In this issue of Blood, Yang et al contribute to fill in the gap of our understanding of cyclooxygenases (COXs) in adaptive immunity by identifying COX-1 as a central player in B-cell development.1
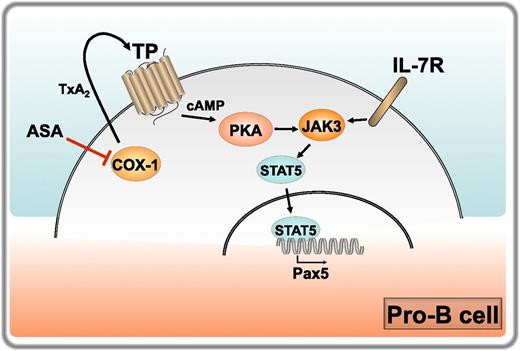
Regulation of B-cell development by COX-1. IL-7 receptor engagement on pro-B cells triggers JAK/STAT5 signaling, resulting in translocation of STAT5 to the nucleus and transcription of target genes. These include the master transcription factor Pax5, which drives the pro-B to pre-B cell transition. COX-1 is expressed at high levels in pro-B cells, where it catalyzes the production of TxA2. Released TxA2 triggers its receptor TP in a cell autonomous manner, promoting the accumulation of cAMP and activation of PKA, which enhances JAK3/STAT5 signaling and Pax5 expression, thereby cooperating with the IL-7 receptor in driving the pro-B to pre-B maturation step. COX-1 inhibition by aspirin (ASA) in healthy volunteers results in a reduction in TxA2 production, which correlates with impaired JAK3/STAT5 signaling and Pax5 expression. Professional illustration by Laura Patrussi.
Regulation of B-cell development by COX-1. IL-7 receptor engagement on pro-B cells triggers JAK/STAT5 signaling, resulting in translocation of STAT5 to the nucleus and transcription of target genes. These include the master transcription factor Pax5, which drives the pro-B to pre-B cell transition. COX-1 is expressed at high levels in pro-B cells, where it catalyzes the production of TxA2. Released TxA2 triggers its receptor TP in a cell autonomous manner, promoting the accumulation of cAMP and activation of PKA, which enhances JAK3/STAT5 signaling and Pax5 expression, thereby cooperating with the IL-7 receptor in driving the pro-B to pre-B maturation step. COX-1 inhibition by aspirin (ASA) in healthy volunteers results in a reduction in TxA2 production, which correlates with impaired JAK3/STAT5 signaling and Pax5 expression. Professional illustration by Laura Patrussi.
COXs, which catalyze the rate-limiting step in the biosynthesis of prostaglandins (PGs) and thromboxanes (TXs), are among the most popular molecules in the biomedical literature, with close to 50 000 references in PubMed since 1975, when the biological activities of these lipids in inflammation and coagulation were first identified. The seminal discovery that COX exists as 2 functionally different isoforms, COX-1 and COX-2, implicated in tissue homeostasis and inflammation, respectively, provided an explanation to the adverse side effects of aspirin on the gastric mucosa, setting the foundations for the development of nonsteroidal anti-inflammatory drugs selectively targeting COX-2.2 This finding, however, faced the scientific community with the difficult challenge of elucidating the mechanisms by which COX-1 and COX-2 play different roles using the same toolbox of lipid mediators, which is confounded by accumulating evidence that the homeostatic vs disease-related function of the 2 enzymes is not as black and white as initially inferred from the effects elicited by their pharmacological blockade.2 Moreover, the widespread expression of COX-1 poses a limit to a full understanding of the growing array of biological functions subserved by this enzyme. The report by Yang et al brings us a step closer to this important objective by implicating COX-1 in the pathway that regulates B-cell development in the bone marrow (BM), on which the ability of the organism to raise an adaptive immune response to pathogens crucially depends.
Although PGs have long been known to suppress T- and B-cell activation in vitro,3 the role of COX-1 in lymphocyte development, activation, and differentiation has been to date largely limited to the T-cell compartment. COX-1 has been shown to participate in thymocyte development, promoting the prostaglandin E2 (PGE2)-dependent transition from the double negative (CD4−CD8−) to the double positive (CD4+CD8+) stage.4 At nonimmunosuppressive concentrations, PGE2 also modulates the differentiation of CD4+ T cells in the periphery, impacting on the T-helper (Th)1/Th2 balance and promoting their polarization to Th17 effectors.3 The relevance of these activities to diseases such as allergic asthma and inflammatory bowel disease has been established with mice lacking the main T-cell PGE2 receptors EP2 and EP4.5,6 As with T cells, PGE2 affects peripheral B-cell differentiation, promoting their maturation to immunoglobulin (Ig)E-secreting cells7 and participating in interleukin (IL)-21–dependent B-cell death during germinal center selection.8 In a recent report, the roles of COX-1 and COX-2 in the humoral immune response have been addressed in vivo in a model of infection with the Lyme disease pathogen Borrelia burgdorferi.9 This study confirmed the implication of COX-1 in the control of class switching, as assessed by the lack of Borrelia-specific IgG in infected COX-1−/− (but not COX-2−/−) mice, which correlated with defective germinal center formation and production of the cytokines IL-6 and IL-17. The report by Yang et al completes this picture by investigating the function of COX-1 in developing B cells.
Starting with the observation that COX-1−/− mice have a reduction in the number of peripheral B cells compared with their wild-type counterparts, which does not result from increased apoptosis, the authors hypothesize an implication of COX-1 in B-cell development, demonstrating that COX-1 regulates the pro-B cell to pre-B cell transition. This was found to correlate with a peak in COX-1 expression in pro-B cells and to be independent of BM stromal cell-derived prostanoids. The maturation of pro-B to pre-B cells is controlled by the cytokine IL-7, which promotes expression of the master transcription factor Pax5 through Janus kinase (JAK)3/signal transducer and activator of transcription (STAT)5 signaling. Based on the finding that COX-1−/− B cells have a defect in Pax5 expression, Yang et al address the potential modulation of IL-7–induced JAK3/STAT5 signaling by COX-1 in in vitro experiments with BM B cells, demonstrating that COX-1 participates in this pathway upstream of STAT5. To identify the underlying mechanism the authors examine the prostanoid profiles in COX-1−/− mice, identifying thromboxane A2 (TxA2) as the main prostanoid altered by COX-1 deficiency and providing evidence that TxA2 and its receptor TP, which is abundantly expressed in developing B cells with a peak at the pro-B stage, participates in B-cell development downstream of COX-1. Finally, they show that TxA2 regulates JAK/STAT5 signaling in B cells by promoting cyclic adenosine monophosphate (cAMP) accumulation and protein kinase A (PKA) activation. Of note, the authors show that healthy volunteers subjected to a low-dose aspirin regimen have a reduction in the number of circulating B cells correlating with decreased levels of urine TxA2 metabolites (see figure).
The report by Yang et al provides important new insights into the IL-7–dependent pathway that regulates a key step in B-cell development. The authors not only implicate COX-1 in the pro-B to B-cell transition but establish a functional link between COX-1 and JAK/STAT5 signaling mediated by the TxA2/TP axis, identifying cAMP as the second messenger responsible for this function. Taken together with the finding that COX-1 is required for the generation of an effective humoral response to infection,9 these data identify COX-1 as a central player in the B-cell compartment. It is noteworthy that the function of COX-1 appears to be mediated by different prostanoids in BM (TxA2) and peripheral (PGE2) B cells. Because immune cells express both TP and the PGE2 receptors EP2 and EP4,10 these results underscore the importance of a lipidomic analysis of the prostanoids to which these cells are physiologically exposed to establish unequivocally which prostanoid is responsible for the specific biological end point. Furthermore, this report shows that, although COX-1 expression is indeed constitutive, it is also dynamic, such that the levels can be substantially different, as exemplified by pro-B and pre-B cells. This must be kept in mind when addressing the function of COX-1. Finally, although the results obtained on healthy volunteers subjected to a low-dose aspirin regimen are limited to a very small number of individuals, they have profound implications for the B-cell response of individuals undergoing preventive antithrombotic therapy. It will be interesting to see whether the reduction in peripheral B cells documented in this report will be confirmed in a larger cohort.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal