In this issue of Blood, Fecteau et al show that lenalidomide inhibits proliferation of chronic lymphocytic leukemia (CLL) cells in vitro by targeting cereblon. Thus far, the clinical activity of this drug in CLL was believed to be mainly via the microenvironment.1
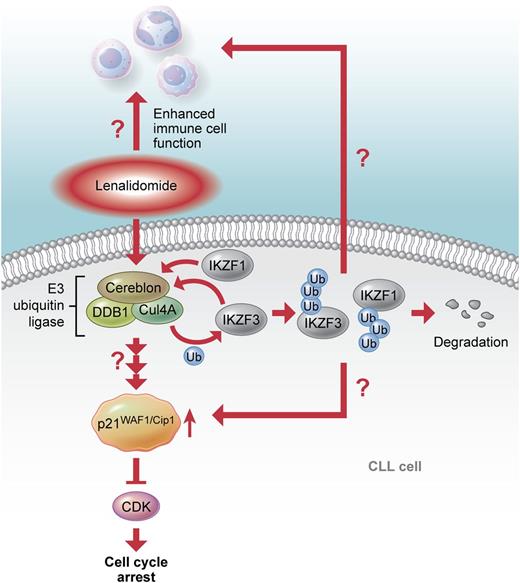
The proposed mode of action of lenalidomide. Binding of lenalidomide to cereblon induces p21WAF1/Cip1 upregulation and cell cycle arrest in CLL cells. Thus far, it is not clear whether ubiquitination and degradation of the transcription factors IKZF1 and 3, as described for multiple myeloma, are involved in the effects of lenalidomide on CLL cells and their microenvironment. Professional illustration by Xavier Studio.
The proposed mode of action of lenalidomide. Binding of lenalidomide to cereblon induces p21WAF1/Cip1 upregulation and cell cycle arrest in CLL cells. Thus far, it is not clear whether ubiquitination and degradation of the transcription factors IKZF1 and 3, as described for multiple myeloma, are involved in the effects of lenalidomide on CLL cells and their microenvironment. Professional illustration by Xavier Studio.
A central role of the microenvironment in CLL pathogenesis is now well established, and several treatment approaches that target the cross-talk of CLL cells with their surroundings are currently being tested. In the majority of patients, CLL is a slowly developing disease with only a small subpopulation of malignant cells that proliferate. The novel findings of this study raise the question of whether inhibition of CLL cell proliferation by lenalidomide is the main driver of the drug’s clinical success.
Lenalidomide is an immunomodulatory drug that was approved for multiple myeloma and myelodysplastic syndrome and that shows clinical activity in patients with CLL, although here its clinical development has been a long and winding road (reviewed in Giannopoulos et al2 ). When properly dosed, lenalidomide is well tolerated and active as single agent even in refractory CLL. More recently, combination trials with rituximab showed promising results for relapsed patients or as initial therapy and also in cases with genomic deletion in chromosome 17p leading to loss of functional tumor suppressor protein p53 and therefore resistance to therapy. Lenalidomide treatment frequently induces an acute inflammatory reaction in CLL patients, the so-called “tumor flare reaction” that is suggestive of an immune activation phenomenon. Therefore, it seems more likely that the efficacy of lenalidomide in CLL results from both an antiproliferative activity on the malignant cells and an immunomodulatory effect on their microenvironment.
In contrast to multiple myeloma cells, lenalidomide has no direct cytotoxic activity for CLL cells in vitro. Therefore, the impact of this drug on the microenvironment was extensively studied, and an enhancement of immune cell functions, as evidenced by altered activity and motility of T cells, natural killer cells, and myeloid cells, was described.3,4 CLL-associated immune dysfunction is reversed by this drug, switching a leukemia-supporting microenvironment into antitumor activity. The question remains of whether this is sufficient to control CLL development.
The data presented by Fecteau et al clearly demonstrate an antiproliferative activity of lenalidomide for CLL cells and suggest that this is at least partly responsible for the clinical success of the drug. This activity has been overlooked by previous in vitro studies, as CLL cells in culture are cell cycle arrested and proliferate only under conditions that mimic stimuli derived from activated T cells or infection. In the present study, such culture conditions were used to demonstrate inhibition of CLL cell proliferation by lenalidomide, which is independent of p53, explaining the good clinical results in cases with 17p deletion. Instead, the effect of lenalidomide depends on p21WAF1/Cip1, a negative regulator of cell cycle progression. This protein is upregulated in CLL cells on lenalidomide treatment in vitro and in patients, and its depletion abrogates the antiproliferative effect. Depletion of cereblon, the only known molecular target of lenalidomide, prevents lenalidomide’s activity.
Cereblon is part of the E3 ubiquitin ligase complex that consists of cullin 4A and damaged DNA binding protein 1 and ubiquitinates specific target proteins that are subsequently degraded by proteasome activity. In this complex, cereblon is responsible for target protein recognition and binding (see figure). Lenalidomide and related compounds like thalidomide directly bind to cereblon and thereby inhibit the autoubiquitination activity of the E3 ligase, which is the basis for the teratogenic activity of thalidomide.5 Recently, 2 groups independently showed that lenalidomide enhances the binding of cereblon to the 2 transcription factors Ikaros (IKZF1) and Aiolos (IKZF3).6,7 These transcription factors are essential for B- and T-cell development and are highly expressed in B-cell malignancies like CLL. Lenalidomide treatment results in ubiquitination and degradation of IKZF1 and 3, which is necessary for the antiproliferative effect of the drug in multiple myeloma. Together, these findings show that lenalidomide can either act as an inhibitor of E3 ubiquitin ligase activity, which results in a stabilization and accumulation of cereblon target proteins, or regulate the degradation of proteins by altering cereblon’s substrate specificity.
As in multiple myeloma, lenalidomide treatment results in increased levels of p21WAF1/Cip1 in CLL, which is dependent on cereblon activity. Thus far, the molecular mechanism of this induction is not known. Data by Fecteau et al suggest a transcriptional upregulation of p21WAF1/Cip1. Future studies need to analyze whether IKZF1 and 3 or another transcription factor that is directly or indirectly regulated by cereblon are involved in p21WAF1/Cip1 expression. IKZF1 and 3 are known to act as repressors or activators of gene expression in different settings. Of interest, the gene coding for p21WAF1/Cip1 contains a potential binding site for IKZF1 in the promoter region, and down-regulation of IKZF1 in acute lymphocytic leukemia cells, resulted in increased p21WAF1/Cip1 levels.8 Alternatively, a posttranslational regulation of p21WAF1/Cip1 by lenalidomide impacting on the stability or degradation of the protein is also conceivable. In this respect, it is of interest that many regulatory proteins of the cell cycle, including p21WAF1/Cip1, are targets of cullin 4–based E3 ubiquitin ligase complexes.9
With the advances in understanding the molecular mechanism of lenalidomide, several questions concerning its therapeutic activity in CLL arise. (1) Are the transcription factors IKZF1 and 3 involved in the antiproliferative effect of lenalidomide in CLL cells? (2) Are cereblon and the transcription factors also responsible for the activity within the CLL microenvironment? An IKZF1- and 3-mediated activation of T cells on lenalidomide treatment has been described. Here, the degradation of both transcription factors results in a derepression of the interleukin-2 gene.10 (3) Why does only a subset of CLL patients respond to lenalidomide treatment? A discrimination of responders and nonresponders might become possible by analyzing the expression and activity of cereblon and its target proteins. On this basis, the rational design of drug combinations including lenalidomide should be advanced.
Within recent years, enormous progress in elucidating the pathomechanisms of CLL and in developing novel targeted compounds has been made. This knowledge now has to be translated into novel treatment protocols. By selecting therapeutics according to the molecular and genetic setup of every patient, we are getting closer to an individualized treatment of CLL with a maximal therapy success.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal