Key Points
Factor VIII antigens can be expressed in chloroplasts and bioencapsulated in plant cells.
Oral delivery of plant cells expressing FVIII domains suppresses and reverses inhibitor formation in mice with hemophilia A by induction of CD4+ regulatory T cells.
Abstract
Hemophilia A is the X-linked bleeding disorder caused by deficiency of coagulation factor VIII (FVIII). To address serious complications of inhibitory antibody formation in current replacement therapy, we created tobacco transplastomic lines expressing FVIII antigens, heavy chain (HC) and C2, fused with the transmucosal carrier, cholera toxin B subunit. Cholera toxin B-HC and cholera toxin B-C2 fusion proteins expressed up to 80 or 370 µg/g in fresh leaves, assembled into pentameric forms, and bound to GM1 receptors. Protection of FVIII antigen through bioencapsulation in plant cells and oral delivery to the gut immune system was confirmed by immunostaining. Feeding of HC/C2 mixture substantially suppressed T helper cell responses and inhibitor formation against FVIII in mice of 2 different strain backgrounds with hemophilia A. Prolonged oral delivery was required to control inhibitor formation long-term. Substantial reduction of inhibitor titers in preimmune mice demonstrated that the protocol could also reverse inhibitor formation. Gene expression and flow cytometry analyses showed upregulation of immune suppressive cytokines (transforming growth factor β and interleukin 10). Adoptive transfer experiments confirmed an active suppression mechanism and revealed induction of CD4+CD25+ and CD4+CD25− T cells that potently suppressed anti-FVIII formation. In sum, these data support plant cell-based oral tolerance for suppression of inhibitor formation against FVIII.
Introduction
Hemophilia is the X-linked bleeding disorder caused by mutations in coagulation factor IX (FIX, hemophilia B) or its cofactor, factor VIII (FVIII, hemophilia A). Because the serine protease FIX has very low activity in the absence of FVIII, mutations in either protein can cause the coagulation defect. This disease affects 1 in 7500 male births worldwide for hemophilia A and 1 in 30 000 for hemophilia B.1-3 Hence, the majority of patients are FVIII-deficient. Current standard treatment is based on IV infusion of plasma-derived or recombinant factor concentrate. A major complication of this therapy is the formation of inhibitory antibodies (“inhibitors”), which occurs in 20% to 30% of patients with severe hemophilia A (as defined by less than 1% coagulation activity) and in ∼5% of patients with severe hemophilia B.1,4-6 Inhibitors seriously complicate treatment and increase morbidity and mortality of this disease. Increased factor doses may be able to restore hemostasis in patients with low-titer inhibitors (less than 5 Bethesda units [BUs]), whereas bypass factors are required to treat a bleed in the presence of high-titer inhibitors. However, these treatments are expensive and have to be carefully dosed. Clinical protocols for reversal of the antibody response via immune tolerance induction consist of frequent high-dose factor administrations for prolonged periods (from months to more than 1 year) and are very expensive (more than $1 000 000), and ∼30% of FVIII inhibitor patients fail to respond.4
Although there are currently no prophylactic protocols against inhibitor formation in patients, preclinical experiments in murine models of hemophilia A have provided proof of principle that preventive immune tolerance to FVIII can be established.6-11 However, such protocols use genetic manipulation or immune suppressive drugs, raising safety concerns for translation to human treatment. In contrast, oral tolerance could be a more readily acceptable form of prophylactic tolerance induction and may be more readily tested in clinical trials.12,13 However, effective tolerogenic delivery of coagulation factor antigen to the gut-associated lymphoid tissue (GALT) is a challenge.14 To address this issue, we have developed a cost-effective system for production of high levels of protein in chloroplasts of transplastomic plant cells, which provide bioencapsulation of the antigen through the cellulose containing cell walls.15,16 Because of the high number of chloroplast genomes per cell and our optimized expression system, transgenic proteins can accumulate in green leaves at much higher levels than is the case for more traditional transgenic plant technologies.17,18 Oral delivery of transplastomic plant cells has been effective in prevention of insulitis in nonobese diabetic mice and of inhibitor formation in mice with hemophilia B.19,20
For FIX inhibitors, immune tolerance induction is often not sustainable because of anaphylactic reactions and the development of nephrotic syndrome. In mice with hemophilia B, we demonstrated that repeated oral delivery of bioencapsulated FIX prevented inhibitor formation and fatal anaphylaxis in subsequent replacement therapy.20 Encouraged by these results, we sought to develop a protocol for hemophilia A. FVIII is a large protein comprising a signal peptide and a 2332-amino acid polypeptide. Structurally, FVIII contains 6 distinct domains, which are organized in the following order: A1-A2-B-A3-C1-C2.21 The large, central B domain is highly glycosylated and aids in secretion of the molecule.22-24 However, recombinant B domain deleted (BDD) FVIII is biologically active and represents one of the products currently used in the clinic. FVIII is secreted as a heterodimer after at least 2 intracellular cleavages within the B domain. As a consequence, circulating FVIII comprises a heavy chain (containing A1-A2-B domains) and a light chain (A3-C1-C2 domains), which are noncovalently linked.21,23
FVIII is a highly immunogenic molecule that can cause potent antibody responses in patients with hemophilia A and in experimental animals at low-antigen doses.6,25 The majority of inhibitors bind to A2, A3, or C2 domains.6,26-28 These highly immunogenic sequences also contain several CD4+ T cell epitopes.6,29,30 Animal studies suggest that inhibitor formation against FVIII can be prevented by tolerization to parts of the molecule, such as a combination of A2 and C2 domains, whereas a single domain may not be sufficient.14,31 Here, we demonstrate that FVIII heavy chain and C2 domain can be expressed as cholera toxin B subunit (CTB) fusion proteins in tobacco chloroplasts. Oral delivery of a mixture of these bioencapsulated antigens suppressed and also reversed inhibitor formation in mice with hemophilia A.
Materials and methods
Design and construction of chloroplast expression vectors
Because efficient delivery of bioencapsulated antigen to the GALT is required for tolerance induction and is facilitated by transmucosal carriers, human FVIII antigens were expressed as CTB fusions, a successful strategy for tolerogenic delivery of FIX and proinsulin.20,32 Because of the large size of the FVIII molecule and the need for CTB fusions to form pentamers to bind to the GM1 receptor on gut epithelial cells, 2 separate FVIII chloroplast transformation vectors were constructed to include either the heavy chain (abbreviated as HC, with an identical amino acid sequence as seen in recombinant BDD-FVIII, and therefore containing A1 and A2 domains and 5 amino acids of B domain) or the C2 domain. The cDNA fragment of human FVIII-HC was amplified by polymerase chain reaction (PCR). PCR products, flanked with a furin cleavage and suitable restriction sites, were cloned into the pCR BluntII Topo vector (Invitrogen), and the sequence was verified. Then, the HC DNA fragment was ligated with plasmid Lee Daniell–chloroplast vector–5 CTB-proinsulin chloroplast transformation vector containing the CTB and glycine-proline-glycine-proline hinge sequences to create the pLD-CTB-HC expression vector.19,33 An analogous pLD-CTB-C2 expression vector was also constructed.
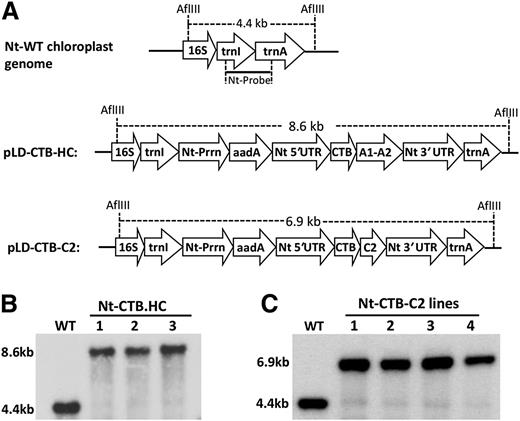
Chloroplast vectors pLD-CTB-HC and pLD-CTB-C2 (Figure 1A) contain homologous flanking sequences 16S/trnI and trnA/23S from tobacco chloroplast genome to facilitate recombination with the native chloroplast genome. Expression of CTB-HC and CTB-C2 is regulated by the highly expressed tobacco chloroplast psbA 5′ untranslated region (UTR)-promoter and 3′ UTR. The CTB-HC and CTB-C2 expression cassettes contain a glycine-proline-glycine-proline hinge between CTB and the HC or C2 element to prevent steric hindrance of the fusion proteins. In addition, a furin cleavage site, arginine-arginine-lysine-arginine, was created at the junction region of the fusion proteins to efficiently release the FVIII domains after internalization by epithelial cells.34 The expression cassettes include the aminoglycoside 3′ adenylyltransferase selection marker gene with a GGAG ribosome binding site, driven by a tobacco plastid ribosomal operon promoter, to confer spectinomycin resistance. The final chloroplast transformation vectors pLD-CTB-HC and pLD-CTB-C2 (Figure 1A) were sequenced and used for transformation.33
Chloroplast transformation vectors and integration of transgenes into the chloroplast genome. (A) Tobacco chloroplast expression vectors. Homologous chloroplast genome flanking sequences comprising 16S (16S rRNA), isoleucine tRNA (trnI), alanine tRNA (trnA) gene sequences. In both vectors, a glycine-proline-glycine-proline hinge and furin cleavage site (RRKR) is included between CTB and the FVIII domain sequence. The restriction site of AflIII and the sizes of Southern blot fragments are indicated. (B) Southern blot, tobacco CTB-HC, wild-type (WT) (untransformed), 1-3 transplastomic lines. Tobacco total genomic DNA was digested with AflIII and probed with 0.81 kb trnI/trnA flanking region fragment. (C) Southern blot, tobacco CTB-C2, WT (untransformed WT), 1-4 transplastomic lines. 3′ UTR, 3′ UTR of tobacco psbA gene; 5′ UTR, promoter and 5′ UTR of tobacco psbA gene; aadA, aminoglycoside 3′-adenylyltransferase gene to confer spectinomycin resistance; Nt, Nicotiana tabacum. Prrn, ribosomal RNA operon promoter with GGAG ribosome binding site; WT, untransformed WT.
Chloroplast transformation vectors and integration of transgenes into the chloroplast genome. (A) Tobacco chloroplast expression vectors. Homologous chloroplast genome flanking sequences comprising 16S (16S rRNA), isoleucine tRNA (trnI), alanine tRNA (trnA) gene sequences. In both vectors, a glycine-proline-glycine-proline hinge and furin cleavage site (RRKR) is included between CTB and the FVIII domain sequence. The restriction site of AflIII and the sizes of Southern blot fragments are indicated. (B) Southern blot, tobacco CTB-HC, wild-type (WT) (untransformed), 1-3 transplastomic lines. Tobacco total genomic DNA was digested with AflIII and probed with 0.81 kb trnI/trnA flanking region fragment. (C) Southern blot, tobacco CTB-C2, WT (untransformed WT), 1-4 transplastomic lines. 3′ UTR, 3′ UTR of tobacco psbA gene; 5′ UTR, promoter and 5′ UTR of tobacco psbA gene; aadA, aminoglycoside 3′-adenylyltransferase gene to confer spectinomycin resistance; Nt, Nicotiana tabacum. Prrn, ribosomal RNA operon promoter with GGAG ribosome binding site; WT, untransformed WT.
Regeneration of transplastomic tobacco plants
Tobacco chloroplast transformation vectors pLD-CTB-HC and pLD-CTB-C2 (Figure 1A) were used to transform tobacco (Nicotiana tabacum) via particle bombardment with gold particles coated with the plasmid DNA.33 The bombarded leaves were then transferred to selection/regeneration medium. Regeneration of FVIII transplastomic tobacco plants was performed as described earlier.33-35
Characterization of FVIII expression in leaf tissues of transplastomic plants
Immunoblot analysis and quantitation of the CTB-HC and CTB-C2 fusion proteins were performed by previously reported protocols.18,34 GM1-ganglioside receptor binding assay was performed as reported earlier.34 The Bis-Tris 3% to 12% gradient native gel electrophoresis followed by immunoblot analysis was carried out by following the instruction manual of the NativePage Novex Bis-Tris Gel System (Life Technologies).
Mouse strains and experiments
Male mice with hemophilia A and targeted deletion of F8 exon 16 (F8e16−/−) on a mixed C57BL6/129 or on a pure BALB/c background were housed under special pathogen-free conditions and were ∼2 months of age at the onset of experiments.9,36 Leaf material was ground in liquid nitrogen and stored at −80°C. A mixture of CTB-HC and CTB-C2 material (total of 125 mg per mouse per dose) was suspended in sterile PBS (200 μL/dose), homogenized, and delivered via oral gavage using a 20-G bulb-tipped gastric gavage needle. For antigen challenge, mice were administrated 1 IU BBD-human FVIII (Xyntha; Pfizer, New York, NY) into the tail vein once a week. Plasma samples were obtained by tail bleed, as published.37 Enzyme-linked immunosorbent assays (ELISAs) for FVIII antigen and anti-FVIII, Bethesda assays, and lymphocyte assays were as published.9,36,37 Additional details, including on flow cytometry analysis of lymphocytes and immunohistochemistry of tissues, are provided in supplemental data, available on the Blood Web site. Animal studies were carried out under approval by University of Florida Institutional Animal Care and Use Committee protocol 201304544.
Results
Characterization of FVIII-transplastomic lines
Putative transplastomic tobacco lines obtained after bombardment of chloroplast vectors were first screened by PCR analysis. Site-specific transgene integration into the chloroplast genome was confirmed with 2 specific primer sets 3P/3M and 5P/2M, which anneal specifically to complementary sequences of transgene cassette and the chloroplast genome.33 Three independent tobacco lines from pLD-CTB-HC transformation and 4 independent lines from pLD-CTB-C2 transformation showed positive PCR products of correct sizes (data not shown). The CTB-HC-transplastomic and CTB-C2-transplastomic tobacco lines were further examined by Southern blot analysis for site-specific stable integration and homoplasmy. Homoplasmy is achieved when all copies of the chloroplast genomes have stably integrated transgenes. The results showed that all 3 tested lines of CTB-HC transplastomic lines had integrated transgenes at specific sites and were homoplasmic, showing only the larger genome fragment (8.6 kb) with the transgene insert when compared with the 4.4-kb fragment in the untransformed control genome (Figure 1B). The CTB-C2-transplastomic tobacco lines also showed integration of transgenes into the chloroplast genome and homoplasmy (Figure 1C).
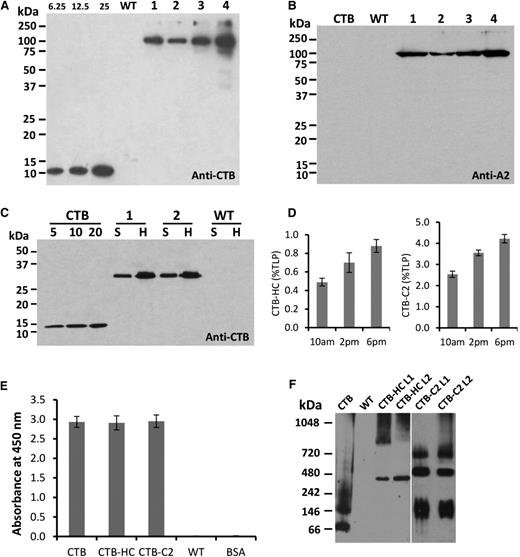
Expression of CTB-HC and CTB-C2 fusion proteins in protein extracts from leaves of transplastomic tobacco plants was evaluated by western blot analysis. Under fully denatured and reducing conditions, blots probed with anti-CTB polyclonal antibody revealed full-length CTB-HC fusion protein with the expected molecular mass of 98 kDa (Figure 2A) in all transplastomic lines. No cleaved products were observed even after solubilization of pentamers and destabilization of disulfide bonds with reducing agents. A similar banding pattern was observed in a parallel blot probed with an anti-A2 domain specific monoclonal antibody. In addition, there was no cross-reactivity of CTB standard protein or any other plant protein in untransformed leaf extracts (both used as negative controls) with the anti-A2 antibody (Figure 2B). Quantitation of the fusion protein was performed by densitometry on western blots of leaf extracts, using known amounts of purified CTB protein as the standard. The CTB-HC fusion protein was found to accumulate up to 0.8% total leaf protein or 80 µg/g fresh leaf tissue (Figure 2A,D). The CTB-C2 fusion protein was similarly analyzed with anti-CTB polyclonal antibody in tobacco plants. As shown in Figure 2C, a 31-kDa polypeptide representing the correct size of CTB-C2 fusion protein was detected in both fractions (supernatant and homogenate) of independent transplastomic lines. The CTB-C2 fusion protein accumulated up to 4.2% in the homogenate (ie, 4.2% total leaf protein or 370 µg per gram fresh leaf; Figure 2D).
Characterization of CTB-HC and CTB-C2 expression in tobacco chloroplasts. (A) Detection of heavy chain fusion protein probed with the CTB antibody. CTB standard, 6.25, 12.5, and 25 ng. Lanes 1 to 4 indicate transplastomic lines. Five micrograms total protein of homogenate fraction per lane was loaded. (B) Detection of heavy chain probed with the A2 antibody. CTB, 25 ng. 1-4, transplastomic lines. Five micrograms total protein of homogenate fraction per lane was loaded. (C) Detection of C2 fusion protein probed with the CTB antibody. CTB standard, 5, 10, and 20 ng. Two micrograms total protein of supernatant or homogenate fraction per lane was loaded. (D) Quantitation of CTB-HC and CTB-C2 expression in tobacco chloroplasts. Proteins were extracted from mature leaves at different time points on the same day. TLP, total leaf protein. (E) Ganglioside GM1 ELISA binding assay. CTB standard (0.1 ng), tobacco CTB-HC (5 µg); tobacco CTB-C2 (1 µg); untransformed tobacco WT (5 µg); BSA, bovine serum albumin (5 µg). (F) Blue native gel electrophoresis and western blot analysis to evaluate pentamer assembly. Pentamer sizes: CTB, 57.5 kDa; CTB-C2: 155 kDa; CTB-HC, 490 kDa. Samples loaded: CTB standard, 100 ng; WT, 40 µg; CTB-HC, 40 µg; CTB-C2, 10 µg. A vertical line is inserted between CTB-HC and CTB-C2 lanes to separate these 2 different blots. H, homogenate fraction; S, supernatant fraction; WT, untransformed WT.
Characterization of CTB-HC and CTB-C2 expression in tobacco chloroplasts. (A) Detection of heavy chain fusion protein probed with the CTB antibody. CTB standard, 6.25, 12.5, and 25 ng. Lanes 1 to 4 indicate transplastomic lines. Five micrograms total protein of homogenate fraction per lane was loaded. (B) Detection of heavy chain probed with the A2 antibody. CTB, 25 ng. 1-4, transplastomic lines. Five micrograms total protein of homogenate fraction per lane was loaded. (C) Detection of C2 fusion protein probed with the CTB antibody. CTB standard, 5, 10, and 20 ng. Two micrograms total protein of supernatant or homogenate fraction per lane was loaded. (D) Quantitation of CTB-HC and CTB-C2 expression in tobacco chloroplasts. Proteins were extracted from mature leaves at different time points on the same day. TLP, total leaf protein. (E) Ganglioside GM1 ELISA binding assay. CTB standard (0.1 ng), tobacco CTB-HC (5 µg); tobacco CTB-C2 (1 µg); untransformed tobacco WT (5 µg); BSA, bovine serum albumin (5 µg). (F) Blue native gel electrophoresis and western blot analysis to evaluate pentamer assembly. Pentamer sizes: CTB, 57.5 kDa; CTB-C2: 155 kDa; CTB-HC, 490 kDa. Samples loaded: CTB standard, 100 ng; WT, 40 µg; CTB-HC, 40 µg; CTB-C2, 10 µg. A vertical line is inserted between CTB-HC and CTB-C2 lanes to separate these 2 different blots. H, homogenate fraction; S, supernatant fraction; WT, untransformed WT.
Pentamer assembly of CTB-HC and CTB-C2 in transgenic chloroplasts
A plasma membrane receptor (GM1-ganglioside) binds CTB in vivo, and a pentameric structure is required for binding to GM1 receptor.38-40 To evaluate the receptor-binding ability of CTB-HC and CTB-C2 fusion proteins produced in tobacco chloroplasts, GM1-binding ELISA was performed. As observed in Figure 2E, CTB-HC and CTB-C2 fusion protein extracts along with purified CTB protein showed strong binding affinity to GM1. Therefore, CTB-HC and CTB-C2 fusion proteins assembled properly to form pentameric structures within transformed chloroplasts. To further evaluate the pentamer assembly directly, we ran blue native gels, and the blots were probed with anti-CTB polyclonal antibody. These results indicate that the pentameric structure (CTB-HC, 490 kDa; CTB-C2, 155 kDa) was formed in both CTB-HC-transformed and CTB-C2-transformed tobacco chloroplasts. In addition, other oligomeric forms larger than pentamers were also observed (Figure 2F). Lack of cleaved products confirmed stability of assembled pentamers or multimers within transformed chloroplasts.
Oral delivery of bioencapsulated FVIII suppresses inhibitor formation in hemophilic mice
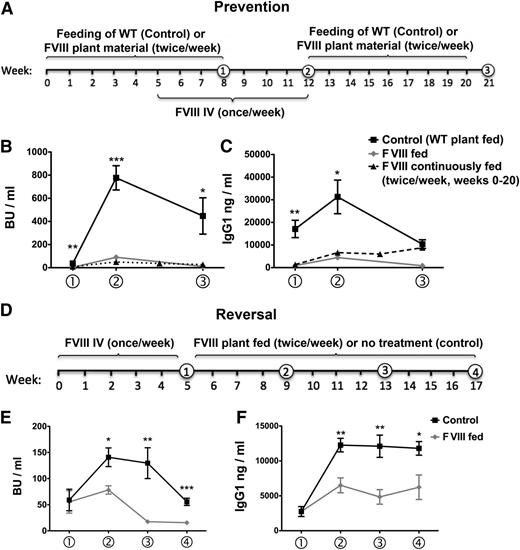
Plant leaf materials were ground in liquid nitrogen, as published.19 CTB-HC and CTB-C2 materials were mixed and suspended in phosphate-buffered saline buffer so that the final product contained approximately equal amounts of both fusion proteins (∼5 µg HC/6 µg C2 per dose/mouse). Male mice with hemophilia A (F8e16−/−) on a C57BL6/129 genetic background received oral gavage of 125 mg mixed material per dose twice per week for 2 months (Figure 3A). During the second month, FVIII concentrate (recombinant BDD-FVIII) was given IV once per week at 1 IU/mouse. As expected on the basis of prior findings, control mice that received no gavage (n = 9) or were fed with WT plant material (n = 6) formed very high-titer inhibitors (50-391 BU/mL; Figure 3B).9,36 These were predominantly immunoglobulin G1 (IgG1) with substantially less IgG2a and IgG2b formation (Figure 3C-E). In contrast, inhibitor formation was significantly suppressed (on average, 7-fold) in those mice that had been fed FVIII plant material (n = 6). These differences in BU correlated with the level of suppression of FVIII-specific IgG1 formation (Figure 3C). IgG2a and IgG2b anti-FVIII became undetectable in FVIII-fed mice (Figure 3D-E).
Suppression of inhibitor formation against FVIII in C57BL6/129 mice with hemophilia A by oral administration of a 1:1 mixture of bioencapsulated CTB-C2 and CTB-HC FVIII antigens. (A) Time line of oral antigen administration and intravenous treatment with BDD-FVIII. Number in circle indicates time-point for tail bleed. (B) Inhibitor titers (in BU per milliliter) after 4 weekly IV injections of FVIII in non-fed animals (“no plant”) or mice fed with WT or FVIII containing plant material. IgG1 (C), IgG2a (D), IgG2b (E) titers against FVIII for the same experimental groups. (B-E) Data are shown for individual mice and as averages ± standard error of the mean (SEM). (F) After the blood draw, mice were killed and spleens collected. Splenocyte cultures for individual mice (n = 3-5 per group) were stimulated in vitro with 10 μg/mL BDD-FVIII for 48 hours. Subsequently, cells were harvested and subjected to quantitative reverse-transcription-PCR analysis. “Fold increase” is change in RNA transcripts of FVIII vs mock-stimulated cultures. The dotted horizontal line indicates the minimally required increase of 2.5-fold for a statistically significant difference. (G) Splenocytes derived from the same experimental mice were subjected to enzyme-linked immunospot analysis for frequency of IL-10 secreting cell population. All data are shown for individual mice and as averages ± SEM. Unpaired 2-tailed Student t tests were used to calculate P values (**P < .01).
Suppression of inhibitor formation against FVIII in C57BL6/129 mice with hemophilia A by oral administration of a 1:1 mixture of bioencapsulated CTB-C2 and CTB-HC FVIII antigens. (A) Time line of oral antigen administration and intravenous treatment with BDD-FVIII. Number in circle indicates time-point for tail bleed. (B) Inhibitor titers (in BU per milliliter) after 4 weekly IV injections of FVIII in non-fed animals (“no plant”) or mice fed with WT or FVIII containing plant material. IgG1 (C), IgG2a (D), IgG2b (E) titers against FVIII for the same experimental groups. (B-E) Data are shown for individual mice and as averages ± standard error of the mean (SEM). (F) After the blood draw, mice were killed and spleens collected. Splenocyte cultures for individual mice (n = 3-5 per group) were stimulated in vitro with 10 μg/mL BDD-FVIII for 48 hours. Subsequently, cells were harvested and subjected to quantitative reverse-transcription-PCR analysis. “Fold increase” is change in RNA transcripts of FVIII vs mock-stimulated cultures. The dotted horizontal line indicates the minimally required increase of 2.5-fold for a statistically significant difference. (G) Splenocytes derived from the same experimental mice were subjected to enzyme-linked immunospot analysis for frequency of IL-10 secreting cell population. All data are shown for individual mice and as averages ± SEM. Unpaired 2-tailed Student t tests were used to calculate P values (**P < .01).
To address the effect of oral antigen delivery on T cell responses to FVIII, we harvested splenocytes from C57BL6/129 F8e16−/− mice that had been fed with WT or FVIII expressing plant material and treated with FVIII. In vitro restimulation with FVIII induced expression of several cytokines associated with different T helper cell responses in cultures from WT-fed mice (Figure 3F). Interleukin 6 (IL-6) was the most highly and consistently expressed cytokine, which we have previously shown to be expressed by CD4+ T cells of this strain in response to FVIII.9 These control mice lacked expression of immune suppressive cytokines or regulatory T cell (Treg) markers. In contrast, splenocytes from FVIII-fed mice did not show expression of IL-6 or other cytokines associated with Th1 (IL-2, interferon γ), Th2 (IL-4, IL-13), or Th17 (IL-17) responses. Instead, upregulation of Treg markers (CD25, FoxP3, CTLA-4) and, more markedly, of suppressive cytokines IL-10 and transforming growth factor β was observed. Hence, the response was shifted from an effector to a suppressive/regulated response. These results were further supported by an increase in IL-10-producing splenocytes in an enzyme-linked immunospot assay (Figure 3G).
Suppression of inhibitor formation is successful in different strain backgrounds
The identical experiment was performed in mice with hemophilia A and the same F8 mutation but backcrossed on a BALB/c background. Inhibitor formation in this strain is not as brisk.36,41 Nonetheless, control mice (n = 8-11/group) invariably formed high-titer inhibitors (8-200 BU/mL) after 4 weekly IV injections of FVIII (Figure 4A-B). The response was again dominated by IgG1, although IgG2a and IgG2b responses were also observed at substantial titers in some of the animals (Figure 4C-E). Among FVIII-fed mice, 7 had undetectable inhibitors and 4 formed low-titer inhibitors (1-4 BU/mL), indicating more complete suppression in this strain by oral antigen administration (Figure 4B). Total IgG formation was suppressed by approximately 1 log, with absent IgG2a and IgG2b and IgG1 reduced to low-titer (Figure 4C-E).
Suppression of inhibitor formation against FVIII in BALB/c mice with hemophilia A by oral administration of a 1:1 mixture of bioencapsulated CTB-C2 and CTB-HC FVIII antigens. (A) Feeding and FVIII treatment schedule. Number in circle indicates time point for tail bleed. (B) Inhibitor titers (in BU per milliliter) after 4 weekly IV injections of BDD-FVIII in No plant, WT plant, and FVIII plant fed groups. IgG1 (C), IgG2a (D), and IgG2b (E) titers against FVIII for the same experimental groups. All data are shown for individual mice and as averages ± SEM. Unpaired 2-tailed Student t tests were used to calculate P values (*P < .05, ** P < .01).
Suppression of inhibitor formation against FVIII in BALB/c mice with hemophilia A by oral administration of a 1:1 mixture of bioencapsulated CTB-C2 and CTB-HC FVIII antigens. (A) Feeding and FVIII treatment schedule. Number in circle indicates time point for tail bleed. (B) Inhibitor titers (in BU per milliliter) after 4 weekly IV injections of BDD-FVIII in No plant, WT plant, and FVIII plant fed groups. IgG1 (C), IgG2a (D), and IgG2b (E) titers against FVIII for the same experimental groups. All data are shown for individual mice and as averages ± SEM. Unpaired 2-tailed Student t tests were used to calculate P values (*P < .05, ** P < .01).
Next, we extended weekly IV administration of FVIII (without additional feeding) for another month in 5 animals previously fed with FVIII plant material (3 of which had initially undetectable inhibitors). All 5 mice showed an increase in Bethesda titers to 35 to 138 BU/mL after 1 month (Figure 5B). As expected, inhibitor and anti-FVIII IgG titers further increased in control animals treated with FVIII in parallel, reaching levels substantially higher (on average, 9-fold) than those in initially FVIII-fed mice (445-998 BU/mL; Figure 5B). Control mice were subsequently fed with WT plant material for 2 months without further exposure to FVIII (Figure 5A). These animals maintained their Bethesda titers and showed a modest decline in IgG1 anti-FVIII (Figure 5B-C). In animals initially tolerized to FVIII, further oral delivery of FVIII plant material for 2 more months reversed inhibitor titers to an average of 11 BU/mL, ranging from undetectable to 20 BU/mL, which correlated with a reversal of IgG1 formation (Figure 5A-C). An additional experimental group (n = 7) was orally tolerized, again followed by weekly IV injections of FVIII starting 1 month after initiation of oral tolerance. However, in this case, the oral tolerance regimen was continued along with replacement therapy (“FVIII continuously fed” group in Figure 5B-C), which resulted in further suppression of the average inhibitor titer at time point 2 (1.7-fold compared with mice with discontinued oral delivery and 15-fold compared with control mice), and suppression was again sustained (Figure 5B). Reminiscent of our published data on FIX, binding antibodies against FVIII remained detectable by ELISA in this group (Figure 5C).20 More studies are needed to determine whether this difference between the 2 FVIII-fed groups represents experimental variability or a difference in the duration of oral FVIII administration, or whether it could be reduced by addition of the entire light chain in the plant material.
Long-term control and reversal of inhibitor formation in BALB/c mice with hemophilia A. (A) Feeding (HC and C2 material) and FVIII administration schedule for prevention of inhibitor formation. Numbers in circles indicate time-points for blood collection. Inhibitor titers in BU per milliliter (B) and IgG1 titers against FVIII (C) at weeks 8, 12, and 21 of the experiment for FVIII-fed mice (n = 5, back square symbols) are compared with control mice (which were fed with WT plant material; n = 7; gray diamonds). Statistically significant differences between these groups for specific time points are indicated (*P < .05; **P < .01; ***P < .001, as calculated by unpaired 2-tailed Student t-test; data are averages ± SEM). A third group of mice (n = 7) was also fed with FVIII material, and FVIII was administered IV once/week starting 1 month after initiation of the oral tolerance regimen. However, FVIII feeding and treatment were continued for the remaining duration of the experiment (ie, 20 weeks of FVIII feeding; these mice are labeled as “FVIII continuously fed” and graphed with black triangle symbols and dotted line in B and C; data are averages ± SEM). (D) FVIII administration and feeding schedule for reversal of inhibitor formation. Inhibitor formation was induced by repeated weekly IV injections of FVIII as indicated. Mice were divided into 2 groups with similar average inhibitor titers. Control mice (n = 5) did not receive any further treatment. The second group (“FVIII fed”; n = 4) was fed with FVIII plant material twice per week for the following 3 months. Inhibitor titers in BU per milliliter (E) and IgG1 titers against FVIII (F) are graphed for weeks 5, 9, 13, and 17 of the experiment, as explained earlier.
Long-term control and reversal of inhibitor formation in BALB/c mice with hemophilia A. (A) Feeding (HC and C2 material) and FVIII administration schedule for prevention of inhibitor formation. Numbers in circles indicate time-points for blood collection. Inhibitor titers in BU per milliliter (B) and IgG1 titers against FVIII (C) at weeks 8, 12, and 21 of the experiment for FVIII-fed mice (n = 5, back square symbols) are compared with control mice (which were fed with WT plant material; n = 7; gray diamonds). Statistically significant differences between these groups for specific time points are indicated (*P < .05; **P < .01; ***P < .001, as calculated by unpaired 2-tailed Student t-test; data are averages ± SEM). A third group of mice (n = 7) was also fed with FVIII material, and FVIII was administered IV once/week starting 1 month after initiation of the oral tolerance regimen. However, FVIII feeding and treatment were continued for the remaining duration of the experiment (ie, 20 weeks of FVIII feeding; these mice are labeled as “FVIII continuously fed” and graphed with black triangle symbols and dotted line in B and C; data are averages ± SEM). (D) FVIII administration and feeding schedule for reversal of inhibitor formation. Inhibitor formation was induced by repeated weekly IV injections of FVIII as indicated. Mice were divided into 2 groups with similar average inhibitor titers. Control mice (n = 5) did not receive any further treatment. The second group (“FVIII fed”; n = 4) was fed with FVIII plant material twice per week for the following 3 months. Inhibitor titers in BU per milliliter (E) and IgG1 titers against FVIII (F) are graphed for weeks 5, 9, 13, and 17 of the experiment, as explained earlier.
Reversal of inhibitor formation
To test whether the oral protocol is effective in preimmune mice, we treated BALB/c mice with hemophilia A with FVIII and divided them into 2 groups (n = 4-5) with similar average Bethesda titers (∼60 BU). One group (control) was not further exposed to FVIII antigen, whereas the other group was subjected to the oral tolerance regimen (Figure 5D). Inhibitor titers in control animals spontaneously rose further, to an average of nearly 150 BU, and eventually contracted to the original titer of ∼60 BU (Figure 5E). IgG1 anti-FVIII titers showed a substantial further increase to a level that was subsequently maintained (Figure 5F). In contrast, oral FVIII delivery slowed and then reversed inhibitor formation, resulting in a 3- to 7-fold decrease compared with controls after 2 to 3 months of feeding (Figure 5E). IgG1 formation was also significantly decreased (by ∼2.5-fold; Figure 5F).
Oral antigen delivery induces a Treg response against FVIII
Lymphocyte assays in the C57BL6/129 strain suggested Treg induction. We sought to obtain more direct evidence for the induction of active immune suppression, using adoptive transfer studies, which was possible in the pure BALB/c background. Lymphocytes were isolated from spleens and mesenteric lymph nodes (MLN) of FVIII-fed hemophilic BALB/c mice at the end of the experiment outlined in Figure 5A. On adoptive transfer to naive mice of the same strain, CD4+CD25+ T cells, and even more so CD4+CD25− T cells (but not CD4− cells) were able to significantly suppress antibody formation to FVIII (Figure 6A). From previous studies, it has become clear that CD4+CD25+FoxP3+ Tregs are critical in tolerance induction to coagulation factors.8,42 To identify potential suppressor cells in the CD4+CD25− T cell population, we performed flow cytometric analyses of various lymphatic tissues in tolerized vs control mice (Figure 6B). We found significant induction of CD4+CD25−LAP+ T cells (which express high levels of transforming growth factor β) in spleens, MLN, and Peyer’s patches, but no induction of type 1 regulatory T cells (which express high levels of IL-10 and are LAG-3+CD49b+).12,13,43 Consistent with in vitro reverse-transcription-PCR array data and our previous findings, overall frequencies of CD4+CD25+FoxP3+ Treg showed only a subtle increase, as antigen-specific cells of this subset function at low cell numbers.42,44
Active suppression of antibody formation against FVIII by induction of regulatory T cells. (A) Adoptive transfer experiments. CD4−, CD4+CD25−, and CD4+CD25+ cells were purified via magnetic sorting from spleens and MLN of FVIII-fed mice (n = 3) at time-point 3 indicated in Figure 5A and pooled (with a final ratio of approximately 30% spleen and 70% MLN-derived CD4+ T cells). Cells (106 per mouse) were adoptively transferred into naive BALB/c mice via tail vein injection. Control cells were from unchallenged naive mice of the same strain. Twenty-four hours later, all recipient mice (n = 5 per group) were challenged with 1 IU FVIII in adjuvant via subcutaneous injection. IgG titers against FVIII were determined 3 weeks later. All data are shown as averages ± SEM (*P < .05; **P < .01). (B) Frequencies of Treg subsets in FVIII fed and control BALB/c mice with hemophilia A. Cells derived from spleens, MLN, inguinal lymph nodes (ILN), and Peyer’s patches (PP) were isolated from mice that had either been fed with FVIII (HC+C2, “FVIII fed”) or WT plant material (“control”), followed by IV treatment with FVIII (“FVIII fed”). Stained cells were first gated for live CD4+ cells (positive CD4-eFluor 450 and negative viability dye eFluor 506 staining). The frequencies of CD4+CD25− Latency Associated Peptide (LAP)+ cells, CD4+CD25+Foxp3+ cells, and type 1 regulatory T cells (CD4+LAG-3+CD49b+) were calculated using flow cytometric analysis. Data for individual animals as well as averages ± SEM are shown (n = 3-5/group). Unpaired 2-tailed Student t tests were used to calculate P values for all panels.
Active suppression of antibody formation against FVIII by induction of regulatory T cells. (A) Adoptive transfer experiments. CD4−, CD4+CD25−, and CD4+CD25+ cells were purified via magnetic sorting from spleens and MLN of FVIII-fed mice (n = 3) at time-point 3 indicated in Figure 5A and pooled (with a final ratio of approximately 30% spleen and 70% MLN-derived CD4+ T cells). Cells (106 per mouse) were adoptively transferred into naive BALB/c mice via tail vein injection. Control cells were from unchallenged naive mice of the same strain. Twenty-four hours later, all recipient mice (n = 5 per group) were challenged with 1 IU FVIII in adjuvant via subcutaneous injection. IgG titers against FVIII were determined 3 weeks later. All data are shown as averages ± SEM (*P < .05; **P < .01). (B) Frequencies of Treg subsets in FVIII fed and control BALB/c mice with hemophilia A. Cells derived from spleens, MLN, inguinal lymph nodes (ILN), and Peyer’s patches (PP) were isolated from mice that had either been fed with FVIII (HC+C2, “FVIII fed”) or WT plant material (“control”), followed by IV treatment with FVIII (“FVIII fed”). Stained cells were first gated for live CD4+ cells (positive CD4-eFluor 450 and negative viability dye eFluor 506 staining). The frequencies of CD4+CD25− Latency Associated Peptide (LAP)+ cells, CD4+CD25+Foxp3+ cells, and type 1 regulatory T cells (CD4+LAG-3+CD49b+) were calculated using flow cytometric analysis. Data for individual animals as well as averages ± SEM are shown (n = 3-5/group). Unpaired 2-tailed Student t tests were used to calculate P values for all panels.
Local and systemic delivery of bioencapsulated FVIII
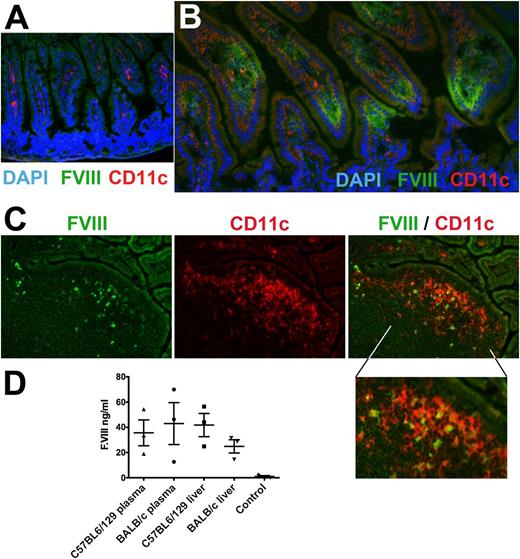
Delivery of FVIII antigen to the GALT was demonstrated by immunostaining, which showed the presence of fed FVIII antigen in epithelial cells and delivery to dendritic cells in the lamina propria and Peyer’s patches of the small intestine (Figure 7A-C). Presence of a furin cleavage site between CTB and FVIII sequences should facilitate systemic delivery of FVIII antigen after uptake in the gut. Indeed, we found HC antigen in plasma samples and liver protein extracts from samples obtained from mice with hemophilia A 5 hours after the last gavage (Figure 7D). Delivery of CTB-HC/CTB-C2 only infrequently elicited systemic antibody responses to CTB, and there was no correlation between anti-CTB and FVIII inhibitor titers (supplemental Figure 1; data not shown). The reason for anti-CTB formation in a subset of C57BL6/129 mice is unclear but may relate to processing of the receptor-bound CTB antigen (cleaved off FVII sequences) or the strength of B- or T-cell epitopes for this antigen/strain combination.
Delivery of FVIII antigen to the GALT and into circulation. (A-C) Immunostains (original magnification, ×200) of ileum cryosections from unfed (A, negative control) or CTB-C2-fed (B, lamina propria; C, Peyer’s patch) BALB/c mice with hemophilia A. Stains are for C2 domain of FVIII (green), CD11c (red), and nuclei (DAPI; blue). (D) Human FVIII antigen levels were measured in plasma or liver protein extract of the CTB-HC-fed C57BL6/129 and BALB/c mice with hemophilia A and WT-fed control mice of the same strain, using HC-specific ELISA. All data are shown for individual mice and as averages ± SEM.
Delivery of FVIII antigen to the GALT and into circulation. (A-C) Immunostains (original magnification, ×200) of ileum cryosections from unfed (A, negative control) or CTB-C2-fed (B, lamina propria; C, Peyer’s patch) BALB/c mice with hemophilia A. Stains are for C2 domain of FVIII (green), CD11c (red), and nuclei (DAPI; blue). (D) Human FVIII antigen levels were measured in plasma or liver protein extract of the CTB-HC-fed C57BL6/129 and BALB/c mice with hemophilia A and WT-fed control mice of the same strain, using HC-specific ELISA. All data are shown for individual mice and as averages ± SEM.
Discussion
Advantages of the plant-based platform for oral tolerance in hemophilia
An oral tolerance protocol would be ideal for he induction of antigen-specific tolerance while avoiding use of genetic manipulation of patient cells or of immune suppressive drugs, which have undesired adverse effects, increase the risk for infection, and may affect development of the immune system.12,14,16,20 Therefore, oral delivery of FVIII antigen may be an acceptable form of prophylactic tolerance induction in pediatric patients. Our current study demonstrates that multiple domains of FVIII can be expressed in plant chloroplasts. Moreover, oral administration of a mixture of bioencapsulated HC and C2 domain antigens substantially suppressed inhibitor formation in subsequent replacement therapy or animals with preexisting response to FVIII infusion.
Oral delivery of plant-made pharmaceutical proteins is emerging as an effective approach.
Bioencapsulation of therapeutic proteins within plant cells protects them from harsh environment of the gastrointestinal tract.15,45,46 In addition, elimination of highly expensive purification, cold storage, transportation, and sterile injections significantly reduces their costs. Although we have not optimized the codons for FVIII expression in the current investigation, it has been reported that protein expression can reach up to 70% of the total leaf protein under optimal conditions.18 Multigene engineering is especially relevant for studies with FVIII because all domains may be simultaneously expressed in a single transformation cassette.47
Further optimization of the system and clinical implications
Although we provide proof of principle for suppression of FVIII inhibitors by oral delivery of transplastomic plant material, inhibitor formation was not completely prevented, especially in the more highly responsive C57BL6/129 background. It is possible that higher-antigen doses or more frequent delivery may be required than what we reported for FIX.20 Transgene codon optimization should increase expression, as observed for several other human genes in chloroplasts.18,32 The C2 domain is expressed 3.6-fold higher than the heavy chain because of higher codon compatibility, underscoring the need for codon optimization to achieve higher levels of expression in chloroplasts. Using a combination of FVIII domains, their ratio becomes important. For example, we observed only a 3-fold reduction in inhibitor titers in hemophilic C57BL6/129 mice when a ratio of HC-C2 of 1:3 (instead of 1:1) was used (data not shown). The tolerogenic antigen mix may be further optimized by testing other ratios or by the addition of more domains, such as A3. Strategies discussed here will allow us to develop optimal materials in lettuce chloroplasts to facilitate clinical translation, which we know from multiple prior studies to be equivalent in delivery of bioencapsulated antigen to the GALT.19,32,35 Further studies of the duration of oral FVIII administration are also needed. Thus far, the data suggest that more continuous oral delivery is required for FVIII than what we had found for FIX.20 Regardless, the new data with FVIII demonstrate that the approach can also be applied to reversal of preexisting inhibitors that have formed during replacement therapy. Therefore, we hope to develop oral tolerance protocols for prevention of inhibitor formation in high-risk patients and as an alternative or an addition to current immune tolerance induction.
Induction of suppressive CD4+ T cell responses
Adoptive transfer studies demonstrate that oral FVIII delivery induced multiple subsets of CD4+ T cells that actively suppress antibody formation. Therefore, this mechanism is distinct from immune tolerance induced by hepatocyte-derived antigen, which primarily induces CD4+CD25+FoxP3+ Treg.44,48,49 Antigen presented in the GALT additionally induced a strongly suppressive CD4+CD25− T cell response. We do not believe this reflects memory effector T cell activity, as transfer of such FVIII-experienced cells from mice that had not received oral delivery increases, rather than suppresses, anti-FVIII formation (X.W., unpublished observations, Aug 9, 2013). Rather, flow cytometric analyses of CD4+ T cells suggest induction of CD4+CD25−LAP+ Treg, which are known to be inducible by antigen presentation in the gut and suppress, by expression of large amounts of transforming growth factor β, a cytokine that is also required for peripheral induction of CD4+CD25+FoxP3+ Treg. Consistent with tologenic oral antigen delivery, induction of CD4+CD25−LAP+ was observed in Peyer’s patches and MLN, which drain the gut, but not nondraining lymph nodes. Increased frequency in the spleen is consistent with suppression of a systemic response, which is required to control inhibitor formation against IV delivered FVIII antigen. We found no evidence for induction of type 1 regulatory T cells. Nonetheless, there was induction of IL-10, a critical anti-inflammatory cytokine in the GALT. Both FoxP3+ Treg and LAP+ Treg are potential sources of IL-10 expression. Codelivery of HC and C2 domain was sufficient to suppress inhibitor formation against the entire FVIII molecule in the BALB/c strain. In humans, additional T-cell epitopes in other domains likely exist. However, efficient induction of Treg may provide sufficient suppression so that not all epitopes have to be covered by the orally delivered antigens.
In conclusion, FVIII domains can be produced at high levels in leaves of transplastomic plants. Oral delivery of modified plant cells induces Treg and suppresses antibody formation to IV-delivered FVIII, and therefore represents a promising approach to control formation of inhibitors.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the following members of the Health Diagnostic Laboratory: Dr Dheeraj Verma for making the tobacco chloroplast vectors, and Andrew Devine, Himabindu Gazula, and Ramya Nityanandam for their technical assistance in the creation and characterization of different transplastomic lines.
This study was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL107904 and R01 HL109442) (H.D. and R.W.H.) and a Bayer Hemophilia award (H.D.).
Authorship
Contribution: R.W.H. and H.D. designed experiments, interpreted data, wrote/edited the manuscript, and supervised the study; and A.S., J.S., S.L., and X.W. performed experiments and interpreted data and wrote parts of the manuscript.
Conflict-of-interest disclosure: H.D. holds several US and international patents on chloroplast transformation technology to produce vaccines and biopharmaceuticals. H.D. and R.W.H. have a pending patent application for chloroplast transgenic plant-based tolerance induction to coagulation factors. The remaining authors declare no competing financial conflicts.
Correspondence: Henry Daniell, University of Pennsylvania, 240 South 40th St, 547 Levy Building, Philadelphia PA 19104-6030; e-mail: hdaniell@upenn.edu; and Roland W. Herzog, University of Florida, Cancer and Genetics Research Center, 2033 Mowry Rd, Room 203, Gainesville, FL 32610; e-mail: rherzog@ufl.edu.
References
Author notes
A.S. and J.S. contributed equally to this study.