In this issue of Blood, Brown et al identify somatic mutations of MAP2K1 capable of driving the RAS-RAF-MEK-ERK pathway in Langerhans cell histiocytosis (LCH). Their findings lend important insight into the pathogenesis of this disease and provide the rationale for exploring targeted approaches in clinical trials.1
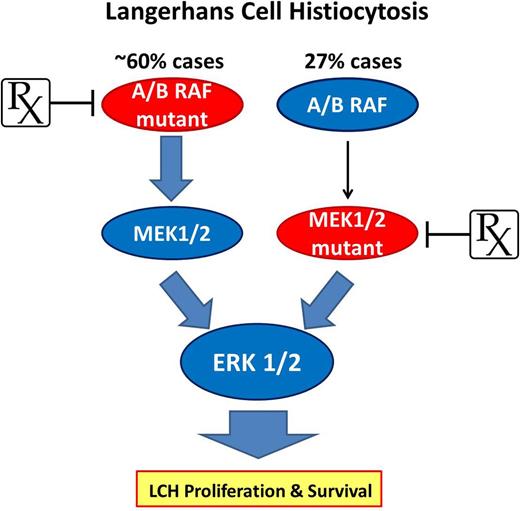
The RAS-RAF-MEK-ERK pathway represents a driver in the pathogenesis of LCH while offering the potential for targeted therapeutic approaches. As much as 60% of LCH patients carry mutated forms of ARAF or BRAF (V600E) capable of constitutive phosphorylation of the downstream effectors MEK1 and MEK2 that ultimately drive ERK activity. Up to 50% BRAF-V600E–negative LCH cases (27% total) harbor a mutation in MAP2K1 that leads to constitutive MEK1 and consequent ERK activation, promoting downstream proliferation and survival networks to drive LCH. The mutually exclusive presence of BRAF and MAP2K1 mutations presents an ideal opportunity to intervene with selective agents like vemurafenib or trametinib to target 2 critical enzymes essential to LCH pathogenesis.
The RAS-RAF-MEK-ERK pathway represents a driver in the pathogenesis of LCH while offering the potential for targeted therapeutic approaches. As much as 60% of LCH patients carry mutated forms of ARAF or BRAF (V600E) capable of constitutive phosphorylation of the downstream effectors MEK1 and MEK2 that ultimately drive ERK activity. Up to 50% BRAF-V600E–negative LCH cases (27% total) harbor a mutation in MAP2K1 that leads to constitutive MEK1 and consequent ERK activation, promoting downstream proliferation and survival networks to drive LCH. The mutually exclusive presence of BRAF and MAP2K1 mutations presents an ideal opportunity to intervene with selective agents like vemurafenib or trametinib to target 2 critical enzymes essential to LCH pathogenesis.
LCH is a rare, often misdiagnosed histiocytic proliferative disorder afflicting pediatric and adult patients. The clonal (CD1a+, S100+, and CD207+) proliferating cells have morphologic features most consistent with bone marrow–derived Langerhans cells that represent an antigen presenting dendritic cell commonly located in the skin and mucosa. The clinical course of LCH is diverse, ranging from solitary skin or bone lesions that may spontaneously resolve to widely disseminated, multisystem disease associated with high morbidity and mortality.2 The broad spectrum of disease activity, inflammatory nature of the lesions, normal karyotype, and associated immune dysregulation suggested that LCH represented an immunoreactive disorder.
However, in 2010 Badalian-Very et al reported 57% of archived LCH lesions harbored a recurrent somatic, activating genetic mutation of the BRAF gene (BRAF-V600E).3 Furthermore, they found evidence of constitutive activation of the RAS-RAF-MAPK-ERK pathway regardless of whether BRAF was mutated or wild-type in LCH lesions. The fact that BRAF-V600E mutations were present as single alleles suggested that this might reflect a driver mutation for this disease. These data presented the first convincing genetic evidence supporting the characterization of LCH as a myeloid neoplastic disorder and provided rationale for considering a targeted approach to treat patients with this disease. Several years later, Haroche et al4 reported impressive clinical responses in 3 patients with BRAF-V600E–mutated LCH treated with the single-agent BRAF inhibitor vemurafenib.
Constitutive ERK activity in all LCH lesions led others to explore additional genetic drivers of ERK activity. Nelson et al5 used whole-exome sequencing of DNA isolated from purified LCH cells from 3 patients with wild-type BRAF. Interestingly, their studies documented the first somatic, activating mutations (F351L and Q347_A3438del) within the kinase-encoded domain of ARAF, leading to both ARAF and MEK constitutive kinase activity. Transfection of mouse fibroblast cells with the ARAF double-mutant clone led to acquired contact-independent growth in soft agar, a hallmark feature of cellular transformation, and further evidence that ERK activation may represent a driver of LCH growth.
In this issue, Brown and colleagues1 provide additional convincing evidence that links constitutive MAPK-ERK signaling to the pathogenesis of LCH. They used targeted next-generation sequencing to evaluate 8 LCH cases and found BRAF-V600E in 3 cases, consistent with prior work, and an E102_I103del mutation in the MAP2K1 gene that encodes for the MEK1 protein kinase, an enzyme directly upstream of extracellular signal-regulated kinases ERK1/2. Interestingly, this MAP2K1 mutation occurred in an LCH case that was BRAF wild-type, which then led the investigators to examine an additional 32 cases by BRAF-V600E allele–specific polymerase chain reaction and Sanger sequencing of exons 2 and 3 of MAP2K1. Surprisingly, 11 of 40 (27%) cases showed somatic MAP2K1 mutations that occurred mutually exclusive to BRAF mutations, with 50% of wild-type BRAF cases showing MAP2K1 mutation. Most mutations identified in this study were deletions within exons 2 and 3 and were previously shown to encode for markedly enhanced MEK1 kinase activity (see Figure 1 in Brown et al).6 Collectively, these results, when taken in the context of studies led by Badalian-Very et al, suggest that the majority of LCH patients harbor a somatic, activating mutation in critical signaling elements of the RAS-RAF-MEK-ERK pathway (see figure).
Do activating mutations of components of the RAS-RAF-MEK-ERK pathway represent true drivers of LCH pathogenesis? Recent work by Berres et al7 examines the biological relevance of recurrent BRAF-V600E mutations in the pathogenesis of LCH. Sixty-four lesions from 100 LCH patients showed the presence of the BRAF-V600E mutation, an event that was linked to increased risk of relapse. In patients with high-risk disease, the BRAF-V600E mutation could be detected in bone marrow CD34+ hematopoietic progenitor cells, whereas patients with low-risk LCH showed presence of the mutation restricted to mature CD207+ dendritic cells (DCs) within primary lesions. The authors went on to develop a novel in vivo mouse model, allowing for conditional expression of BRAF-V600E in various subsets of DCs. Mice with conditional expression of BRAF-V600E in DC progenitors (under CD11c promoter) rapidly developed an aggressive, multisystem LCH-like disease. Interestingly, granulomatous lesions in these mice showed inflammatory infiltrates with accumulation of regulatory T cells and abundant expression of inflammatory cytokines that are observed in the lesions of humans with aggressive LCH. In stark contrast, mice with conditional expression of BRAF-V600E in mature DCs (under langerin promoter) developed a low-grade, localized LCH-like disease. By definition, driver mutations contribute toward the initiation of cellular transformation and the progression of malignant disease, 2 features clearly illustrated in this interesting preclinical model of LCH. Elegant models such as this will certainly improve our understanding of the complex pathogenesis of LCH and provide a useful setting to test novel targeted therapies exploiting this pathway.
Additional studies supporting the driver nature of these mutated signaling components have illustrated the capacity of activating ARAF mutations to drive fibroblast transformation,5 MAP2K1 mutations driving constitutive ERK phosphorylation in melanomas6 and hairy cell leukemia,8 and MAP kinase activity to drive transformation of mammalian cells.9
Perhaps the most intriguing implications reported by Brown et al1 and others3,5 lie within the potential for pursuing targeted therapeutic strategies. Current up-front therapeutic modalities to manage patients with multisystem LCH have traditionally used a risk-stratified approach (single vs multisystem LCH), often using intensive cytotoxic chemotherapy.10 Patients with relapsed or refractory disease have benefitted from single-agent modalities delivering cytarabine or cladribine. Although these regimens have generally improved outcome, toxicity remains a challenge and patients with high-risk, multisystem disease still face a high mortality rate. The identification of activating mutations in A/BRAF and MAP2K1, genes encoding 2 critical signaling enzymes in the ERK pathway, allows for strategic approaches using novel targeted agents. Promising Food and Drug Administration–approved, selective agents like vemurafenib and trametinib to target mutant A/BRAF and MAP2K1/2, respectively, provide an ideal opportunity to develop well-designed, multicenter, genetically stratified (using targeted sequencing approaches) clinical trials. The collective findings reported by Brown et al1 and others3,5 identifying activating somatic mutations in the majority of LCH patients have shed light on the complex pathogenesis of LCH while offering patients with LCH hope for improved treatment strategies in the near future.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal