Key Points
This study demonstrated an essential role of COX-1 in early B-cell development.
Low-dose aspirin may have a potential suppressive effect on B-cell development in humans.
Abstract
Cyclooxygenases (COXs) and their prostanoid products play important roles in a diverse range of physiological processes, including in the immune system. Here, we provide evidence that COX-1 is an essential regulator in early stages of B-cell development. COX-1–deficient mice displayed systematic reduction in total B cells, which was attributed to the arrest of early B-cell development from pro-B to pre-B stage. We further demonstrated that this defect was mediated through downregulation of the Janus kinase/signal transducer and activator of transcription 5 (JAK/STAT5) signaling and its target genes, including Pax5, in COX-1−/− mice. Mechanistic studies revealed that COX-1–derived thromboxane A2 (TxA2) could regulate JAK3/STAT5 signaling through the cyclic adenosine monophosphate-protein kinase A pathway, via binding with its receptor thromboxane A2 receptor (TP). Administration of the TP agonist could rescue the defective B-cell development and JAK/STAT5 signaling activity in COX-1–deficient mice. Moreover, administration of low-dose aspirin caused a significant reduction in total B cells in peripheral blood of healthy human volunteers, coincidentally with reduced TxA2 production and downregulation of JAK/STAT5 signaling. Taken together, our results demonstrate that COX-1–derived TxA2 plays a critical role in the stage transition of early B-cell development through regulation of JAK/STAT5 signaling and indicate a potential immune-suppressive effect of low-dose aspirin in humans.
Introduction
B-cell development is a stepwise process that requires tight coordination between cytokines and induced transcription factors, as well as cross-talk between hematopoietic progenitors and the bone marrow (BM) microenvironment in which they reside. The earliest step in B-cell commitment is the generation of common lymphoid progenitors (CLPs) from hematopoietic stem cells (HSCs) through multiple steps. CLPs give rise to pre-pro-B cells, pro-B cells, pre-B cells, immature B cells, and finally develop into mature B cells in BM. Mature B cells migrate to peripheral lymphoid tissues, where they undergo activation and produce specific antibodies in response to antigen exposure.1,2
The molecular mechanism dictating early B-cell development within the BM has been extensively investigated, and a small set of transcription factors has been identified as essential regulators of this process.3,4 Among these, the role of interleukin (IL)-7–induced activation of the Janus kinase/signal transducer and activator of transcription 5 (JAK/STAT5) pathway has been well documented.5,6 Specifically, CLPs express the receptor for IL-7 (IL-7R),7 and IL-7 can be produced by both stromal cells and some progenitor cells in BM environment.8 Binding of IL-7 to IL-7R on CLPs activates JAK/STAT5 signaling, which induces the transcription of genes essential for B-cell development, including Pax5 and Ebf1.9-11 The necessity of the JAK/STAT5 pathway in B-cell development has been firmly established; however, the upstream mechanism regulating JAK/STAT5 activity remains poorly understood.
Cyclooxygenases (COXs) are enzymes that metabolize arachidonic acid and produce prostanoids, including prostaglandins (PGs) and thromboxanes (TXs), in combination with specific PG and TX synthases.12 Prostanoids, acting through G-protein–coupled receptors, elicit intracellular signaling pathways and play crucial roles in a variety of (patho)physiological processes, depending on specific metabolite and also on the cell type.13,14 Two isoforms of COX enzymes, COX-1 and COX-2, have been identified. They differ in terms of gene structure, tissue distribution, and protein functions.15 COX-1 is constitutively expressed in most cell types, whereas COX-2 is induced in response to certain stimuli.16 Nonsteroidal anti-inflammatory drugs, including aspirin, exhibit clinically antithrombotic and anti-inflammatory efficacies by inhibition of COX-derived prostanoid production.17,18
In this study, we wished to investigate the role of COX-1 in the development of immune cells. We found that COX-1 deficiency specifically impairs early B-cell development through downregulation of thromboxane A2 (TxA2)-TxA2 receptor (TP)–mediated JAK3/STAT5 signaling via the cyclic adenosine monophosphate (cAMP)-protein kinase A pathway (PKA) axis. Furthermore, administration of low-dose aspirin reduced B-cell levels in humans. Thus, our observations demonstrated that COX-1–derived TxA2 represents a novel regulator of early B-cell development.
Methods and materials
Mouse strains
All experiments performed on mice were approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University. COX-1−/− mice were maintained on a mixed C57BL/6 × Sv129 genetic background (50%:50%) for >20 generations,19 TP−/− mice were maintained on a C57BL/6 background. All mice were housed in a specific pathogen-free facility, and age-matched littermates (6-8 weeks) were used as controls in this study.
Flow cytometric analysis and sorting
Single-cell suspensions were prepared and were stained with fluorochrome-conjugated antibodies. Data were collected on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR). Data were acquired as the fraction of labeled cells within a live-cell gate set for 50 000 events. For flow cytometric sorting, cells were stained with specific antibodies and isolated on a BD FACSAria cell sorter (BD Bioscience).
Aspirin administration in humans
Healthy volunteers who had no reported history of chronic illness, no drug allergy, or no bleeding tendency were enrolled. They are administrated daily oral aspirin (100 mg/day) for 10 days. Peripheral blood mononuclear cells were isolated by Ficoll centrifugation before and at the end of administration. The study was approved by Ethics Review Board of Sun Yat-Sen University, and all participants gave written informed consent in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical tests were performed using GraphPad Prism version 5.0a software and SPSS 170. Unpaired Student t tests and analysis of variance (ANOVA) were used to confirm most comparisons. Statistical analysis was performed using the paired Student t test in the human aspirin experiment. Correlations between different parameters were analyzed using the Spearman rank test. P < .05 was considered significant.
Results
COX-1 deficiency impairs B-cell homeostasis
To investigate the physiological role of COX-1 during hematopoiesis, we analyzed the effect of COX-1 deficiency on the hematopoietic system by comparing COX-1−/− mice with wild-type (WT) littermates. The absence of COX-1 expression in the BM and spleen from the COX-1−/− mice was confirmed by immunoblotting (supplemental Figure 1 available on the Blood Web site).
We first evaluated the frequencies of B cells and other immune cells in several tissues, including BM, spleen, peripheral blood, and lymph nodes, by flow cytometric analysis. The total numbers of nucleated cells in multiple tissues remained largely unchanged in COX-1−/− mice compared with WT controls (data not shown). We found that COX-1−/− mice displayed a twofold reduction in the percentage of B220+CD19+ B cells (Figure 1A-B); the absolute number of B cells was consistently reduced in various tissues of COX-1−/− mice, reaching only ∼50% of the level present in BM of WT mice (Figure 1C). In contrast, the proportions and absolute cell counts of T lymphocytes were not noticeably different between COX-1−/− and WT mice (Figure 1A-C). The proportion of different types of myeloid cells, including immature myeloid cells, dendritic cells, and macrophages, did not display any changes between WT and COX-1−/− mice (supplemental Figure 2). Taken together, these observations indicated that COX-1 is specifically essential for B-cell homeostasis.
COX-1 deficiency impairs B-cell homeostasis. (A) Representative flow cytometry profiles of BM, spleen (SP), peripheral blood (PB), and lymph node (LN) tissues from homozygous COX-1−/− mice and WT littermates to identify total B (B220+CD19+) and T (CD3e+) lymphocytes. Numbers in the plots indicate percentages in each gate. (B) Percentages and (C) absolute cell counts of B cells, T cells, and T-cell subsets in the indicated tissues of WT and COX-1−/− mice. (A-C) Each group included 6 mice, and measurements were repeated 3 times for each mouse; data are shown as mean ± standard error of the mean (SEM). *P < .05, **P < .01 compared with controls using unpaired Student t tests.
COX-1 deficiency impairs B-cell homeostasis. (A) Representative flow cytometry profiles of BM, spleen (SP), peripheral blood (PB), and lymph node (LN) tissues from homozygous COX-1−/− mice and WT littermates to identify total B (B220+CD19+) and T (CD3e+) lymphocytes. Numbers in the plots indicate percentages in each gate. (B) Percentages and (C) absolute cell counts of B cells, T cells, and T-cell subsets in the indicated tissues of WT and COX-1−/− mice. (A-C) Each group included 6 mice, and measurements were repeated 3 times for each mouse; data are shown as mean ± standard error of the mean (SEM). *P < .05, **P < .01 compared with controls using unpaired Student t tests.
COX-1 is required for early B-cell development
Generation of B cells is coordinated by B-cell development and B-cell survival.20 The B-cell reduction in COX-1−/− mice prompted us to investigate the underlying mechanism. We first examined whether it was due to enhanced B-cell apoptosis caused by COX-1 deficiency. We failed to observe any difference in the frequency of apoptotic cells in the presence or absence of COX-1, in either B cells or T cells, by annexin V staining (Figure 2A).
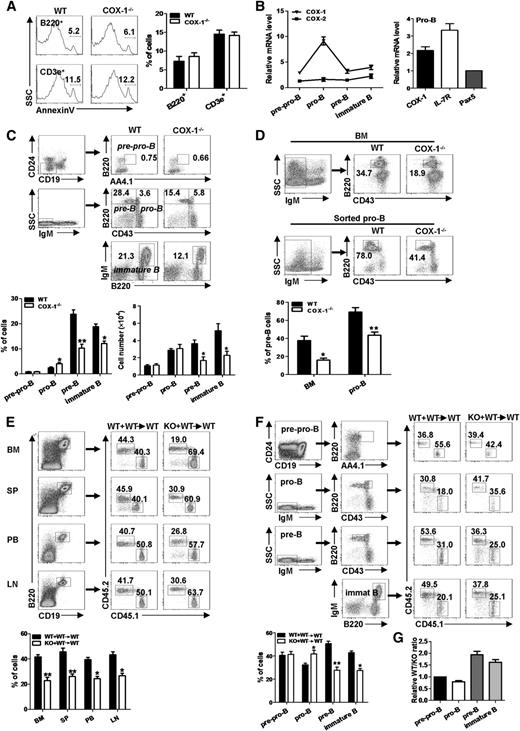
COX-1 is required for early B-cell development. (A) Flow cytometric analysis of apoptosis (annexin V+) in B220+ B cells and CD3e+ T lymphocytes from BM in COX-1−/− and WT mice. (Left) Representative from a single experiment. (Right) Mean ± SEMs from 4 independent experiments. (B) (Left) mRNA expression of COX-1 and COX-2 in distinct stages of developing B cells from mouse BM was evaluated by qRT-PCR. The subpopulations were purified by flow cytometric sorting based on the following surface markers: pre-pro-B (AA4.1+B220+CD19−CD24−), pro-B (B220+CD43+IgM−), pre-B(B220+CD43−IgM−), and immature B (B220+IgM+). (Right) Relative expression of COX-1 and other genes in sorted pro-B cells was determined by qRT-PCR. β-actin was used to normalize gene expression, the group with lowest expression was artificially set as 1. Mean ± SEMs of 3 independent experiments are shown. (C) Flow cytometry profiles of distinct B-cell compartments in BM from COX-1−/− and WT mice. (D) BM cells were cocultured on OP9 stromal cells with IL-7 for 6 days (upper), or purified pro-B cells were cultured with IL-7, SCF, and Flt3L cytokines (lower), from COX-1−/− and WT mice; the frequencies of pre-B cells generated were then determined by flow cytometric analysis. (E-G) BM transplantation: A 1:1 mixture of BM cells from COX-1−/− or WT mice (CD45.2+) with BM from syngenic (CD45.1+) were injected into irradiated syngenic mice (5 × 106 cells per mouse, n = 6). Mice were killed 6 weeks after transplantation. The levels of (E) total B cells and (F) developing B cells among CD45.2+ cells in recipients were determined by flow cytometry analysis. (G) Relative WT/knockout (KO) ratios of B-cell subpopulations in F were normalized against pre-pro-B cells. (C-G) (Upper) Representative from 1 single experiment; numbers in quadrants indicate percentage of total living cells. (Lower) Mean ± SEMs from (C,E-G) 6 mice or (D) 3 independent experiments. *P < .05 and **P < .01, using unpaired Student t tests.
COX-1 is required for early B-cell development. (A) Flow cytometric analysis of apoptosis (annexin V+) in B220+ B cells and CD3e+ T lymphocytes from BM in COX-1−/− and WT mice. (Left) Representative from a single experiment. (Right) Mean ± SEMs from 4 independent experiments. (B) (Left) mRNA expression of COX-1 and COX-2 in distinct stages of developing B cells from mouse BM was evaluated by qRT-PCR. The subpopulations were purified by flow cytometric sorting based on the following surface markers: pre-pro-B (AA4.1+B220+CD19−CD24−), pro-B (B220+CD43+IgM−), pre-B(B220+CD43−IgM−), and immature B (B220+IgM+). (Right) Relative expression of COX-1 and other genes in sorted pro-B cells was determined by qRT-PCR. β-actin was used to normalize gene expression, the group with lowest expression was artificially set as 1. Mean ± SEMs of 3 independent experiments are shown. (C) Flow cytometry profiles of distinct B-cell compartments in BM from COX-1−/− and WT mice. (D) BM cells were cocultured on OP9 stromal cells with IL-7 for 6 days (upper), or purified pro-B cells were cultured with IL-7, SCF, and Flt3L cytokines (lower), from COX-1−/− and WT mice; the frequencies of pre-B cells generated were then determined by flow cytometric analysis. (E-G) BM transplantation: A 1:1 mixture of BM cells from COX-1−/− or WT mice (CD45.2+) with BM from syngenic (CD45.1+) were injected into irradiated syngenic mice (5 × 106 cells per mouse, n = 6). Mice were killed 6 weeks after transplantation. The levels of (E) total B cells and (F) developing B cells among CD45.2+ cells in recipients were determined by flow cytometry analysis. (G) Relative WT/knockout (KO) ratios of B-cell subpopulations in F were normalized against pre-pro-B cells. (C-G) (Upper) Representative from 1 single experiment; numbers in quadrants indicate percentage of total living cells. (Lower) Mean ± SEMs from (C,E-G) 6 mice or (D) 3 independent experiments. *P < .05 and **P < .01, using unpaired Student t tests.
Flow cytometric analysis revealed that the frequency of hematopoietic progenitors, including HSCs, CLPs, and common myeloid cells, did not differ between WT and COX-1−/− mice (supplemental Figure 3), indicating that the B-cell defect caused by COX-1 deficiency may occur at a stage later than CLPs.
To gain insights into the relationship between COX-1 and early B-cell development, we first evaluated the expression of COXs in developing B cells using quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). In contrast to the steadily low expression of COX-2 in these B-cell compartments, the expression of COX-1 was found to be dynamic in developing B cells, peaking at the pro-B stage. The mRNA expression level of COX-1 in pro-B cells was comparable to that of IL-7R and Pax5, 2 crucial regulators of early B-cell development (Figure 2B). These results indicate a potential role for COX-1 in the stage transition of developing B cells.
We next examined B cells in the mouse BM, the primary tissue in which early B-cell development occurs. The frequencies and absolute numbers of pre-B and immature B cells were markedly reduced in COX-1−/− mice. The proportion of pro-B cells displayed a moderate but significant increase in COX-1−/− mice, whereas the level of pre-pro-B cells remained essentially unchanged (Figure 2C). These observations collectively indicated that COX-1 deficiency impacts the transition from pro-B to pre-B stage.
In an aim to further specify the effect of COX-1 on early B-cell development, an in vitro B-cell differentiation experiment was performed. As expected, the capability of progenitors in BM to generate pre-B cells was significantly lower in COX-1−/− mice than in WT controls (Figure 2D). Consistently, purified pro-B cells from COX-1−/− mice also displayed impairment in differentiating into pre-B cells compared with the corresponding controls (Figure 2D). Taken together, our data demonstrated that COX-1 is required for the transition from pro-B to pre-B stage during early B-cell development.
To investigate whether the B-cell defect in COX-1−/− mice was cell intrinsic, we performed BM transplantation experiments. We mixed BM cells from WT or COX-1−/− (CD45.2+) with BM from syngenic (CD45.1+), at a 1:1 ratio, and cells were then transplanted into lethally irradiated syngenic mice.21,22 After 6 weeks, flow cytometry analysis was used to evaluate the relative percentages of B cells expressing the CD45.2 (donor)- or CD45.1 (recipient)-derived population. Results showed that the ability of COX-1−/− BM to generate B220+CD19+ cells was significantly decreased compared with the BM cells from WT mice (Figure 2E). The levels of pre-B and immature B cells were decreased almost twofold in the COX-1−/− BM compared with their WT control (Figure 2F-G).
These data indicated that the defect of B-cell development in COX-1–deficient mice is cell autonomous rather than being associated with the BM microenvironment.
JAK/STAT5 signaling mediates the B-cell defect in the absence of COX-1
To explore the molecular mechanism underlying the impaired B-cell development caused by COX-1 deficiency, we first profiled the expression of some transcription factors that are crucial for B-cell commitment.4 qRT-PCR showed a marked reduction in mRNA levels of Pax5 and Ebf1 in the purified pro-B and pre-B cells from COX-1−/− mice compared with those from WT controls. In contrast, the expression levels of other transcription factors did not display marked differences (Figure 3A). The downregulation of Ebf1 and Pax5 by COX-1 deletion was further confirmed by immunoblotting (Figure 3B).
JAK/STAT5 signaling mediates B-cell defect in the absence of COX-1. (A) The expression of the indicated transcription factors in purified pro-B and pre-B cells from COX-1−/− and WT BM was determined by qRT-PCR. Mean ± SEMs from 4 independent experiments are shown. (B) Ebf1 and Pax5 expression was further confirmed by western blotting (WB). (Upper) From single representative experiments using mixed samples from 5 mice. (Lower) Mean ± SEMs from 3 independent experiments. (C) mRNA expression of Pax5 and Ebf1 in the distinct B-cell subpopulations was examined by qRT-PCR. We used β-actin for normalization, and the group with lowest expression was artificially set as 1. Data are presented as mean ± SEM from 3 independent experiments. (D) Purified B-cell subpopulations from mouse BM were stimulated with IL-7 for 15 min. The phosphorylation of STAT5 was analyzed by flow cytometry. (Left) Representative data from a single experiment. Solid, unstimulated; dotted, IL-7 stimulated. (Right) Mean ± SEMs from 3 independent experiments. MFI, mean fluorescence intensity. (E) Purified B-cell subpopulations were stimulated with IL-7 for 24 hours, and expression of STAT5 target genes were determined by qRT-PCR. Results represent mean ± SEM of 3 independent experiments. (F) BM cells were pretreated with the JAK3 inhibitor (WHI-P131, 15 μM) or dimethylsulfoxide (DMSO) for 12 hours, followed by administration of COX-1 inhibitor (SC-560, 20 μM). Cells were cultured under conditions to induce B-cell differentiation for 6 days. The percentages of pre-B cells (IgM−CD43−B220+) were analyzed by flow cytometry. Numbers indicate the frequencies of total living cells. (G-H) BM cells from COX-1−/− and WT mice were transduced with lentiviral plasmid (with GFP tag) expressing (G) constitutively activated STAT5 or (H) Pax5, and empty vector CPPT was used as the control. Infected cells were cultured under conditions for 6 days to induce B-cell differentiation. The percentages of total B cells and pre-B cells among GFP+ cells were evaluated by flow cytometric analysis. (F-H) (Left) Representative from a single experiment. (Right) Mean ± SEMs from 3 independent experiments. *P < .05 and **P < .01 using unpaired Student t tests and 1-way ANOVA.
JAK/STAT5 signaling mediates B-cell defect in the absence of COX-1. (A) The expression of the indicated transcription factors in purified pro-B and pre-B cells from COX-1−/− and WT BM was determined by qRT-PCR. Mean ± SEMs from 4 independent experiments are shown. (B) Ebf1 and Pax5 expression was further confirmed by western blotting (WB). (Upper) From single representative experiments using mixed samples from 5 mice. (Lower) Mean ± SEMs from 3 independent experiments. (C) mRNA expression of Pax5 and Ebf1 in the distinct B-cell subpopulations was examined by qRT-PCR. We used β-actin for normalization, and the group with lowest expression was artificially set as 1. Data are presented as mean ± SEM from 3 independent experiments. (D) Purified B-cell subpopulations from mouse BM were stimulated with IL-7 for 15 min. The phosphorylation of STAT5 was analyzed by flow cytometry. (Left) Representative data from a single experiment. Solid, unstimulated; dotted, IL-7 stimulated. (Right) Mean ± SEMs from 3 independent experiments. MFI, mean fluorescence intensity. (E) Purified B-cell subpopulations were stimulated with IL-7 for 24 hours, and expression of STAT5 target genes were determined by qRT-PCR. Results represent mean ± SEM of 3 independent experiments. (F) BM cells were pretreated with the JAK3 inhibitor (WHI-P131, 15 μM) or dimethylsulfoxide (DMSO) for 12 hours, followed by administration of COX-1 inhibitor (SC-560, 20 μM). Cells were cultured under conditions to induce B-cell differentiation for 6 days. The percentages of pre-B cells (IgM−CD43−B220+) were analyzed by flow cytometry. Numbers indicate the frequencies of total living cells. (G-H) BM cells from COX-1−/− and WT mice were transduced with lentiviral plasmid (with GFP tag) expressing (G) constitutively activated STAT5 or (H) Pax5, and empty vector CPPT was used as the control. Infected cells were cultured under conditions for 6 days to induce B-cell differentiation. The percentages of total B cells and pre-B cells among GFP+ cells were evaluated by flow cytometric analysis. (F-H) (Left) Representative from a single experiment. (Right) Mean ± SEMs from 3 independent experiments. *P < .05 and **P < .01 using unpaired Student t tests and 1-way ANOVA.
To specify the stage at which gene downregulation occurs, the expression levels of Pax5 and Ebf1 in developing B cells were measured by qRT-PCR. A dramatic reduction in both Pax5 and Ebf1 expression levels was observed in pre-B and immature B cells from COX-1−/− mice in comparison with those from WT controls (Figure 3C).
Because both Pax5 and Ebf1 are transcriptional targets of IL-7–induced JAK/STAT5 signaling in B cells,11 we therefore proposed that COX-1 deficiency may downregulate JAK/STAT5 signaling. Flow cytometric analysis showed that the pro-B and pre-B cells from COX-1−/− mice failed to show much difference in STAT5 phosphorylation (p-STAT5) levels before and after IL-7 stimulation, whereas B cells from WT control expectedly displayed enhanced STAT5 phosphorylation on IL-7 treatment (Figure 3D). Moreover, we also observed a failure in the induction of STAT5 target genes in these B-cell populations from COX-1−/− mice in response to IL-7 treatment (Figure 3E). Collectively, these results demonstrated that COX-1 deletion impaired IL-7–induced JAK/STAT5 signaling in B cells.
To investigate whether JAK/STAT5 signaling plays an essential role in perturbed B-cell development of COX-1–deficient mice, we modulated STAT5 signaling, either pharmacologically or genetically, in B cells. First, we used the JAK inhibitor WHI-P131 to block phosphorylation of STAT5 in differentiating B cells.23 We found that WHI-P131 blocks the development of B cells to the same extent as cells treated with SC-560, a specific inhibitor of COX-1, although WHI-P131 did not enhance the inhibitory effect of SC-560. These results indicated that JAK/STAT5 signaling mediates the regulatory effect of COX-1 on B cells (Figure 3F). Second, lentiviral plasmid expressing constitutively activated STAT5 was used to infect BM cells cultured for B-cell differentiation. The transduction efficiency was >20% (data not shown), and B-cell phenotype was evaluated among green fluorescent protein (GFP)+ cells. Results showed that transduction of constitutively activated STAT5 almost completely rescued the impaired B-cell differentiation in COX-1−/− mice to the level of WT control (Figure 3G). However, overexpression of Pax5 only partially recovered the defect (Figure 3H). Our observations therefore demonstrated that the JAK/STAT5 signaling pathway plays an essential role in mediating the effect of COX-1 on B-cell development.
TxA2-TP axis participates in the early B-cell development
In an attempt to investigate the mechanism underlying COX-1–mediated B-cell development, we first examined the prostanoid profiles of sorted pro-B and pre-B cells from COX-1−/− and WT mice using mass spectrum assays. Results showed that the content of thromboxane (Tx)B2, the stable metabolite of TxA2, in B cells from COX-1−/− mice was reduced to ∼50% of the corresponding control; PGE2 and PGD2 levels were also reduced to some extent, whereas the amounts of PGF2 and 6-keto-PGF1α remained largely unchanged (Figure 4A). We further measured the amount of Leukotriene B4 in B220+ B cells to determine whether there was compensatory overproduction of leukotrienes through an alternative arachidonic acid metabolic pathway in COX-1−/− mice.12 No significant difference was observed between COX-1−/− and WT mice (supplemental Figure 4).
TxA2-TP axis participates in the early B-cell development. (A) Prostanoid profiles in purified B-cell subpopulations from BM were examined by mass spectrum assays. Results show the mean ± SEM of 6 independent experiments. (B) mRNA expression of receptors for prostanoids in purified B cells was evaluated by semiquantitative RT-PCR. Data are representative of 4 independent experiments. (C) mRNA expression of TP in distinct stages of developing B cells derived from BM of naïve mice was evaluated by RT-PCR. We used β-actin for normalization, and the group with lowest expression was artificially set as 1. Results show the mean ± SEM of 3 independent experiments from 3 mice. (D) Flow cytometric analysis of distinct B-cell populations in BM from TP−/− and WT controls (n = 6). (E) BM transplantation experiments: BM cells from TP−/− or WT mice (CD45.2+) were mixed with BM from syngenic mice (CD45.1+) at a 1:1 ratio, and cells were then injected into irradiated syngenic mice (n = 6). The percentages of distinct B-cell compartments among CD45.2+ cells in BM were analyzed 6 weeks after transplantation. (F) The relative WT/KO ratios of B-cell subpopulations in E were normalized against pre-pro-B cells. Mean ± SEM from 6 mice is shown. (D-E) (Upper) Representative results from 1 single experiment; numbers adjacent indicate percentages of total living cells. (Lower) Mean ± SEM from all mice analyzed. *P < .05 and **P < .01, using unpaired Student t tests.
TxA2-TP axis participates in the early B-cell development. (A) Prostanoid profiles in purified B-cell subpopulations from BM were examined by mass spectrum assays. Results show the mean ± SEM of 6 independent experiments. (B) mRNA expression of receptors for prostanoids in purified B cells was evaluated by semiquantitative RT-PCR. Data are representative of 4 independent experiments. (C) mRNA expression of TP in distinct stages of developing B cells derived from BM of naïve mice was evaluated by RT-PCR. We used β-actin for normalization, and the group with lowest expression was artificially set as 1. Results show the mean ± SEM of 3 independent experiments from 3 mice. (D) Flow cytometric analysis of distinct B-cell populations in BM from TP−/− and WT controls (n = 6). (E) BM transplantation experiments: BM cells from TP−/− or WT mice (CD45.2+) were mixed with BM from syngenic mice (CD45.1+) at a 1:1 ratio, and cells were then injected into irradiated syngenic mice (n = 6). The percentages of distinct B-cell compartments among CD45.2+ cells in BM were analyzed 6 weeks after transplantation. (F) The relative WT/KO ratios of B-cell subpopulations in E were normalized against pre-pro-B cells. Mean ± SEM from 6 mice is shown. (D-E) (Upper) Representative results from 1 single experiment; numbers adjacent indicate percentages of total living cells. (Lower) Mean ± SEM from all mice analyzed. *P < .05 and **P < .01, using unpaired Student t tests.
Further in vitro B-cell differentiation experiments showed that administration of PGE2 or PGD2 did not cause any effect on B-cell development (supplemental Figure 5), which excluded their possible participation in the COX-1 effect on B cells.
TxA2 exerts its action through the specific G-protein–coupled TxA2 receptor (TP).24 qRT-PCR results showed that TP was abundantly expressed in both pro-B and pre-B cells from mouse BM (Figure 4B). Interestingly, TP expression was dynamic in developing B cells, peaking at the pro-B cell stage (Figure 4C), raising the possibility that TxA2-TP signaling may play a potential role in B-cell development. Flow cytometric analysis showed that the frequencies of total B, pre-B, and immature B cells were consistently reduced in BM from TP−/− mice compared with that in WT controls. The proportions of pro-B and pre-pro-B cells in TP−/− BM were largely unchanged (Figure 4D). The total B-cell numbers in spleen, peripheral blood, and lymph nodes were also clearly reduced in TP−/− mice (supplemental Figure 6). A further mixed BM transplantation experiment confirmed that the B-cell defect in TP−/− mice was cell autonomous (Figure 4E, 4F). These observations demonstrated that TP deficiency caused a disturbed early B-cell development.
TxA2-TP mediates the effect of COX-1 on B-cell development
We next investigated whether TxA2 mediates the effect of COX-1 on B-cell development. TP agonist U46619 and TP antagonist SQ29548 were used to modulate TP signaling in differentiating B cells. As expected, administration of SQ29548 in BM from WT mice significantly suppressed B-cell differentiation, whereas activation of TP by U46619 almost completely rescued the impaired B-cell differentiation in COX-1−/− mice to that seen in WT littermates, as evidenced by the frequencies of pre-B cells in BM cultures in vitro (Figure 5A).
TxA2-TP mediates the effect of COX-1 on B-cell development. (A) BM cells from WT and COX-1−/− mice were treated with the TP antagonist SQ29548 and the TP agonist U46619, respectively. Cells were then cultured for B-cell differentiation. The frequency of pre-B cells was evaluated by flow cytometric analysis. Both representative results from (left) 1 experiment and (right) the mean ± SEM of 3 independent experiments are shown. (B) WT or COX-1−/− mice (n = 6) were intraperitoneally injected with U46619 (5 μg/kg body weight) or phosphate-buffered saline twice weekly. The proportions of total B cells and pre-B cells were examined by flow cytometry 3 weeks after injection. (Left) Representative results from 1 mouse; the numbers in the plots indicate percentages of total living cells. (Right) Mean ± SEM from all 6 mice. (C) Purified B-cell subpopulations from BM of TP−/− and WT controls were stimulated with IL-7 for 15 minutes; the level of p-STAT5 was determined by flow cytometric analysis. (D) BM cells from WT and COX-1−/− mice were treated with the TP agonist U46619 or DMSO. Cells were then stimulated with IL-7 for 15 minutes. The levels of p-STAT5 in B220+ cells were determined by flow cytometric analysis. (C-D) (Left) Representative data from a single experiment. (Right) Mean ± SEM of MFI from 3 independent experiments. *P < .05 and **P < .01 using unpaired Student t tests.
TxA2-TP mediates the effect of COX-1 on B-cell development. (A) BM cells from WT and COX-1−/− mice were treated with the TP antagonist SQ29548 and the TP agonist U46619, respectively. Cells were then cultured for B-cell differentiation. The frequency of pre-B cells was evaluated by flow cytometric analysis. Both representative results from (left) 1 experiment and (right) the mean ± SEM of 3 independent experiments are shown. (B) WT or COX-1−/− mice (n = 6) were intraperitoneally injected with U46619 (5 μg/kg body weight) or phosphate-buffered saline twice weekly. The proportions of total B cells and pre-B cells were examined by flow cytometry 3 weeks after injection. (Left) Representative results from 1 mouse; the numbers in the plots indicate percentages of total living cells. (Right) Mean ± SEM from all 6 mice. (C) Purified B-cell subpopulations from BM of TP−/− and WT controls were stimulated with IL-7 for 15 minutes; the level of p-STAT5 was determined by flow cytometric analysis. (D) BM cells from WT and COX-1−/− mice were treated with the TP agonist U46619 or DMSO. Cells were then stimulated with IL-7 for 15 minutes. The levels of p-STAT5 in B220+ cells were determined by flow cytometric analysis. (C-D) (Left) Representative data from a single experiment. (Right) Mean ± SEM of MFI from 3 independent experiments. *P < .05 and **P < .01 using unpaired Student t tests.
The rescue effect of TP agonist on B-cell differentiation in COX-1−/− mice was further confirmed in vivo. U46619 was administered to COX-1−/− and WT mice by intraperitoneal injection twice weekly. B-cell phenotype was evaluated by flow cytometry 3 weeks after injection. Results showed that the frequencies of total B cells and pre-B cells in BM from COX-1−/− mice were recovered to the levels of those in phosphate-buffered saline–treated WT mice, after administration of TP agonist U46619 (Figure 5B). These observations strongly indicated that TxA2 mediates the effect of COX-1 on B-cell development through the TP receptor.
Based on our observation that JAK/STAT5 signaling mediated the impaired B-cell development caused by COX-1 deletion, we were then interested to establish whether JAK/STAT5 signaling was regulated by COX-1–derived TxA2. Results showed that the level of p-STAT5 in sorted pro-B and pre-B cells from TP−/− mice was markedly reduced on IL-7 stimulation compared with the corresponding control cells from WT mice (Figure 5C). Expression of the STAT5 target gene Pax5 showed a similar trend (data not shown). The TP agonist enhanced the p-STAT5 level in B cells from WT mice, whereas a TP antagonist displayed reverse change (supplemental Figure 7). More importantly, the TP agonist could rescue defective JAK/STAT5 signaling in B cells from COX-1−/− mice (Figure 5D). These observations demonstrated that JAK/STAT5 signaling was subject to regulation by the TxA2-TP axis in developing B cells.
JAK/STAT5 signaling is regulated by TxA2-TP through cAMP-PKA axis in B cells
On binding to TxA2, TP couples with G-proteins and elicits the activation of intracellular effector molecules and signaling pathways, including at least adenylyl cyclase (AC), phosphatidylinositol-specific phospholipase C, and small G-protein ρ-specific guanine nucleotide exchange factor.25,26 AC catalyzes the production of the second messenger cAMP, which activates downstream kinases, primarily PKA; phosphatidylinositol-specific phospholipase C leads to elevation of intracellular Ca2+ concentration and activation of phospholipase C (PKC); and small G-protein ρ-specific guanine nucleotide exchange factor activates the ρ-mediated signaling.27,28 To investigate which molecule mediates the regulatory effect of TxA2-TP on JAK/STAT5 signaling, we used specific inhibitors for these molecules in WT BM cells pretreated with the TP agonist U46619. Flow cytometric analysis showed that the PKA inhibitor H89 clearly counteracted the effect of U46619 on B-cell differentiation, which was almost comparable to the effect of JAK3 inhibitor WHI-P131. Other inhibitors, however, did not display any noticeable effects (Figure 6A). Consistently, administration of H89 abrogated the effect of TP agonist on STAT5 phosphorylation (Figure 6B) and Pax5 expression (data not shown). These observations support the possibility that TP may function through PKA in regulating JAK/STAT5 signaling in developing B cells.
JAK/STAT5 signaling is regulated by TxA2-TP through cAMP-PKA axis in B cells. (A) BM cells from naive mice were pretreated with the TP agonist U46619 (10 μM) or DMSO for 15 minutes, followed by administration with the indicated pathway inhibitors. Cells were then cultured under conditions to induce B-cell differentiation. The proportions of pre-B cells (IgM−CD43−B220+) were analyzed by flow cytometry after a 6-day culture. The concentration of PKA inhibitor was 5 μM, PKC inhibitor chelerythrine chloride (CC) was 1 μM, and ROCK inhibitor Y-27632 was 5 μM. The JAK3 inhibitor WHI-P131 (15 μM) was used as the positive control. (Left) Representative data from a single experiment; numbers in the plots indicate the percentage of pre-B cells among total living cells. (Right) Mean ± SEM of 3 independent experiments. (B) BM cells from naive mice were pretreated with TP agonist U46619 or DMSO for 15 minutes, followed by administration of PKA inhibitor H89 or vehicle for another 15 minutes; B220+ cells were gated, and the levels of p-STAT5 was determined by flow cytometric analysis. (Upper) Representative data from a single experiment. (Lower) Mean ± SEM of MFI from 3 independent experiments. (C) B220+ cells from BM of naive mice were stimulated with arachidonic acid (1 μM) for 30 minutes, followed by the indicated treatments for 15 minutes. The (upper) intracellular cAMP level and (lower) PKA activity were measured by enzyme-linked immunosorbent assay. TP agonist, U46619 (10 μM); adenylyl cyclase (AC) inhibitor, MDL 12330A (20 μM). The AC activator forskolin (Fsk, 10 μM) was used as the positive control. (D) B220+ cells from COX-1−/− and WT mice BM were stimulated with arachidonic acid for 30 minutes, followed by treatment with U46619 or vehicle for 15 minutes. The cellular cAMP levels and PKA activity were measured by enzyme-linked immunosorbent assay. (C-D) The results are presented as mean ± SEM from 3 independent experiments. PKA activity was normalized against the control group. (E) BM cells were cultured with IL-7 and treated with JAK3 inhibitor WHI-P131 (15 μM) or vehicle. Cells were then administrated with or without PKA activator 6-Bnz-cAMP (200 μM) for 15 minutes. The levels of p-STAT5 in B220+ cells were examined by flow cytometric analysis. Both representative results from (upper) a single experiment and (lower) mean ± SEM of MFI from 3 independent experiments were included. (F) BM cells were stimulated with IL-7 for 15 minutes, following with treatments of PKA activator 6-Bnz-cAMP and/or PKA inhibitor H89 (5 μM) for 15 minutes. The phosphorylation levels of JAK3 and JAK1 were determined by immunoblotting. (G) BM cells from COX-1−/− and WT mice were stimulated with IL-7 for 15 minutes, followed by treatment in the presence or absence of U46619 for 15 minutes. Immunoblotting was used to determine the phosphorylation of JAK3. (F-G) Results are representative of 3 independent experiments. (Right) The relative intensity of WB bands normalized to β-actin. (A-E) *P < .05 and **P < .01, using unpaired Student t tests and 1-way ANOVA.
JAK/STAT5 signaling is regulated by TxA2-TP through cAMP-PKA axis in B cells. (A) BM cells from naive mice were pretreated with the TP agonist U46619 (10 μM) or DMSO for 15 minutes, followed by administration with the indicated pathway inhibitors. Cells were then cultured under conditions to induce B-cell differentiation. The proportions of pre-B cells (IgM−CD43−B220+) were analyzed by flow cytometry after a 6-day culture. The concentration of PKA inhibitor was 5 μM, PKC inhibitor chelerythrine chloride (CC) was 1 μM, and ROCK inhibitor Y-27632 was 5 μM. The JAK3 inhibitor WHI-P131 (15 μM) was used as the positive control. (Left) Representative data from a single experiment; numbers in the plots indicate the percentage of pre-B cells among total living cells. (Right) Mean ± SEM of 3 independent experiments. (B) BM cells from naive mice were pretreated with TP agonist U46619 or DMSO for 15 minutes, followed by administration of PKA inhibitor H89 or vehicle for another 15 minutes; B220+ cells were gated, and the levels of p-STAT5 was determined by flow cytometric analysis. (Upper) Representative data from a single experiment. (Lower) Mean ± SEM of MFI from 3 independent experiments. (C) B220+ cells from BM of naive mice were stimulated with arachidonic acid (1 μM) for 30 minutes, followed by the indicated treatments for 15 minutes. The (upper) intracellular cAMP level and (lower) PKA activity were measured by enzyme-linked immunosorbent assay. TP agonist, U46619 (10 μM); adenylyl cyclase (AC) inhibitor, MDL 12330A (20 μM). The AC activator forskolin (Fsk, 10 μM) was used as the positive control. (D) B220+ cells from COX-1−/− and WT mice BM were stimulated with arachidonic acid for 30 minutes, followed by treatment with U46619 or vehicle for 15 minutes. The cellular cAMP levels and PKA activity were measured by enzyme-linked immunosorbent assay. (C-D) The results are presented as mean ± SEM from 3 independent experiments. PKA activity was normalized against the control group. (E) BM cells were cultured with IL-7 and treated with JAK3 inhibitor WHI-P131 (15 μM) or vehicle. Cells were then administrated with or without PKA activator 6-Bnz-cAMP (200 μM) for 15 minutes. The levels of p-STAT5 in B220+ cells were examined by flow cytometric analysis. Both representative results from (upper) a single experiment and (lower) mean ± SEM of MFI from 3 independent experiments were included. (F) BM cells were stimulated with IL-7 for 15 minutes, following with treatments of PKA activator 6-Bnz-cAMP and/or PKA inhibitor H89 (5 μM) for 15 minutes. The phosphorylation levels of JAK3 and JAK1 were determined by immunoblotting. (G) BM cells from COX-1−/− and WT mice were stimulated with IL-7 for 15 minutes, followed by treatment in the presence or absence of U46619 for 15 minutes. Immunoblotting was used to determine the phosphorylation of JAK3. (F-G) Results are representative of 3 independent experiments. (Right) The relative intensity of WB bands normalized to β-actin. (A-E) *P < .05 and **P < .01, using unpaired Student t tests and 1-way ANOVA.
We next investigated whether TP could elicit cAMP-PKA events in B cells, Data showed that the TP agonist U46619 markedly increased the intracellular cAMP level and PKA kinase activity in B cells stimulated with arachidonic acid, which could be virtually completely abrogated by the AC inhibitor MDL12330A; the AC activator forskolin stimulated the activation of the cAMP-PKA axis as expected (Figure 6C). In line with this, B cells from COX-1−/− mice displayed marked downregulation of cAMP-PKA signaling, whereas the TP agonist could recover this defect (Figure 6D). These observations suggested that TP functionally couples with the Gs α protein in B cells.
We next asked whether PKA phosphorylated STAT5 directly or through the upstream kinase JAKs. Data showed that the PKA activator 6-Bnz-cAMP enhanced the level of p-STAT5 on IL-7 stimulation compared with the corresponding control cells, whereas pretreatment with the JAK3 inhibitor WHI-P131 abrogated this effect (Figure 6E). These results indicated that PKA phosphorylates STAT5 through JAK3. Further immunoblotting experiments demonstrated that the PKA activator led to an increase in phosphorylation of JAK3 (tyrosine 980/981), but not JAK1, whereas a PKA inhibitor reversed this effect (Figure 6F). Consistently, we observed a clear reduction in the level of p-JAK3 in IL-7–stimulated BM cells from COX-1−/− mice compared with that in WT controls. The TP agonist, however, abrogated this difference (Figure 6G). These results indicate that JAK3 is a potential substrate of PKA in B cells. Collectively, these observations demonstrated that the JAK/STAT5 pathway is regulated by TxA2-TP through the cAMP-PKA axis in B cells.
Low-dose aspirin causes a significant reduction in B cells in humans
Low-dose (50-100 mg/day) aspirin has a clinically well-established effect in reducing the incidence of myocardial infarction and stroke,29 which is attributed to the inhibition of COX-1–mediated TxA2 synthesis. To determine whether low-dose aspirin has any effects on human B-cell levels, we investigated the levels of B cells in peripheral blood from healthy volunteers (n = 8) before and after a 10-day low-dose aspirin regime. As expected, the TxA2 metabolites in the urine were markedly reduced in all participants after aspirin administration (supplemental Figure 8). Both the frequencies and absolute numbers of total B cells were decreased significantly after taking aspirin compared with those before administration (Figure 7A-B). Interestingly, we found that the B-cell levels correlated with urinary TxA2 metabolites, both before (r = 0.6154, P = .0332) and after (r = 0.7665, P = .0368) aspirin uptake. The reduction in B cell levels by aspirin was not caused by enhanced B-cell apoptosis (supplemental Figure 8). Instead, the phosphorylation of STAT5, as well as the expression of its target genes, decreased significantly in human B cells after aspirin (Figure 7C). Stimulation of human B cells with arachidonic acid clearly enhanced the activity of STAT5 signaling. In line with the absence of IL7R expression on human B cells, administration of IL-7 failed to display any effect on STAT5 signaling (Figure 7D). These data suggest an important role of arachidonic acid metabolites in the regulation of JAK/STAT5 signaling in human B cells. Collectively, our observations indicated a potential adverse effect of low-dose aspirin on B-cell development.
Low-dose aspirin causes a significant reduction in B cells in humans. (A-B) Healthy human volunteers (n = 8) were administered with 100 mg/day of aspirin for 10 days. PB was collected before and at the end of the aspirin regime. The levels of total B cells (CD19+IgD+CD38−) were evaluated by flow cytometric analysis. (A) Representative results from 1 individual, as well as (B) (left) frequencies and (right) absolute cell counts of B cells in all participants were shown. (C) (Left) Representative results of p-STAT5 level in human B cells before and after taking aspirin. (Center) MFI from 3 individuals. (Right) mRNA expression of STAT5 target genes in B cells by qRT-PCR (n = 3). (D) Human peripheral blood mononuclear cells were stimulated with arachidonic acid (1 μM), human IL-7 (10 ng/mL), or both; the level of pSTAT5 in B cells was evaluated by flow cytometry analysis. (Left) Results are representative of 3 independent experiments. (Right) Expression of STAT5 target genes in sorted B cells was determined by qRT-PCR; data are mean ± SEM from 3 independent experiments (right). *P < .05 and **P < .01, using paired Student t tests and 1-way ANOVA. (E) A model for the role of COX-1 in early B-cell development: during the transition from pro-B to pre-B stage in early B-cell development, COX-1–derived TxA2 could activate the JAK/STAT5 pathway through eliciting cAMP-PKA signaling, on binding with TP, which leads to the transcription of STAT5 target genes, including Pax5, and eventually promotes B-cell development.
Low-dose aspirin causes a significant reduction in B cells in humans. (A-B) Healthy human volunteers (n = 8) were administered with 100 mg/day of aspirin for 10 days. PB was collected before and at the end of the aspirin regime. The levels of total B cells (CD19+IgD+CD38−) were evaluated by flow cytometric analysis. (A) Representative results from 1 individual, as well as (B) (left) frequencies and (right) absolute cell counts of B cells in all participants were shown. (C) (Left) Representative results of p-STAT5 level in human B cells before and after taking aspirin. (Center) MFI from 3 individuals. (Right) mRNA expression of STAT5 target genes in B cells by qRT-PCR (n = 3). (D) Human peripheral blood mononuclear cells were stimulated with arachidonic acid (1 μM), human IL-7 (10 ng/mL), or both; the level of pSTAT5 in B cells was evaluated by flow cytometry analysis. (Left) Results are representative of 3 independent experiments. (Right) Expression of STAT5 target genes in sorted B cells was determined by qRT-PCR; data are mean ± SEM from 3 independent experiments (right). *P < .05 and **P < .01, using paired Student t tests and 1-way ANOVA. (E) A model for the role of COX-1 in early B-cell development: during the transition from pro-B to pre-B stage in early B-cell development, COX-1–derived TxA2 could activate the JAK/STAT5 pathway through eliciting cAMP-PKA signaling, on binding with TP, which leads to the transcription of STAT5 target genes, including Pax5, and eventually promotes B-cell development.
Discussion
Besides their well-established effects on inflammation and the cardiovascular system, emerging roles for COXs and their metabolites in the immune system are gaining increasing attention in recent years, especially for COX-2 and its metabolites.30-33 The roles of COX-1 in immune system, however, are far from being understood.34,35 In this study, we demonstrated a novel role of COX-1 in early B-cell development and uncovered the underlying molecular mechanism.
IL-7–induced JAK/STAT5 signaling plays fundamental roles in the regulation of B-cell lineage commitment and development.10 The mechanisms regulating the activity of JAK/STAT5 signaling in B cells remains unclear. In this study, we found that COX-1–derived TxA2 could regulate JAK/STAT5 signaling via cAMP-PKA, on binding its receptor TP. The expression of both COX-1 and TP is inducible in developing B cells, which causes the activation of the JAK/STAT5 pathway through the intracellular cAMP-PKA event and finally leads to B-cell development. A model for the role of COX-1 in the regulation of early B-cell development is proposed in Figure 7E. The mechanisms regulating the transcription of COX-1 and TP in developing B cells, however, remain to be investigated.
TxA2 has established platelet aggregating and vessel-contracting activities.27 To date, reports about the role of TxA2 in the immune system have been limited. TxA2 plays a key role in neutrophil-mediated T-cell immune responses to infections and vaccinations.36 TxA2-TP signaling also negatively regulates dendritic cell–T cell interaction and modulates acquired immunity.37 Interaction between TxA2 with its receptor in thymic microenvironment may be important in the apoptosis of activated T cells.38 The role of the TxA2-TP axis in B-cell biology remains poorly understood. We found that TP-deficient mice display disturbed early B-cell development and that administration of a TP agonist or antagonist could modulate B-cell differentiation. These observations demonstrated that TxA2-TP signaling plays essential roles in early B-cell development.
The sources of TxA2 production and the manner in which TxA2 exerts its role on developing B cells in vivo is an interesting question. A variety of cell types in the BM microenvironment could produce TxA2, including dendritic cells, T cells, macrophages, and stromal cells.37-39 Deletion of COX-1 selectively impairs the production of TxA2 in B cells but not in T cells (data not shown), supporting the possibility that TxA2 functions in an autocrine manner in developing B cells. In vitro culture of purified B cells using a stromal-free system would help to clarify whether B cells produce TxA2 and activate the signaling axis we observed. In contrast with a previous report that B cells from mouse spleen express very low levels of TP,37 we found that TP expression in B cells from mouse BM was abundant and much higher than those from the spleen (data not shown); more interestingly, TP expression was dynamic during the course of B-cell development. These observations raise the possibility that some stimuli within the BM may induce the transcription of genes essential for TxA2 signaling in B cells, which facilitate B-cell development.
Our investigations showed that PKA plays a dominant role in the regulation of the JAK/STAT5 signal, in the presence of IL-7 stimulation, with JAK3 serving as the substrate of PKA in this axis. It has been reported that the cAMP-PKA pathway could negatively regulate JAK3/STAT5 activation in response to IL-2 stimulation in T lymphocytes, and functionally, inactivation of JAK3 by PKA is the mechanism.40,41 Our study demonstrated that PKA positively regulates JAK/STAT5 signaling in developing B cells, and PKA can activate JAK3 through tyrosine phosphorylation. It is therefore plausible that distinct cell types use different mechanisms in regulating the same signaling molecule, which may be context dependent.
The immunoregulatory effects of aspirin are gaining increasing attention.29,36 Aspirin is generally considered to have immune-suppressive capabilities.42 Clinical administration of aspirin has beneficial effects on certain autoimmune diseases,43 as well as allograft rejection.44 Aspirin and other nonsteroidal anti-inflammatory drugs were reported to suppress the humoral immune response by impairing antibody synthesis.45,46 The mechanisms underlying aspirin’s immunomodulatory effects, however, deserve further exploration. Our study indicates that low-dose aspirin may suppress early B-cell development, providing an alternative explanation for the beneficial effects of aspirin in some autoimmune diseases. Given that low-dose aspirin has been routinely prescribed to prevent myocardial infarction and stroke,29 our work raises concerns about the potential adverse effect of this maneuver on B-cell development.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Dr Toshio Kitamura (University of Tokyo, Tokyo, Japan) for providing the constitutively activated STAT5 construct (STAT5A1*6).
This work was supported by the following grants to J.Z.: National Key Basic Research Program of China grant 2012CB524900; Guangdong Innovative Research Team Program grant 2009010058; Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme grant GDUPS, 2014; Key Research Projects of National 12th Five-year Plan for the Prevention and Treatment of Major Infectious Diseases grant 2012ZX10001003; National Natural Science Foundation of China grants 81072397 and 31270921; Natural Science Foundation of Guangdong grant S2011020006072; and Fundamental Research Funds for the Central Universities, 111 Project grant B12003. Y.Y. was supported by the following grants: Ministry of Science and Technology of China grants 2012CB945100, 2011CB503906, and 2011ZX09307-302-01; National Natural Science Foundation of China grants 81030004 and 31200860; National Natural Science Foundation of China–Canadian Institutes of Health Research joint grant 81161120538; and One Hundred Talents Program of the Chinese Academy of Sciences grant 2010OHTP10. Y.Y. is a Fellow at the Jiangsu Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Authorship
Contribution: Q.Y. carried out the experiments, analyzed the data, and participated in figure organization and manuscript writing; M.S. and S.Z. performed the bone marrow transplantation experiment; Y.C. assisted with the low-dose aspirin experiment; Y.S. helped with mouse strain maintaining; C.Z. performed mass spectrometry assay; H.Z. and D.I.G. provided helpful suggestions in the experimental design and manuscript writing; Y.Y. provided all the mouse strains, participated in experimental design, and edited the manuscript; and J.Z. designed the overall study, supervised all the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jie Zhou, Institute of Human Virology, Zhongshan School of Medicine, Sun Yat-Sen University, 74 Zhongshan 2nd Rd, Guangzhou 510080, China; e-mail: zhouj72@mail.sysu.edu.cn; or Ying Yu, Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 294 Taiyuan Rd, Shanghai 200031, China; e-mail: yuying@sibs.ac.cn.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal