutations and 8 patients (24%) with c-KIT mutations received interventional DLI (P = .10, χ2). The 2-year CIR was 9% and 32% (P = .01, Figure 4A); the 2-year OS and LFS were 71% vs 61% (P = .32) and 70% vs 55% (P = .12, Figure 4B) for the 2 groups, respectively.
Subgroup analysis of effect of c-KIT mutations on outcomes. (A) CIR by c-KIT mutations. (B) LFS by c-KIT mutations.
Subgroup analysis of effect of c-KIT mutations on outcomes. (A) CIR by c-KIT mutations. (B) LFS by c-KIT mutations.
Furthermore, among the 17 patients with fusion gene level >10% (as mentioned in “Patients and methods”) directly before transplant, 6 of the 7 patients without c-KIT mutations and 4 of the 10 patients with c-KIT mutations achieved >3-log reduction at each of the first 3 months after transplantation (P = .13, Fisher’s test); whereas among the 17 patients at relapse, 2 of the 4 patients without c-KIT mutations and 4 of the 13 patients with c-KIT mutations achieved >3-log reduction at each of the first 3 months after transplantation (P = .58, Fisher’s test). No disparity regarding the status of c-KIT mutations was found at diagnosis, directly before transplant, or at relapse among the previously mentioned small patient population; thus c-KIT mutational status at diagnosis was entered into factor analysis.
Univariate and multivariate analysis
Univariate analyses for CIR and LFS are indicated in Table 2. Apart from post-HSCT MRD and c-KIT mutations, another factor influencing both CIR and LFS (a marked trend) was the number of courses required to achieve CR. Furthermore, in an attempt to determine whether the combination of predictive factors (serial MRD at the first 3 months after HSCT, c-KIT mutations, and number of courses required to achieve CR) could further identify patients at high risk of relapse, we divided the patients into 3 groups: low risk (possessing none of the factors, n = 19), intermediate risk (possessing any one of the factors, n = 41), or high risk (possessing at least 2 factors, n = 21). This variable is significant and is associated with a 2-year CIR of 0%, 8%, and 64% (P < .001) as well as a 2-year LFS of 82%, 77%, and 29% (P < .001), respectively. Based on multivariate analysis, serial MRD at the first 3 months after HSCT, number of courses required to achieve CR, and interventional DLI are all independent risk factors for CIR and LFS (Table 3).
Significant factors in multivariate analysis
| Outcome . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| Relapse | ||
| Achieving MMR at all of the first 3 mo, yes vs no | 0.07 (0.02-0.26) | .001 |
| Courses required to achieve CR, 1 vs >1 | 0.17 (0.04-0.64) | .009 |
| Interventional DLI, yes vs no | 0.13 (0.03-0.54) | .005 |
| LFS | ||
| Achieving MMR at all of the first 3 mo, yes vs no | 0.13 (0.05-0.34) | .001 |
| Courses required to achieve CR, 1 vs >1 | 0.36 (0.14-0.90) | .03 |
| Interventional DLI, yes vs no | 0.22 (0.07-0.71) | .01 |
| Outcome . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| Relapse | ||
| Achieving MMR at all of the first 3 mo, yes vs no | 0.07 (0.02-0.26) | .001 |
| Courses required to achieve CR, 1 vs >1 | 0.17 (0.04-0.64) | .009 |
| Interventional DLI, yes vs no | 0.13 (0.03-0.54) | .005 |
| LFS | ||
| Achieving MMR at all of the first 3 mo, yes vs no | 0.13 (0.05-0.34) | .001 |
| Courses required to achieve CR, 1 vs >1 | 0.36 (0.14-0.90) | .03 |
| Interventional DLI, yes vs no | 0.22 (0.07-0.71) | .01 |
Discussion
In the current study, we demonstrated for the first time that RUNX1/RUNX1T1-based MRD status during the first 3 months after HSCT both separately and jointly is highly predictive of post-HSCT relapse for t(8;21) patients. Our data show that achievement of MMR throughout the first 3 months post-HSCT was associated with a CIR of only 8%, whereas patients not in MMR at least once during the first 3 months had a CIR of 56%; for patients not achieving MMR persistently at all of the first 3 months, relapse seems inevitable (4/5 relapsed, and the other patient achieved post-DLI MMR after 3 months). Although 17 patients received interventional DLI, which may be an effective intervention for the post-HSCT relapse and thus may bias the predictive value of MRD, more patients (36%) not achieving MMR at least once early after HSCT received DLI than patients (12%) achieving MMR at each of the first 3 months. In other words, the predictive value of MRD should be more prominent without DLI. More importantly, MRD status early after HSCT was proved to be an independent factor both for CIR as well as for LFS in multivariate analysis. Nevertheless, our results strongly support MRD monitoring early after HSCT as indicative of post-HSCT relapse, and closer monitoring with multiple markers should be required, which might help to identify the best time for intervention therapy.
MRD status early after transplant was more informative than c-KIT mutations. This observation may be explained by the fact that c-KIT mutations tended to be adversely associated with early MMR achievement after HSCT (P = .08); in other words, MRD status was a more comprehensive marker. Unfortunately, there are no data on c-KIT mutational status in ∼10% of the patients that would allow us to explore this phenomenon further. However, our findings still indicate that the adverse effect of c-KIT mutations might be alleviated to some extent early after HSCT, but the value of c-KIT mutations might be partly obscured by MRD status. Altogether, our results support monthly monitoring for MRD in t(8;21) AML early after HSCT.
In addition, the current results show that the median time from a <3-log reduction in transcript level to morphologic relapse is 90 days, which suggests that, once the <3-log reduction occurs, close monitoring should be started, and the optimal measurement schedules need further investigation. Blood is more easily obtained than marrow; thus, Liu Yin et al’s9 preliminary evidence that blood might substitute for BM during follow-up might allow for more frequent MRD monitoring (perhaps weekly). Moreover, simultaneous monitoring of multiple markers is another means of testing optimization. For example, chimerism analysis was valuable to predicting relapse in a small subgroup of patients, especially when combined with RUNX1/RUNX1T1-based MRD monitoring, and resulted in higher sensitivity. Although it is difficult to compare the accuracy of monitoring MRD for t(8;21) with the chimerism analysis in predicting post-HSCT relapse because of the paucity of data, chimerism evaluation is at least a feasible method.
Moreover, MRD monitoring is a predictive marker in terms of DLI. The CIR was significantly lower for patients receiving DLI compared with patients without DLI. The basic characteristics were balanced between patients with or without DLI; thus, considering the definite antileukemia effect of DLI and the effectiveness of interventional DLI at molecular relapse after HSCT for patients without t(8;21),16 these results at least partly reflect the MRD-based risk stratification–directed interventions including DLI that may decrease post-HSCT relapse and improve survival for t(8;21) patients. The role of DLI deserves further investigation with a larger population.
Additionally, the loss or acquisition of a c-KIT mutation during the course of AML was not encountered among a small number of patients with c-KIT mutational status tested at diagnosis, directly before HSCT, or at relapse. The possible evolution of c-KIT mutations should be further explored and analyzed in future studies.
Based on the previous results as well as our previous findings on MRD status and c-KIT mutations,12 a possible management strategy for RUNX1/RUNX1T1-positive patients in the near future might be as follows: screen for c-KIT mutations and measure the baseline level of RUNX1/RUNX1T1 transcripts, start with conventional therapy consisting of cytarabine plus an anthracycline, then test MRD after each course of chemotherapy, and determine risk based on the factors of c-KIT mutations, MRD after the second consolidation course, and number of courses required to achieve CR. If HSCT is necessary, start MRD monitoring early after HSCT at monthly intervals and consider intervention for MRD status if still not achieving MMR or losing MMR post-HSCT. Interventional therapy including DLI needs further exploration.
In conclusion, a >3-log reduction at the first 3 months after HSCT in RUNX1/RUNX1T1 transcripts from diagnosis is highly predictive. Rather than c-KIT mutations, MRD monitoring by qRT-PCR at regular early time points post-HSCT in t(8;21) AML allows further rapid identification of patients at high risk of relapse even after allo-HSCT. This study strongly supports implementing prospective MRD monitoring after HSCT in the design of t(8;21) AML trials, and further evaluation of the role of risk-directed preemptive therapy could be incorporated in future clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Martin Tallman for reviewing this paper.
This work was partly supported by the Collaborative Innovation Center of Hematology China, the Key Program of National Natural Science Foundation of China (grant 81230013), Scientific Research Foundation for Capital Medicine Development (grant 2011-4022-08), and Beijing Municipal Science & Technology Commission (nos. Z121107002812033, Z121107002612035, and Z111107067311070).
Authorship
Contribution: X.-J.H. designed the research; Y.W. and X.-J.H. analyzed the data and wrote the manuscript; and all authors provided patient data and gave final approval for the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Jun Huang, Peking University People’s Hospital, Peking University Institute of Hematology, Beijing 100044, China; e-mail: xjhrm@medmail.com.cn.
References
Author notes
Y.W. and D.-P.W. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal