Key Points
ALPS predisposes to invasive bacterial infections, notably following splenectomy, and is associated with defective B-cell function.
Poor anti-polysaccharide IgM antibody production and spleen MZ disorganization correlate with lymphoproliferation.
Abstract
Autoimmune lymphoproliferative syndrome (ALPS) caused by impaired FAS-mediated apoptosis of lymphocytes is characterized by lymphoproliferation, autoimmunity, but also an increased risk of invasive bacterial infection, notably following splenectomy. We surveyed a cohort of 100 ALPS patients (including 33 splenectomized) and found that 12 (10 splenectomized) had experienced 23 invasive bacterial infections mainly caused by Streptococcus pneumoniae. This vulnerability was associated with evidence of defective B-cell function characterized by low serum immunoglobulin (Ig) M, low IgM antibody production in response to S pneumoniae following nonconjugated immunization, and low blood memory B-cells counts (including marginal zone [MZ] B-cell counts). This immunodeficiency strongly correlated with intensity of lymphoproliferation. Spleen sections from 9 ALPS patients revealed double-negative T-cell (DN-T) infiltration of the MZ, which was depleted of B cells. MZ in ALPS patients contained an abnormally thick layer of MAdCAM-1(+) stromal cells and an excess of DN-Ts. DN-Ts were shown to express MAdCAM-1 ligand, the α4β7 integrin. These observations suggest that accumulating DN-Ts are trapped within stromal cell meshwork and interfere with correct localization of MZ B cells. Similar observations were made in spleens of fas-deficient mice. Our data revealed an unexpected mechanism by which ALPS results in anti-polysaccharide IgM antibody production–specific defect. Splenectomy should be avoided.
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) is characterized by early onset lymphoproliferation and (in ∼70% of patients) autoimmune cytopenia. In most cases, ALPS is the consequence of germ-line or somatic mutations of the TNFRSF6 FAS death receptor gene (ALPS-FAS and ALPS-sFAS, respectively).1-3 Elevated counts of circulating TCRαβ+ double-negative CD4−CD8− T lymphocyte cells (DN-Ts) are hallmarks of the disease. Splenomegaly and lymphadenopathy develop early in life, notably as a consequence of DN-Ts accumulation in paracortical areas.4 There is marked expansion of both the white and red pulp of the spleen, which becomes filled with DN-Ts.4 In some ALPS patients, splenectomy can be used to treat refractory cytopenia related to autoimmunity or hypersplenism.5 Studies of the clinical and immunologic characteristics of ALPS-FAS have identified an elevated risk of severe postsplenectomy bacterial infection5-8 ; indeed, up to 30% of splenectomized patients experienced invasive bacterial infections. No other susceptibilities to infection have been noted in these patients. The B-cell compartment and B-cell function have not been extensively studied in ALPS patients. Immunoglobulin (Ig) G and IgA hypergammaglobulinemia is commonly observed, whereas hypogammaglobulinemia has been reported in few patients.5,9
The spleen exerts a key role in host defense against blood-borne pathogens, notably encapsulated bacteria.10 Susceptibility to fulminant, potentially life-threatening infections with encapsulated bacteria is a major risk associated with splenectomy and congenital asplenia.11 The postsplenectomy infection and mortality rates vary from study to study and depend on age at splenectomy, nature of the underlying disease, and duration of follow-up.11-14 The spleen comprises red pulp (an open circulatory system of blood-filled spaces known as splenic cords) and white pulp (the lymphoid compartment).15 The latter is composed of T- and B-cell compartments. T lymphocytes are concentrated in periarteriolar lymphoid sheathes (PALSs), close to B-cell follicles surrounded by the marginal zone (MZ) and perifollicular zone (PFZ). The MZ and PFZ are strategically located at the interface between the white pulp and the circulation and serve as transit areas. The MZ is the niche for a B-cell subset. Along with other resident cells (such as macrophages, dendritic cells, and granulocytes), these MZ B cells are enmeshed within a network of stromal cells.16 When comparing humans and rodents, the splenic microvasculature and white pulp architecture have both similarities and differences.15,17 MZ B cells have a unique ability to produce natural antibodies and can initiate T-cell–independent immune responses against infections or vaccination with capsular polysaccharide antigens.18-22 The MZ B cells are able to recirculate in the periphery (at least in humans). Children under the age of 2 years display a low circulating MZB cells count, which is considered to be related to MZ immaturity.23
In the present work, we sought to describe the immunologic basis of the susceptibility to infections observed in ALPS patients in general and splenectomized ALPS patients in particular. To this end, we examined blood and splenic B-cell characteristics in a cohort of ALPS-FAS patients and in asymptomatic FAS mutation carriers.
Patients, materials, and methods
Study populations
All study participants met criteria for ALPS-FAS or ALPS-sFAS (mosaic patients with heterozygous somatic FAS mutations).24 Overall, 3 groups of patients were studied: ALPS-FAS patients with homozygous germ-line TNFRSF6 mutations (n = 5), ALPS-FAS and ALPS-sFAS patients carrying heterozygous germ-line or somatic TNFRSF6 mutations (n = 95), and asymptomatic TNFRSF6 mutation–positive relatives (MPRs, n = 16). The main characteristics of these patients have been reported elsewhere.5 Patient cases are identified by numbers. Patients from the same family are identified by letters following the number. Patients with ALPS related to heterozygous germ-line FAS mutation are indicated “P,” patients with homozygous TNFRSF6 mutations are indicated “P(HZ),” and patients with ALPS-sFAS as “Pm.” Each patient’s personal medical history (age at onset, lymphoproliferation, autoimmunity, treatment modalities, splenectomy status, etc.) was noted with special attention to compliance with prophylactic anti-infective recommendations in splenectomized patients (postsplenectomy prophylaxis for at least 5 years in children and at least 2 years in adults, and up-to-date vaccination schedules for Streptococcus pneumoniae, Hemophilus influenzae, and Neisseria meningitidis), the occurrence of invasive bacterial infections (defined as bacteremia and/or life-threatening infection), and documented bacteriologic information. Data on disease biomarkers (DN-Ts and plasma interleukin 10 and FAS-ligand [FAS-L] levels)25,26 were also collected. Thirty-three patients had undergone splenectomy at a median age of 10 years (range: 0.5-43 years). The median follow-up time since splenectomy was 14 years (range: 2-52 years), and the cumulative follow-up time since splenectomy was 588 years. Three additional patients had undergone a partial splenectomy. The characteristics of splenectomized patients are summarized in supplemental Table 1 (see the Blood Web site). The study was performed in accordance with the Declaration of Helsinki and was approved by the Necker Hospital Review Board.
Spleen specimen
A total of 9 spleen specimens from ALPS patients and 3 control spleens were studied for histology and immunohistochemistry. The patients’ clinical characteristics, indications for splenectomy, and treatments prior to splenectomy are given in supplemental Table 2. Only 1 patient (P-46) received immunosuppressive treatment prior to splenectomy. Control spleens (C1 to C3) were obtained from children aged 2, 5, and 6 years, respectively. The indications for splenectomy were congenital cysts of the spleen in C1, sickle cell disease in C2, and trauma in C3. In addition, splenocytes from 3 ALPS patients (Pm-1, Pm-10, and P-46) and 1 additional control (a 6-year-old child with spherocytosis) were available for flow cytometry analysis. Cryostats from 2 ALPS patients (Pm-1 and Pm-2) and 1 control spleen (C1) were available for immunofluorescence studies.
Methods
Methods used for flow cytometry studies (peripheral blood mononuclear cells and splenocytes), human spleen immunohistology and immunofluorescence studies, antibody measurement (anti–S pneumoniae antibody response and anti-phosphatidylcholine), and mice studies are given in the supplemental Methods.
Statistical analysis
All analyses were performed using PRISM software (GraphPad software). Populations were compared using a 2-tailed Student t test or a Mann-Whitney U test. For correlation between biomarkers (FAS-L and DN-Ts) and memory B-cell counts, a Spearman correlation coefficient was calculated. The threshold for statistical significance was set to P < .05.
Results
Patients with ALPS are exposed to a high risk of invasive bacterial infection
We retrospectively screened our ALPS cohorts for invasive bacterial infections. Twelve of the 100 ALPS patients presented a total of 23 episodes of severe bacterial infections (Table 1). Two nonsplenectomized patients had invasive S pneumoniae infections during their first year of life. Pm-1 (ALPS-sFAS) had never received any immunosuppressive drugs but had S pneumoniae meningitis at 9 months of age. P(HZ)-3 (homozygous ALPS-FAS) had S pneumoniae sepsis at 6 months of age. The prevalence of an invasive S pneumoniae infection under the age of 2 in our cohort of ALPS patients was thus 2%.
Characteristics of invasive bacterial infections in ALPS patients
| Infection . | ALPS manifestations* . | Age of onset (y) . | Age at splenect (y) . | Age at infection (y) . | Elapsed time from splenect (y) . | Infectious agent . | Prophylaxis . | Last immunization (y) . | Ongoing treatment at time of infection . | Previous treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before splenectomy | |||||||||||
| Pm-1 | SM ADP ITP NHL | 0.2 | 6 | 0.8 | NA | S pneumoniae | — | None | None | None | Alive |
| P(HZ)-3 | SM ADP AIHA NHL | <0.5 | NA | 0.5 | NA | S pneumoniae | — | None | None | None | Alive |
| After splenectomy | |||||||||||
| Index cases | |||||||||||
| P-lb | SM | 3 | 13 | 27 | 14 | NI | P | 5 | None | None | Died |
| P-9 | HSM ITP | 6 | 12 | 35 | 23 | S agalactiae | None | 3 | None | None | Alive |
| P-14 | Hydrops, HSM, ADP Osteoporosis | 2.5 | 2 (×2) | S pneumoniae | P | 1.5 | 6MP | 6MP from 0.5 y, ongoing HSCT (6 y)* | Alive | ||
| Birth | 0.5 | 10.5 | 10 (×2) | NI | P | <2 | 6MP | Alive | |||
| 12.5 | 12 | S pneumoniae | P/TS/IVIG | <2 | 6MP | Alive | |||||
| P-35 | SM ADP AIHA AIN ITP | 1.5 | 3 | 14 | 11 | S pneumoniae | P | 5 | None | None | Alive |
| P-46 | SM ADP AIHA | 0.3 | 3 | 4.8 | 1.8 | S pneumoniae | P | 2 | None | CS anti-CD20** azathioprin | Died |
| Pm-16 | SM AIHA | 1.5 | 4.5 | 5.5 | 1 | S pneumoniae | None*** | 1 | None | None | Alive |
| 11 | 6.5 | S pneumoniae | Cephalo | 7.5 | None | None | Alive | ||||
| HL | 23 | 4.5 | 27 | 22.5 (×2) | S pneumoniae | None | 0.2 | None | Chemotherapy | Alive | |
| Relatives | |||||||||||
| P-4b | SM ADP | 13 | 14 | 37 | 23 | S pneumoniae | — | >5 | None | None | Alive |
| P-18b | HL | 14 | 14 | 38 | 24 | S pneumoniae | — | <5 | None | Radiotherapy chemotherapy | Died |
| P-75c | HSM ADP Anemia ITP | <2 | 2 | 4, episodes 4-6 | 2-4 | S pneumoniae | — | None | None | None | Alive |
| NHL | 38 | 46 | 44 | S pneumoniae | — | >5 | None | Axillar local radiotherapy | Alive | ||
| P-86b | SM anemia | Infancy | 6 | 34 | 28 | S pneumoniae | — | None | None | None | Died |
| Infection . | ALPS manifestations* . | Age of onset (y) . | Age at splenect (y) . | Age at infection (y) . | Elapsed time from splenect (y) . | Infectious agent . | Prophylaxis . | Last immunization (y) . | Ongoing treatment at time of infection . | Previous treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before splenectomy | |||||||||||
| Pm-1 | SM ADP ITP NHL | 0.2 | 6 | 0.8 | NA | S pneumoniae | — | None | None | None | Alive |
| P(HZ)-3 | SM ADP AIHA NHL | <0.5 | NA | 0.5 | NA | S pneumoniae | — | None | None | None | Alive |
| After splenectomy | |||||||||||
| Index cases | |||||||||||
| P-lb | SM | 3 | 13 | 27 | 14 | NI | P | 5 | None | None | Died |
| P-9 | HSM ITP | 6 | 12 | 35 | 23 | S agalactiae | None | 3 | None | None | Alive |
| P-14 | Hydrops, HSM, ADP Osteoporosis | 2.5 | 2 (×2) | S pneumoniae | P | 1.5 | 6MP | 6MP from 0.5 y, ongoing HSCT (6 y)* | Alive | ||
| Birth | 0.5 | 10.5 | 10 (×2) | NI | P | <2 | 6MP | Alive | |||
| 12.5 | 12 | S pneumoniae | P/TS/IVIG | <2 | 6MP | Alive | |||||
| P-35 | SM ADP AIHA AIN ITP | 1.5 | 3 | 14 | 11 | S pneumoniae | P | 5 | None | None | Alive |
| P-46 | SM ADP AIHA | 0.3 | 3 | 4.8 | 1.8 | S pneumoniae | P | 2 | None | CS anti-CD20** azathioprin | Died |
| Pm-16 | SM AIHA | 1.5 | 4.5 | 5.5 | 1 | S pneumoniae | None*** | 1 | None | None | Alive |
| 11 | 6.5 | S pneumoniae | Cephalo | 7.5 | None | None | Alive | ||||
| HL | 23 | 4.5 | 27 | 22.5 (×2) | S pneumoniae | None | 0.2 | None | Chemotherapy | Alive | |
| Relatives | |||||||||||
| P-4b | SM ADP | 13 | 14 | 37 | 23 | S pneumoniae | — | >5 | None | None | Alive |
| P-18b | HL | 14 | 14 | 38 | 24 | S pneumoniae | — | <5 | None | Radiotherapy chemotherapy | Died |
| P-75c | HSM ADP Anemia ITP | <2 | 2 | 4, episodes 4-6 | 2-4 | S pneumoniae | — | None | None | None | Alive |
| NHL | 38 | 46 | 44 | S pneumoniae | — | >5 | None | Axillar local radiotherapy | Alive | ||
| P-86b | SM anemia | Infancy | 6 | 34 | 28 | S pneumoniae | — | None | None | None | Died |
6MP, 6-mercaptopurine; ADP, adenopathy; AIHA, autoimmune hemolytic anemia; AIN, autoimmune neutropenia; anti-CD20, anti-CD20 monoclonal antibodies; cephalo, cephalosporin; CS, corticosteroids; HL, Hodgkin lymphoma; HSCT, hematopoietic sterm cell transplantation; ITP, idiopathic thrombocytopenic purpura; IVIG, intravenous immunoglobulin, NHL, non-Hodgkin lymphoma; P, penicillin; SM, spenomegaly; Splenect, splenectomy; TS, trimethoprim-sulfamethoxazol.
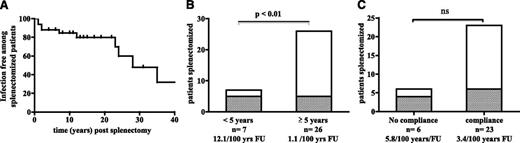
Ten splenectomized patients developed a total of 21 episodes of sepsis (7 patients had 1 episode, and 3 patients had more than 1 episode). Thus, 10 out of 33 splenectomized patients had sepsis, yielding a risk of postsplenectomy infection of 3.6 per 100 patient years of follow-up (Figure 1A). S pneumoniae was the most frequent causal microorganism, whereas Streptococcus agalactiae was identified once. In 3 of the 21 episodes of sepsis, the causative microorganism could not be identified. The median time interval between splenectomy to infection was 10 years (range: 1-44 years). Four of the 33 patients died as a result of an infection (12.1%). Young age at splenectomy was associated with a high risk of infection because 5 of the 7 patients splenectomized under the age of 5 years had invasive bacterial infections (Figure 1B). Three of these patients had several infectious episodes (4 in Pm-16, 5 in P-14, and 5 in P-75c). The risk of infections was thus 12.1 per 100 patient years of follow-up in patients splenectomized before the age of 5 years and 1.1 per 100 for patients splenectomized after that age. It is noteworthy that 2 ALPS-FAS homozygous patients splenectomized at 6 months of age who subsequently underwent hematopoietic stem cell transplantation with full donor chimerism had not developed invasive bacterial infection at last follow-up (10 and 14 years, respectively). Immunosuppressive treatment of splenectomized patients did not appear as an additional risk factor for invasive bacterial infection (data not shown). In view of the unusually high susceptibility of ALPS patients to invasive bacterial infections, we then searched for the underlying pathological cause.
Frequency of sepsis in splenectomized ALPS patients and an analysis of risk factors. (A) Sepsis-free survival in 33 splenectomized ALPS patients. (B) Risk of sepsis as a function of age at splenectomy (<5 years or ≥5 years). In each column, the number of patients with sepsis is presented in gray (5 out of 7 patients splenectomized before the age of 5, and 5 out of 26 patients splenectomized at the age of 5 or older). The risk is 12.1 and 1.1 per 100 patient years of follow-up, respectively. (C) Risk of sepsis as a function of compliance with recommended prophylaxis (antibiotic prophylaxis for at least 5 years in children and at least 2 years in adults and up-to-date vaccination schedules for S pneumoniae, H influenzae, and N meningitidis). In each column, the number of patients with sepsis is presented in gray (4 out of 6 noncompliant patients and 6 out of 23 compliant patients). The risk is 5.8 and 3.4 per 100 patient years of follow-up, respectively.
Frequency of sepsis in splenectomized ALPS patients and an analysis of risk factors. (A) Sepsis-free survival in 33 splenectomized ALPS patients. (B) Risk of sepsis as a function of age at splenectomy (<5 years or ≥5 years). In each column, the number of patients with sepsis is presented in gray (5 out of 7 patients splenectomized before the age of 5, and 5 out of 26 patients splenectomized at the age of 5 or older). The risk is 12.1 and 1.1 per 100 patient years of follow-up, respectively. (C) Risk of sepsis as a function of compliance with recommended prophylaxis (antibiotic prophylaxis for at least 5 years in children and at least 2 years in adults and up-to-date vaccination schedules for S pneumoniae, H influenzae, and N meningitidis). In each column, the number of patients with sepsis is presented in gray (4 out of 6 noncompliant patients and 6 out of 23 compliant patients). The risk is 5.8 and 3.4 per 100 patient years of follow-up, respectively.
ALPS patients display a B-cell deficiency that is related to lymphoproliferation
The narrow-spectrum susceptibility to infection in ALPS patients prompted us to (1) rule out functional asplenia in nonsplenectomized ALPS patients and (2) screen for immunodeficiencies that conferred a risk of invasive S pneumoniae infection. In a peripheral blood smear, a screen for Howell-Joly bodies was negative in 10 nonsplenectomized ALPS patients including the 2 patients who had invasive S pneumoniae infection in infancy). The classic complement activation pathway (CH50) was normal (n = 11, including 4 patients who had invasive S pneumoniae infection). The response to Toll-like-receptor ligands was evaluated in 2 patients and was found to be normal (data not shown).
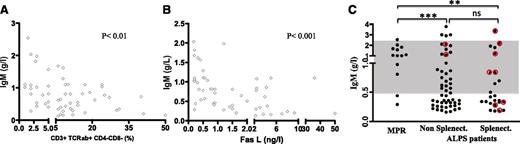
Patients were then investigated for possible antibody deficiencies. Low serum IgM is frequently noted in ALPS,5 whereas serum IgG and IgA levels tend to be elevated. In our cohort, approximately half of the patients (38 out of 73) displayed abnormally low serum IgM values during follow-up; low serum IgM was more pronounced in patients with active disease, as shown by the inverse correlation between elevated plasma FAS-L levels, the proportion of DN-Ts, and serum IgM levels (Figure 2A-B). Low serum IgM was not more frequent or more severe in splenectomized patients (Figure 2C).
Low serum IgM is a marker of ALPS disease activity. (A-B) The serum IgM level (g/L) is inversely correlated with (A) the proportion of circulating DN-Ts (CD3+TCRαβ+CD4−CD8−) and (B) the plasma FAS-L concentration (ng/L) measured concomitantly. (C) The serum IgM level (g/L) in asymptomatic MPRs and nonsplenectomized and splenectomized ALPS patients. In relevant patients, IgM levels at the time of sepsis are highlighted in red. The normal range is indicated by the gray zone.
Low serum IgM is a marker of ALPS disease activity. (A-B) The serum IgM level (g/L) is inversely correlated with (A) the proportion of circulating DN-Ts (CD3+TCRαβ+CD4−CD8−) and (B) the plasma FAS-L concentration (ng/L) measured concomitantly. (C) The serum IgM level (g/L) in asymptomatic MPRs and nonsplenectomized and splenectomized ALPS patients. In relevant patients, IgM levels at the time of sepsis are highlighted in red. The normal range is indicated by the gray zone.
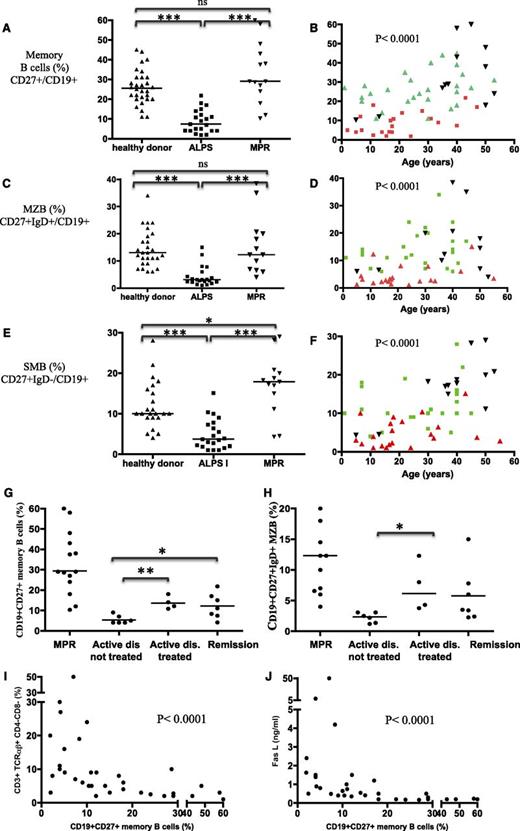
Blood B-cell subsets, naïve B cells (CD27−IgD+/CD19+), memory B cells (CD27+/CD19+), MZ B cells (CD27+IgD+/CD19+), and switched memory (SM) B cells (CD27+IgD−/CD19+) were analyzed in 21 ALPS patients, 16 MPRs, and 31 age-matched controls (Figure 3A-F). The 3 groups did not differ significantly in terms of the absolute B-cell count (data not shown). ALPS-FAS patients displayed low absolute counts (data not shown) and relative proportion of memory CD27+ B cells, MZ B cells, and SM B cells, relative to controls (P < .0001) (Figure 3A-F). Strikingly, CD27+ memory B-cell, MZ B-cell, and SM B-cell counts in MPRs were within the normal range (P = ns when compared with controls, and P < .0001 when compared with ALPS patients). CD27+ memory B-cell counts were significantly lower in splenectomized ALPS patients (P < .05) than in nonsplenectomized ALPS patients. The MZ B-cell counts were low in all patients, regardless of whether they were splenectomized (supplemental Figure 1A-B).
ALPS patients (but not asymptomatic, mutation-positive carriers) display decreased circulating memory B-cell counts, which correlate with levels of lymphoproliferation markers. The proportion of memory B cells (CD19+CD27+ cells) (A-B), MZ B cells (CD19+CD27+IgD+) (C-D), and SM B cells (CD19+CD27+IgD−) in healthy controls, ALPS patients, and asymptomatic MPRs (E-F). Values are plotted as a function of age for each group in panels B, D, and F. ns, nonsignificant. *P < .05; ***P < .001. In panels B, D, and F, healthy donors are plotted in green, ALPS patients in red, and MPR in black. Proportions of memory B cells CD19+CD27+ (G) and MZ B cells CD19+CD27+IgD+ (H) are depicted as a function of disease activity (nontreated patients with active disease, treated patients, and patients in remission) and compared with the values in asymptomatic MPRs. The proportion of CD19+CD27+ memory B cells was inversely correlated with (I) the proportion of circulating DN-Ts (CD3+TCRαβ+CD4−CD8−) and (J) the plasma FAS-L concentration (ng/L). *P < .05; **P < .01.
ALPS patients (but not asymptomatic, mutation-positive carriers) display decreased circulating memory B-cell counts, which correlate with levels of lymphoproliferation markers. The proportion of memory B cells (CD19+CD27+ cells) (A-B), MZ B cells (CD19+CD27+IgD+) (C-D), and SM B cells (CD19+CD27+IgD−) in healthy controls, ALPS patients, and asymptomatic MPRs (E-F). Values are plotted as a function of age for each group in panels B, D, and F. ns, nonsignificant. *P < .05; ***P < .001. In panels B, D, and F, healthy donors are plotted in green, ALPS patients in red, and MPR in black. Proportions of memory B cells CD19+CD27+ (G) and MZ B cells CD19+CD27+IgD+ (H) are depicted as a function of disease activity (nontreated patients with active disease, treated patients, and patients in remission) and compared with the values in asymptomatic MPRs. The proportion of CD19+CD27+ memory B cells was inversely correlated with (I) the proportion of circulating DN-Ts (CD3+TCRαβ+CD4−CD8−) and (J) the plasma FAS-L concentration (ng/L). *P < .05; **P < .01.
To test whether these abnormalities were related to disease activity (as suggested by B-cell phenotype of MPRs), the CD27+ memory B-cell and MZ B-cell counts were analyzed as a function of ALPS disease status (ie, active/remission) and treatment status (Figure 3G). Remission of lymphoproliferation (whether spontaneous or achieved through immunosuppressive therapy) was associated with higher counts of CD27+ memory B cells and MZ B cells (P < .05) (Figure 3G-H and supplemental Figure 1C). Similarly, there was an inverse correlation between plasma FAS-L levels, the proportion of DN-Ts, and the proportion of memory B cells (Figure 3I-J). To further validate the observed correlation between the abnormal peripheral B-cell subset distribution and disease activity, we retrospectively immunophenotyped peripheral blood mononuclear cells from frozen samples collected from the same patient (P-54) at the time of active lymphoproliferation and 18 months later (after 6MP therapy had resulted in a significant reduction in spleen size and levels of disease markers, including IgM) (supplemental Table 3). Clinical and biochemical improvements were accompanied by increased relative and absolute counts of CD27+ memory B cells and CD27+IgD+ MZ B cells.
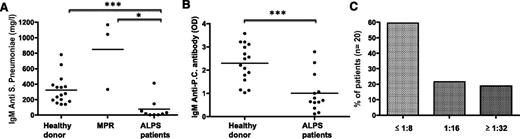
To assess the MZ B cells’ antibody response, nonconjugated pneumococcal vaccine was administered to 9 heterozygous ALPS patients (aged 2 to 18), 3 adults MPRs (see supplemental Table 4 for details about vaccinated subjects), and healthy controls. Specific anti-pneumococcal IgM antibodies were significantly lower in patients with active disease but were normal in the tested MPRs (Figure 4A), suggesting that the defect in specific IgM production was a consequence of disease activity rather than an intrinsic consequence of the TNFRSF6 mutation. It is noteworthy that the patients’ anti-pneumococcal IgG levels were similar to those observed in controls (data not shown). The ability to produce other natural IgM antibodies (such as anti-phosphatidylcholine and isohemagglutinin antibodies) was also significantly impaired (Figure 4B-C) in ALPS patients with active disease.
Nonsplenectomized ALPS patients display a low serum IgM antibody response after immunization with a nonconjugated pneumococcal vaccine. (A) Serum anti-pneumococcal IgM (“IgM antipneumo”) levels measured 3 to 4 weeks after immunization with a nonconjugated vaccine were measured in 16 healthy adult controls, 3 asymptomatic MPRs, and 9 nonsplenectomized ALPS patients. *P < .05; ***P < .001. Horizontal bars represent mean values. (B) Levels of anti-phosphatidylcholine IgM antibodies were measured in healthy controls and ALPS patients. Horizontal bars represent mean values. OD, optic density; P.C., phosphatidylcholine. (C) Isohemaglutinin IgM antiA and antiB measured in 20 patients (total of 37 values) showing the percentage of measures with a titer of ≤1:8, 1:16, or ≥1:32. Normal values in healthy controls are ≥1/32.
Nonsplenectomized ALPS patients display a low serum IgM antibody response after immunization with a nonconjugated pneumococcal vaccine. (A) Serum anti-pneumococcal IgM (“IgM antipneumo”) levels measured 3 to 4 weeks after immunization with a nonconjugated vaccine were measured in 16 healthy adult controls, 3 asymptomatic MPRs, and 9 nonsplenectomized ALPS patients. *P < .05; ***P < .001. Horizontal bars represent mean values. (B) Levels of anti-phosphatidylcholine IgM antibodies were measured in healthy controls and ALPS patients. Horizontal bars represent mean values. OD, optic density; P.C., phosphatidylcholine. (C) Isohemaglutinin IgM antiA and antiB measured in 20 patients (total of 37 values) showing the percentage of measures with a titer of ≤1:8, 1:16, or ≥1:32. Normal values in healthy controls are ≥1/32.
In vitro, naïve B cells (sorted from ALPS splenocytes) exhibited normal proliferation and normal differentiation into plasmablasts; they were able to produce IgM after cytosine guanine dinucleotide and CD40 ligand plus interleukin-21 stimulation (supplemental Figure 2). Hence, these data concur to suggest that the defects in B-cell immune function observed in vivo are not intrinsic but related to the disease environment.
The spleen MZ of ALPS patients is abnormal
Histopathological studies of the spleen were performed in 9 ALPS patients and 3 controls. Red pulp and white pulp (including PALSs, follicles with the MZ and the PFZ) were systematically assessed. Staining with hematoxylin-eosin-safran (HES) showed that the ALPS patients’ red pulp was morphologically normal but larger than in controls (Figure 5A-D). In ALPS cases, lymphoid follicles were sparse (Figure 5A-D and supplemental Figure 3A), whereas the germinal center and mantle zone were morphologically normal. The MZ was prominent, with medium to large cells and an abnormally high frequency of mitosis. The PALSs were hypertrophic. In 1 specimen (from Pm-1), lymphoid follicles had been replaced by large nodules filled with medium-to-large cells with frequent mitotic features (Figure 5D).
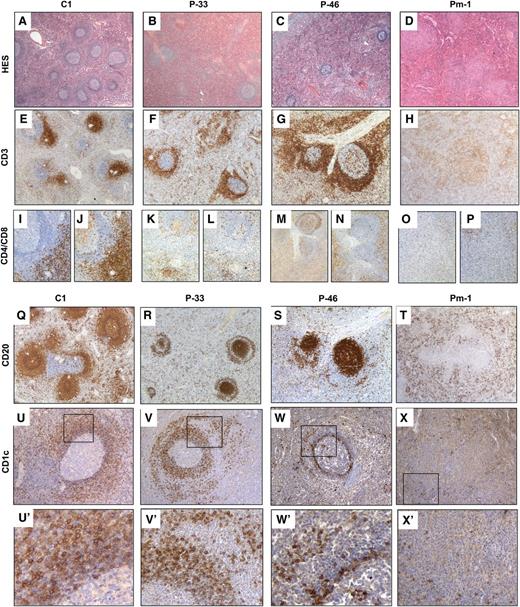
The architecture of the splenic white pulp is abnormal in ALPS patients. HES staining (A-D) and immunohistochemistry (IHC) (E-X) of paraffin sections of spleen specimens from control (A,E,I,J,Q,U) and from P-33 (B,F,K,L,R,V), P-46 (C,G,M,N,S,W), and Pm-1 (D,H,O,P,T,X). (A-D) HES staining revealed that patients had fewer follicles than controls (magnitude ×16). (E-H) IHC with an anti-CD3 antibody (magnitude ×25) showed normal PALSs in a control specimen (E) but revealed a striking expansion of the T-cell zone around the follicles (in place of MZ) in P-33 and P-46 (F-G). In Pm-1, nodules stained positive for CD3 (H). (K-P) As revealed by staining with specific antibodies, most of the T cells expressed neither CD4 (K,M,O) nor CD8 (L,N,P) (magnitude ×50) in ALPS patients. (Q-T) IHC with an anti-CD20 antibody (magnitude ×25) stained the follicles, mantle zone, and MZ in control spleen (Q). In P-33 and P-46, staining was positive in the follicles and the mantle zone but negative in the MZ. A ring of positive CD20+ cells (P-33) (R) or a few CD20(+) cells around the MZ were found in P-46 (S). In Pm-1, a few CD20+ cells were found in the follicle-free red pulp (T). (U-X,U’-X’) IHC with anti-CD1c antibody (U-X: magnitude ×100; U’-X’: amplification of framed area). Naïve B cells in the mantle zone are CD1c low, whereas MZ B cells are CD1c bright in control spleen (U,U’), P-33 (V,V’), P-46 (W,W’), and Pm-1 (X,X’).
The architecture of the splenic white pulp is abnormal in ALPS patients. HES staining (A-D) and immunohistochemistry (IHC) (E-X) of paraffin sections of spleen specimens from control (A,E,I,J,Q,U) and from P-33 (B,F,K,L,R,V), P-46 (C,G,M,N,S,W), and Pm-1 (D,H,O,P,T,X). (A-D) HES staining revealed that patients had fewer follicles than controls (magnitude ×16). (E-H) IHC with an anti-CD3 antibody (magnitude ×25) showed normal PALSs in a control specimen (E) but revealed a striking expansion of the T-cell zone around the follicles (in place of MZ) in P-33 and P-46 (F-G). In Pm-1, nodules stained positive for CD3 (H). (K-P) As revealed by staining with specific antibodies, most of the T cells expressed neither CD4 (K,M,O) nor CD8 (L,N,P) (magnitude ×50) in ALPS patients. (Q-T) IHC with an anti-CD20 antibody (magnitude ×25) stained the follicles, mantle zone, and MZ in control spleen (Q). In P-33 and P-46, staining was positive in the follicles and the mantle zone but negative in the MZ. A ring of positive CD20+ cells (P-33) (R) or a few CD20(+) cells around the MZ were found in P-46 (S). In Pm-1, a few CD20+ cells were found in the follicle-free red pulp (T). (U-X,U’-X’) IHC with anti-CD1c antibody (U-X: magnitude ×100; U’-X’: amplification of framed area). Naïve B cells in the mantle zone are CD1c low, whereas MZ B cells are CD1c bright in control spleen (U,U’), P-33 (V,V’), P-46 (W,W’), and Pm-1 (X,X’).
In 8 of the 9 ALPS specimens, CD3 staining (Figure 5E-H) revealed expanded T-cell zones around the follicles in place of the MZ (Figure 5E-G and supplemental Figure 4A). Many of these T cells expressed neither CD4 nor CD8 (Figure 5I-N). There were also many T cells within the red pulp. In 3 of the 8 specimens (Pm-2, P-22, and Pm-10) (supplemental Table 2), CD20 staining (Figure 5Q-T and supplemental Figure 4A) was not detected in the MZ. In other specimens (P-9, P-46, and Pm-3) (Figure 5S), a few CD20(+) cells were found around the MZ. In P-4 and P-33 specimens, CD20 staining was present in the follicles (the germinal centers and mantle zone), and a ring of CD20+ cells was observed outside the MZ (Figure 5R). These CD20+ cells were IgD(+) and IgM(+) and thus were either naïve B cells or MZ B cells (supplemental Figure 4B). The strong expression of CD1c suggests that they were MZ cells (Figure 5U-X,U’-X’). Immunophenotyping of spleen cells in suspension (performed in 3 cases) showed a reduced B-cell pool with normal proportions of MZ and SM memory CD27+ B cells, suggesting that MZ B cells could be ectopically located outside the MZ (supplemental Figure 5). Although there were many plasma cells (CD138+) in the red pulp, few were IgM(+) (data not shown). In 1 case (Pm-1), few CD20+ cells and no germinal centers were detected in the red pulp (Figure 5T). Instead, there were nodules containing CD3 and mainly DN-Ts (Figure 5H, O-P). Pm-1 was the most severely affected patient, with a long history of chronic lymphoproliferation and cytopenia at the time of splenectomy. In contrast, P-4 and P-33 had the mildest clinical phenotype, with a short history of lymphoproliferation prior to splenectomy. Taken as a whole, these data strongly suggest a correlation between clinical severity and the intensity of the splenic tissue lesions.
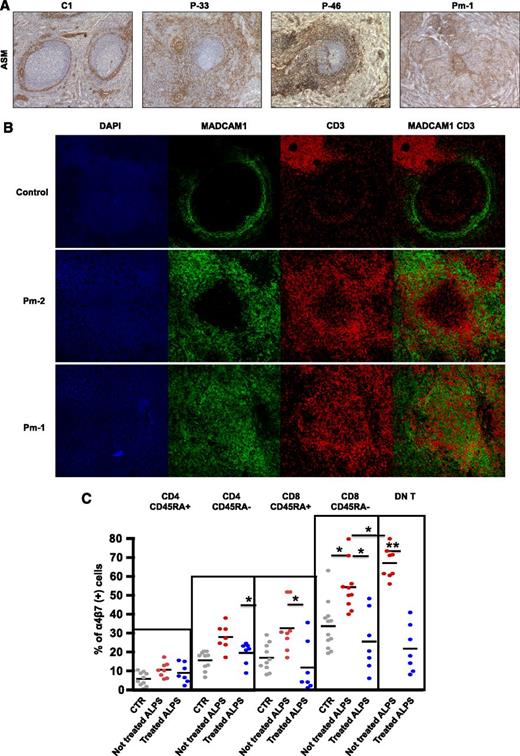
In humans (unlike rodents), B-cell follicles are not separated from the MZ by the marginal sinus.15 The MZ is divided into an inner and an outer compartment by a layer of specialized, mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1)(+) fibroblasts.16,23 These fibroblasts also express α actin (smooth muscle) (ASM) and may have a role in guiding lymphocytes from the circulation back into the splenic white pulp.16 In all 9 of the ALPS specimens, ASM-staining on paraffin sections showed a striking extension of this layer, which formed a meshwork around the follicles (Figure 6A) from the MZ to the PFZ. Figure 6B shows MAdCAM-1 and CD3 expression in spleen specimens from 1 control and 2 ALPS patients. In control spleens, staining revealed a well-delimited meshwork of MAdCAM-1(+) cells outside and few CD3(+) cells inside follicles. In contrast, both patient specimens were characterized by an extension of the MAdCAM-1 meshwork with many CD3(+)cells enmeshed. These findings prompted us to study the expression of α4β7 integrins (the ligand for MAdCAM-1) by T-lymphocyte subsets in ALPS patients (both with active and nonactive disease) and healthy controls. The α4β7 expression was significantly higher in nonnaïve T cells and maximal in DN-Ts (Figure 6C). It is thus plausible that the many α4β7- integrin–positive DN-Ts bind to MAdCAM-1(+) stromal cells and disorganize the local splenic architecture.
Extension of the MAdCAM-1(+) meshwork in ALPS spleen is associated with high expression of integrin α4β7 on DN-Ts. (A) Staining with anti-ASM antibody (magnitude ×50) on control spleen, P-33, P-46, and Pm-1 revealed a thin ring of positive cells within the MZ of control spleen. In all patients, a striking expansion of this layer was observed around the follicles in the marginal and PFZs. (B) Immunofluorescence staining with 4',6-diamidino-2-phenylindole (DAPI) (blue), anti-MAdCAM-1 antibody (green), and anti-CD3 antibody (red) in cryostat sections of a control spleen specimen (first row) and 2 ALPS spleen specimens (second and third rows). In the control spleen, staining revealed a well-delimited meshwork of MAdCAM-1(+) cells with a few CD3(+) cells inside follicles and outside the MZ. In both patient specimens, the extended MAdCAM-1(+) meshwork was in close contact with many CD3(+) cells. GC denotes germinal center. (C) The proportion of cells expressing both α4β7 integrins in various different T-cell subsets in 3 populations: healthy controls and treated and nontreated ALPS patients. DN T, double-negative T cells.
Extension of the MAdCAM-1(+) meshwork in ALPS spleen is associated with high expression of integrin α4β7 on DN-Ts. (A) Staining with anti-ASM antibody (magnitude ×50) on control spleen, P-33, P-46, and Pm-1 revealed a thin ring of positive cells within the MZ of control spleen. In all patients, a striking expansion of this layer was observed around the follicles in the marginal and PFZs. (B) Immunofluorescence staining with 4',6-diamidino-2-phenylindole (DAPI) (blue), anti-MAdCAM-1 antibody (green), and anti-CD3 antibody (red) in cryostat sections of a control spleen specimen (first row) and 2 ALPS spleen specimens (second and third rows). In the control spleen, staining revealed a well-delimited meshwork of MAdCAM-1(+) cells with a few CD3(+) cells inside follicles and outside the MZ. In both patient specimens, the extended MAdCAM-1(+) meshwork was in close contact with many CD3(+) cells. GC denotes germinal center. (C) The proportion of cells expressing both α4β7 integrins in various different T-cell subsets in 3 populations: healthy controls and treated and nontreated ALPS patients. DN T, double-negative T cells.
Age-related structural changes in the spleen of lprcg mice
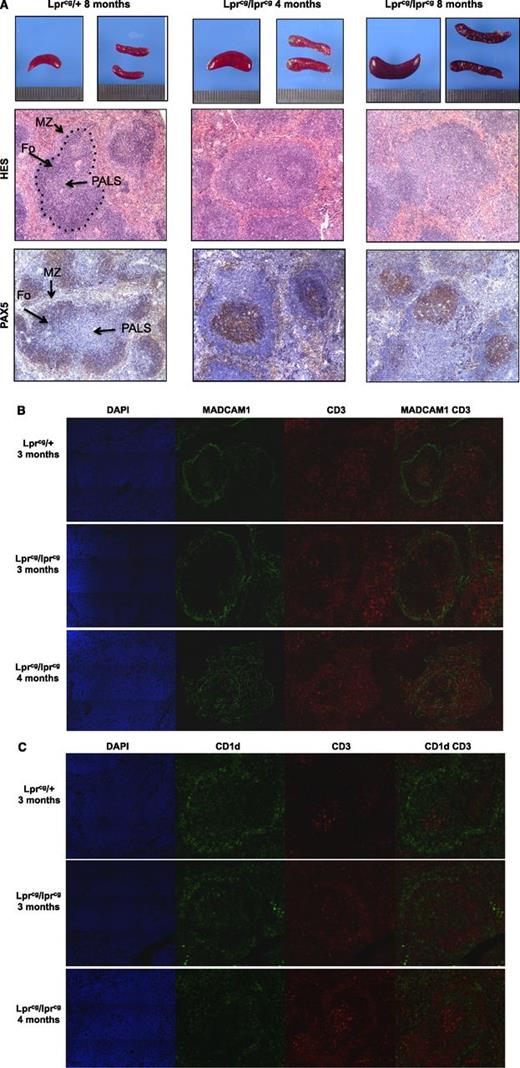
Spleen specimens from 4-, 8-, and 10-month-old homozygous lprcg mice were examined and compared with asymptomatic heterozygous lprcg mice of the same age. Although the splenic architecture was normal in all heterozygous animals, age-related structural changes in the white pulp were observed in homozygous lprcg mice (Figure 7). The marginal sinus delineated by MAdCAM-1(+) stromal cells was characterized by an age-dependent thickening of the meshwork that was filled with CD3(+) cells, whereas CD1d+ MZ B cells were very scarce (Figure 7B-C). Thus, despite human vs murine differences in the architecture of the MZ, similar levels of disorganization were observed in the ALPS patients and homozygous lprcg mice.
Age-related structural changes in spleen of lpr mice. (A) Spleen sections of the indicated mice (upper row), HES staining (middle row), and immunohistochemical analysis with anti-PAX5 antibody (lower row). Fo: B-cell follicles. The location of the marginal sinus is indicated by the dashed black line. (B) Spleen sections from the indicated mice stained with DAPI (blue), anti-MAdCAM-1 (green), and anti-CD3 antibody (red). The MAdCAM-1(+) cells delineate the marginal sinus and reveal age-dependent extension of this meshwork, which is filled with CD3+ cells in homozygous lprcg mice. (C) Spleen sections from the indicated mice stained with DAPI (blue), anti-CD1d (green), and anti-CD3 antibody (red). CD1d identifies MZ B cells.
Age-related structural changes in spleen of lpr mice. (A) Spleen sections of the indicated mice (upper row), HES staining (middle row), and immunohistochemical analysis with anti-PAX5 antibody (lower row). Fo: B-cell follicles. The location of the marginal sinus is indicated by the dashed black line. (B) Spleen sections from the indicated mice stained with DAPI (blue), anti-MAdCAM-1 (green), and anti-CD3 antibody (red). The MAdCAM-1(+) cells delineate the marginal sinus and reveal age-dependent extension of this meshwork, which is filled with CD3+ cells in homozygous lprcg mice. (C) Spleen sections from the indicated mice stained with DAPI (blue), anti-CD1d (green), and anti-CD3 antibody (red). CD1d identifies MZ B cells.
Discussion
In the present work, we reported on the occurrence of a high rate of invasive bacterial infections (mainly caused by S pneumoniae) in ALPS patients. These infections were more frequent in splenectomized patients but also occurred in nonsplenectomized patients. The elevated risk of infection was observed in patients with active disease and was associated with a B-cell immunodeficiency characterized by low serum IgM levels, poor production of IgM (but not IgG) anti–S pneumoniae antibodies, low circulating SM B-cells counts, very low circulating MZ B cells, and profound disorganization of the B-cell compartment in the spleen.
Two of the 100 patients presented invasive S pneumoniae infections in infancy; both had shown the first features of ALPS before the age of 6 months. This incidence rate is therefore much higher than that reported for healthy infants (0.2% to 0.5% per year) before the systematic use of a conjugated pediatric vaccine against S pneumoniae was implemented.27,28 The rate of invasive bacterial infection in splenectomized patients was as high as 30%. A similar risk of severe, postsplenectomy sepsis in ALPS was recently reported by Rao et al.8 This risk is much higher than the values of 2% and 11.6% observed after posttrauma splenectomy and in splenectomized thalassemia patients, respectively.12-14 It is noteworthy that invasive bacterial infections occurred in all ALPS subsets, including patients with homozygous TNFRSF6 mutations, heterozygous germ-line mutations, and heterozygous somatic mutations. Young age at splenectomy and poor adherence to anti-infectious prophylaxis appeared to be additional risk factors in our cohort of patients, as has previously been noted in other settings.11-14 Furthermore, patients in our cohort splenectomized before the age of 5 years had a risk as high as 12.1 per 100 years of follow-up, similar to that reported in patients with congenital asplenia.29 A similar risk of severe postsplenectomy sepsis was observed in a cohort of splenectomized patients with Wiskott-Aldrich syndrome30 (a primary immunodeficiency characterized among other abnormalities by absent or hypotrophic MZ and serum hypo IgM31 ). In our cohort, severe sepsis postsplenectomy was the main cause of death (n = 4, 12.5%; 0.7 per 100 years of follow-up), whereas the expected rate in splenectomized populations ranges between 0.1 and 0.3 per 100 years of follow-up.13 This observation points to the need for strict prophylaxis and monitoring of splenectomized ALPS patients. Antibiotic prophylaxis should not be discontinued, and splenectomy should be avoided as much as possible in order to preserve other anti-infectious spleen functions as well as residual MZ activity. Splenectomy should be replaced by treatment with antiproliferative drugs (such as rapamycin) and/or proapoptotic drugs (such as 6MP, azathioprin, and mycophenolate mofetil).5,8,32
The unusually high susceptibility to infection by encapsulated bacteria observed here prompted us to screen our patients for a predisposing immunodeficiency. Low circulating counts of memory B cells (both MZ and SM B cells) in nonsplenectomized ALPS patients were noted, as previously reported in a small cohort of ALPS patients.9 Poor anti–S pneumoniae IgM production following administration of nonconjugated vaccine was also documented. Taken as a whole, these data suggest that defective function of the MZ B-cell compartment accounts for the observed vulnerability to S pneumoniae.18,19,23 Given the inverse correlation between disease activity and B-cell immunodeficiency, we further hypothesize that the B-cell deficiency is a consequence of the ALPS activity. Absence of B-cell anomaly in asymptomatic MPR further emphasizes this hypothesis. In order to understand the underlying mechanism, we examined available spleen specimens from ALPS patients. The T-cell zone (including a majority of DN-Ts) was abnormally large, and germinal centers were rare, which perhaps accounts for the low circulating SM B-cell count. The disorganization of the MZ was striking. In fact, B cells with an MZ phenotype were present but were not in the usual location. The MZ was filled with an enmeshed mixture of T cells and stromal cells. These abnormalities were more pronounced in patients with more advanced disease. This is reminiscent of the previous observations33 of progressive atrophy of follicles in peripheral lymph nodes (with hyperplasia of the T-cell interfollicular zone instead) in patients who later were diagnosed with ALPS-FAS (B. Neven, unpublished data). Histopathological studies in homozygous lprcg mice confirmed the occurrence of age-related structural changes in the spleen, as previously described in the spleen, mesenteric lymph nodes, and Peyer’s patches of MRL/lpr mice.34,35
The margin between red and white splenic pulp displays species-specific characteristics.15 In rodents, the MZ is surrounded by the marginal sinus and a lining of MAdCAM-1(+) cells. In contrast, humans B follicles are not separated from the MZ by a marginal sinus; instead, a layer of MAdCAM-1(+) stromal cells divides the MZ in an inner and an outer part.16 The MZ is surrounded by a large PFZ. Lymphocytes may exit the circulation via this structure and then cross the MZ in their journey back to the white pulp. It has been suggested that CD4+ lymphocytes are guided by this stromal layer.16 In ALPS spleen specimens, a striking expansion of the stromal layer was noted, with the presence of a meshwork of MAdCAM-1(+) cells in MZ and the PFZ. A high proportion of DN-Ts (the hallmark lymphocyte population in ALPS, present in large numbers in lymphoid organs such as the spleen) were found to express the MAdCAM-1 ligand α4β7 integrin. One can hypothesize that the accumulation of expanding DN-Ts around the MAdCAM-1(+) cell meshwork in the MZ excludes MZ B cells from their usual functional location and thus leads to a functional, T-independent B-cell immunodeficiency. Therapeutic measures aimed at reducing the T-cell mass might therefore contribute to restore B-cell competence. Antiproliferative (as rapamycine) or cytotoxic drugs (such as azathiorpine, mycophenolate mofetil, 6MP, etc.) may clear accumulating T cells from the MZ, thus restoring an appropriate environment for effective B-cell response.
In conclusion, ALPS is also characterized by an antibody deficiency that is related (at least in part) to disorganization of the splenic MZ. This antibody deficiency deserves more attention because it may cause life-threatening invasive bacterial infections. Our observations may have several practical consequences for the care of patients with ALPS, that is, prevention of S pneumoniae and encapsulated bacterial infections by recurrent immunization with conjugated vaccine and concomitant oral penicillin treatment, avoidance of splenectomy, and reduction of lymphoproliferative syndromes with a view to restoring MZ B-cell function.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their cooperation, S. Ehl for scientific discussion, and M. Forveille, V Gaba, and C. Daussy for excellent technical assistance. The authors also thank the “plateforme d’imagerie,” Institut Imagine, Raphaëlle Devaux and Nicolas Goudin, and the tumorothèques, from Necker Hospital.
This work was funded by grants from the Institut National de la Santé et de la Recherche Médicale, the Fondation pour la Recherche Médicale (B.N.), the poste d’accueil APHP/CNRS/CEA, Département de la Recherche Clinique et du Développement Ile de France (J.B.), the Agence Nationale pour la Recherche (F.R.-L.), an Advanced Senior Grant from the European Research Council (PID Immune 249816) (A. Fischer). I.M. is supported by a KOF of the Catholic University of Leuven.
Authorship
Contribution: B.N. designed the research, performed experiments, collected and analyzed data, and participated in the drafting of the manuscript and clinical care; J.B. performed pathology and immunohistochemical and immunofluorescence studies, collected and analyzed data, and participated in the drafting of the manuscript; N.B. and T.J.M. analyzed pathology and immunohistochemical data; M.-C.S., I.M., A.M.-C., N.L., S.W., D.A., and L.M. participated in experiments and data collection and analysis; I.M., B.F., B.B.-M., G.L., A. Ferster, C.C., S.B., and C.P. participated in clinical care and data collection and analysis; A.D., M.R., and X.B. designed the research and participated in data analyses and drafting of the manuscript; A. Fischer designed the research and participated in data analyses, as well as the drafting of the manuscript and clinical care; F.R.-L. designed the research and participated in data analyses and the drafting of the manuscript; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bénédicte Neven, 149 rue de Sèvres, Hopital Necker, 75015 Paris, France; e-mail: benedicte.neven@nck.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal