To the editor:
JAK2V617F-positive myeloproliferative neoplasms (MPNs), especially polycythemia vera (PV), may occur concomitantly with chronic lymphocytic leukemia (CLL). This association has not been described with a variant of essential thrombocythemia (ET), prefibrotic-stage myelofibrosis (preF-MF) or primary myelofibrosis (PMF) or with calreticulin (CALR)-mutated MPNs. JAK2V617F has been reported in MPNs, but not in CLL clones.1,2 We previously reported 3 unrelated women with concomitant PV and CLL, in which these 2 diseases used different X chromosome alleles, indicating their origin from different pre-JAK2V617F hematopoietic stem cells.2 Herein, we present additional observations in 2 patients suggesting an independent relation between MPN and coexisting CLL clones.
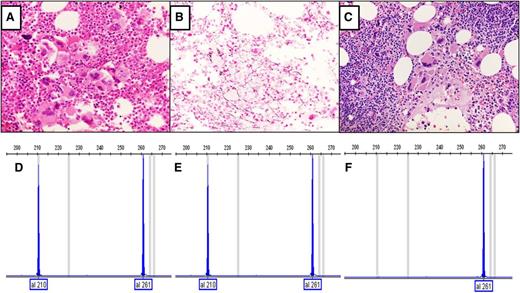
The first is a 67-year-old man with thrombocytosis who presented 9 years ago with a platelet count of 707 × 106/μL, a leukocyte count of 9.55/μL (lymphocytes 19%), and a hemoglobin level of 13.5 g/dL, but no history of thromboembolic events, aquagenic pruritus, erythromelalgia, lymphadenopathy, or splenomegaly. There was no evidence of reactive thrombocytosis or detectable JAK2V617F or the thrombopoietin receptor (cMPL) mutations. Marrow was 90% cellular and consistent with preF-MF (Figure 1A-B). Although no increased lymphocytes were appreciated by marrow morphological evaluation, marrow and blood flow cytometry revealed a small CD5, CD19, dim CD20, CD23, dim κ light chain-positive B-cell clone accounting for ∼4% of lymphoid cells. This clone resembled CLL but was deemed to be of unknown clinical significance. Imaging studies failed to detect any evidence of lymphoproliferative disorder. The propositus remained asymptomatic and in hematological remission on hydroxyurea for the subsequent 9 years. On 3 successive occasions, the same small B-lymphocyte clone was consistently detectable at a low level in blood. After 9 years, the patient developed progressive lymphocytosis (white blood cells of 25.12 × 103/μL, with 72% lymphocytes and 23% neutrophils, platelet count of 281 × 106/μL, and hemoglobin level of 12 g/dL) and a moderately enlarged spleen but was asymptomatic with no lymphadenopathy. Marrow showed persistent morphology of preF- MF, with expansion of the previous B-cell clone to 30% of marrow cells (Figure 1C) and increased fibrosis, now judged “moderate” (2+). Flow cytometry again revealed the same B-cell clone, now comprising 48% of leukocytes and consistent with CLL.
Analyses of CALR mutations and bone marrow morphology of the first subject. (A) Hematoxylin and eosin stain of initial marrow core biopsy and hypercellular marrow with increased megakaryocytes with variable size and shape and hyperchromatic hypolobate nuclei that are arranged in dense clustering with no overt CLL cells. (B) Reticulin stain of initial marrow biopsy with no significant increase in reticulin fibers. (C) Hematoxylin and eosin stain of follow-up marrow core biopsy after 9 years, showing megakaryocytic histotopography consistent with the prefibrotic stage of primary myelofibrosis. An increased number of small lymphocytes in aggregates are present in the upper right and lower left corners of the field, compatible with CLL (original magnification, ×40). Analysis of CALR mutations in exon 9 was performed by fragment analysis, which is a semiquantitative analysis.3 (D) Fragment analysis of CALR from granulocytes obtained 3 years after diagnosis of type 1 CALR deletion. (E) Fragment analysis data of current granulocyte DNA (9-year follow-up after diagnosis), with the same CALR deletion. (F) Fragment analysis of DNA from CD19+ cells; the type 1 CALR deletion is not present.
Analyses of CALR mutations and bone marrow morphology of the first subject. (A) Hematoxylin and eosin stain of initial marrow core biopsy and hypercellular marrow with increased megakaryocytes with variable size and shape and hyperchromatic hypolobate nuclei that are arranged in dense clustering with no overt CLL cells. (B) Reticulin stain of initial marrow biopsy with no significant increase in reticulin fibers. (C) Hematoxylin and eosin stain of follow-up marrow core biopsy after 9 years, showing megakaryocytic histotopography consistent with the prefibrotic stage of primary myelofibrosis. An increased number of small lymphocytes in aggregates are present in the upper right and lower left corners of the field, compatible with CLL (original magnification, ×40). Analysis of CALR mutations in exon 9 was performed by fragment analysis, which is a semiquantitative analysis.3 (D) Fragment analysis of CALR from granulocytes obtained 3 years after diagnosis of type 1 CALR deletion. (E) Fragment analysis data of current granulocyte DNA (9-year follow-up after diagnosis), with the same CALR deletion. (F) Fragment analysis of DNA from CD19+ cells; the type 1 CALR deletion is not present.
The second case is a 69-year-old man with a 14-year history of CLL who recently was referred to us. A year ago, while his CLL was in complete remission after chemotherapy, he developed transfusion-dependent anemia and progressive splenomegaly and underwent a splenectomy resulting in a decreased but persistent red cell transfusion requirement. On referral, we found leukoerythroblastic peripheral blood morphology with dacrocytes, and his marrow also indicated PMF, without JAK2 or cMPL mutations.
Somatic CALR mutations have been identified in patients with ET and MF who lacked JAK2V617F or cMPL mutations.3,4 We detected a CALR 52-bp deletion type 1 mutation3 in the granulocytes of both patients, but the CD19-positive sorted CLL cells had no detectable CALR mutation (Figure 1D-F). It is not known if CALR mutations in MF and ET are disease-originating mutations or more similar to the JAK2V617F-positive MPNs, wherein other somatic or germ line mutations are thought to precede the MPN phenotypes.5
CALR mutations are reported to be associated with a lower risk in MPNs and thus are likely to be acquired earlier,3,4 raising the possibility that preF-MF and CLL may arise from the same pluripotent stem cell. However, the lack of CALR mutation in these patients’ CLL cells suggests that they represent independent clones. However, as the X chromosome–based clonality assays can be performed only in women, a common precursor origin of MPN and CLL clones cannot be completely excluded in these male patients.
Authorship
Contribution: M.E.S. and J.T.P. designed the studies, performed experiments, collected clinical information, analyzed data, and wrote the manuscript; and S.S., N.S.R., T.T., and C.A.W. performed/designed laboratory experiments or reviewed and analyzed clinical data and contributed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohamed E. Salama, Associated Regional University Pathologists Laboratories and University of Utah, Department of Pathology, Salt Lake City, UT 84108; e-mail: Mohamed.salama@aruplab.com; or Josef T. Prchal, Hematology, SOM 5C310, University of Utah School of Medicine, Salt Lake City, UT 84132-2408; e-mail: josef.prchal@hsc.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal