To the editor:
The use of low-molecular-weight heparin (LMWH) during anticancer therapy has been studied by several authors, who speculated about a direct antitumor effect of LMWH, which might be translated into a survival benefit in patients with cancer.1 Other authors have suggested that the antitumor activity of LMWH was fortuitous and independent of the anticoagulant effect.2
According to this theory, the advantage in terms of survival should be weighted differently if one is evaluating the anticancer or antithrombotic effects of LMWH.2
Based on this assumption, we conducted a post hoc analysis of the PROTECHT (PROphylaxis of ThromboEmbolism during CHemoTherapy; #NCT00951574) study3 to evaluate if prophylaxis with nadroparin conferred any additional benefit in terms of survival, depending on whether chemotherapy disease control was achieved (complete response, partial response, or stable disease).
In the PROTECHT study, nadroparin (3800 anti-Xa IU once daily for 120 days) has been demonstrated to reduce the incidence of venous and arterial thrombotic events by about 50% in 1150 cancer outpatients receiving chemotherapy (P = .024).
In our post hoc analysis, individual patient data were reviewed and analyzed to assess response to chemotherapy and overall survival. Overall survival was calculated 1 year after study treatment (nadroparin or placebo) initiation by considering the time from randomization to the occurrence of death or last follow-up visit if the event did not occur (right censored). Survival analysis was performed using a Cox regression model with treatment (nadroparin or placebo), response to chemotherapy (disease control or no disease control), and their interaction as covariates. A statistically significant interaction indicates that an overall comparison between the 2 treatments is inappropriate, and therefore hazard ratios and their statistical significance must be evaluated separately in each group (disease control/no disease control).
Randomized patients are distributed as responders and nonresponders (according to RECIST [Response Evaluation Criteria In Solid Tumors] criteria) and perfectly balanced across the 2 treatments (P = .9961): 41.2% (317/769) and 58.8% (452/769) of patients receiving nadroparin and 41.2% (157/381) and 58.8% (224/381) receiving placebo were responders and nonresponders, respectively. Key statistical findings are summarized as follows, with 2-sided 95% confidence limits indicated in brackets:
A statistically significant interaction between treatment and response to chemotherapy (P = .0435) was found, thus supporting the hypothesis that the between-treatment difference in survival depends on the response to chemotherapy.
A statistically significant treatment difference was found in the subgroup of patients with disease control (P = .05) with an estimated hazard ratio equal to 0.665 [0.442-1.00], but not in the subgroup of patients without disease control (P = .525) with an estimated hazard ratio of 1.077 [0.857-1.35].
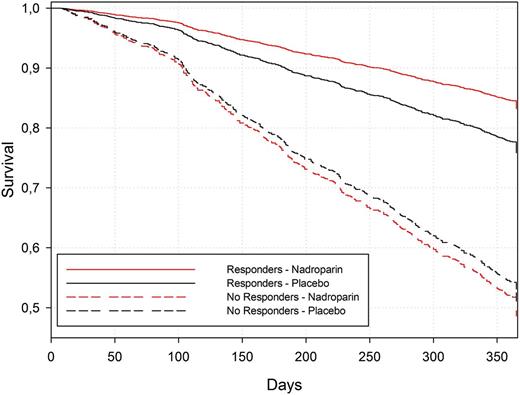
In patients with disease control, the 1-year survival rate estimated from the Cox model was 83% [79% to 87%] for nadroparin and 76% [70% to 83%;] for placebo, whereas in patients without disease control it was 49% [44% to 53%] for nadroparin and 51% [45% to 58%] for placebo (Figure 1).
Survival curves for the Cox model estimated based on the Breslow approach.
These findings are consistent with the possibility that nadroparin may ensure a survival benefit, even if limited to patients experiencing disease control, and they highlight a potentially new way to evaluate overall survival in clinical trials assessing the efficacy of thromboprophylaxis.
It may be of clinical interest to evaluate if the survival advantage detected in the disease-control PROTECHT subpopulation could be confirmed in other published randomized trials assessing the efficacy of thromboprophylaxis.
Authorship
Contribution: E.B. planned and reviewed the statistical analysis. All authors read and approved the final manuscript.
Conflict-of-interest disclosure: G.A. had a consultant or advisory role for Bayer and Boehringer-Ingelheim and received honoraria from Bayer, Boehringer-Ingelheim, Daiichi-Sankio, Pfizer, and Sanofi. T.P. is employee of Italfarmaco S.p.A., Italy. This study was supported by Italfarmaco S.p.A.
Correspondence: Sandro Barni, Medical Oncology Department, Treviglio-Caravaggio Hospital, Treviglio, Italy; e-mail: sandro.barni@ospedale.treviglio.bg.it.
References
Author notes
S.B., M.V., G.G., and G.A. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal