In this issue of Blood, Castleton et al show that mesenchymal stromal cells (MSCs) can deliver oncolytic measles virotherapy directly into acute lymphoblastic leukemia (ALL) cells, even in the face of high-titer humoral antiviral immunity, leading to remarkable therapeutic success in a murine model of disseminated ALL.1
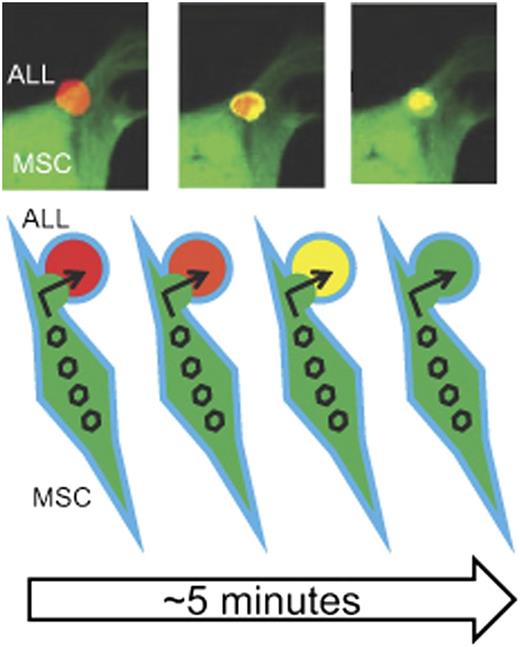
MSC delivery of MV to ALL cells. The top portion of the figure depicts live cell confocal microscopy showing the transfer (“handoff”) of GFP-expressing MV from a virus-loaded MSC (green) to an RFP-expressing Nalm-6 ALL cell. The ALL cells shift from red to yellow (and eventually green) fluorescence as more GFP-expressing virus enters within the RFP-expressing cell. Both cells will eventually undergo viral-mediated lysis. The bottom portion of the figure is a schematic representation of the cell MSC-ALL cell fusion and passage of GFP-expressing virus (open hexagon) into the RFP-expressing Nalm 6 ALL cell depicted by the red-orange-yellow-green color shift as the viral (green) fluorescence blends with and then overtakes the ALL cell (red) fluorescence. GFP, green fluorescent protein; RFP, red fluorescent protein. The top three panels have been adapted from Figure 4 in the article by Castleton et al that begins on page 1327.
MSC delivery of MV to ALL cells. The top portion of the figure depicts live cell confocal microscopy showing the transfer (“handoff”) of GFP-expressing MV from a virus-loaded MSC (green) to an RFP-expressing Nalm-6 ALL cell. The ALL cells shift from red to yellow (and eventually green) fluorescence as more GFP-expressing virus enters within the RFP-expressing cell. Both cells will eventually undergo viral-mediated lysis. The bottom portion of the figure is a schematic representation of the cell MSC-ALL cell fusion and passage of GFP-expressing virus (open hexagon) into the RFP-expressing Nalm 6 ALL cell depicted by the red-orange-yellow-green color shift as the viral (green) fluorescence blends with and then overtakes the ALL cell (red) fluorescence. GFP, green fluorescent protein; RFP, red fluorescent protein. The top three panels have been adapted from Figure 4 in the article by Castleton et al that begins on page 1327.
Oncolytic virotherapy is widely studied for the treatment of solid tumors. The attraction of this approach lies in the mechanism of action and resistance distinct from other therapies as well as the nonoverlapping toxicities and generally acceptable safety profile.2 Indeed, the promise of this therapy has been established in early-phase clinical trials.3 Far less attention in this field has focused on hematologic malignancies, possibly due to the disseminated nature of leukemia in contrast to discrete masses of solid tumor, inferring that leukemia is less suitable as an oncolytic viral target. A notable exception is the investigation of vaccine-strain, live, attenuated measles virus (MV) as oncolytic therapy for B-cell malignances.4,5 However, the high frequency of antiviral immunity due to prior infection, vaccination, or recurrent therapeutic exposure may present an obstacle to any successful systemic virotherapy, but especially with the MV given the widely immunized population.6
Castleton et al use bone marrow–derived MSCs as carriers (effectively ALL-targeting producer cells) of the oncolytic MV to circumvent the anti-measles humoral immunity found in most patients which does not seem to be abrogated by their prior chemotherapy.1 MSCs are an attractive cell carrier as they are easily isolated from a bone marrow aspirate, readily ex vivo–expanded under good manufacturing practice conditions, and may be infused across HLA barriers. The hundreds of clinical trials worldwide over the last 2 decades persuasively support the safety of these cells for clinical applications.
Castleton et al demonstrated that clinically relevant anti-MV immunglobulin G antibody titers persisted in 16 study patients treated in the UK ALL 14 Multicenter Trial, illustrating the impending challenge of using oncolytic measles virotherapy in these patients. In an effort to investigate MSCs as a carrier to overcome the neutralizing effect of the antibodies, they first demonstrated that these cells expressed CD46, the vaccine strain receptor, proving the susceptibility of MSCs to MV infection and the potential to use MSCs as carriers. They generated human MSCs from bone marrow, defining their cells according to the widely accepted International Society for Cellular Therapy (ISCT) criteria,7 and then optimized the conditions for in vitro MV infection. Importantly, they characterized the time course for maximal viral production within the cells, delineating the conditions needed for production and infusion of the viral-loaded MSCs. Using an established murine model of ALL (Nalm-6 cells [precursor B-lineage leukemia cell line] intravenously infused into SCID mice), they showed by unambiguous bioluminescence that MV-loaded MSCs localize to sites of proven bone marrow ALL after intravenous infusion. Then, using 2-color live cell confocal microscopy of cells in culture, they elegantly demonstrated that viable replicating MV is directly transferred (“handoff”) from the MSCs to Nalm-6 cells by a mechanism that involves, at least in part, an MSC-Nalm-6 cell fusion (see figure). Finally, to demonstrate the clinical applicability of MSC delivery of MV to ALL, they treated SCID mice with established Nalm-6 cell ALL with MV or MV-loaded MSCs, with and without anti-MV antibodies. The original idea that persisting anti-MV antibodies would block any benefit of MV therapy was validated as the survival benefit of MV therapy was abolished by anti-MV antibodies. Additionally, mice receiving MSC-MV showed a benefit, demonstrating that MSCs can deliver the oncolytic virus to ALL cells in vivo. Importantly, the central thesis of this work, the neutralizing effect of anti-MV antibodies could be overcome using cell carriers, was proven correct as mice receiving MSC-MV maintained the observed benefit in the presence of anti-MV antibodies.
These observations have enormous implications for biologic therapy of leukemia. Although the specific message conveyed by this work is that MSCs can be used to deliver oncolytic measles virotherapy to ALL cell targets and shelter the virus from the patient’s humoral immunity, the extension of these results may have far-reaching consequences. First, these data establish proof-of-concept that MSCs can target disseminated leukemia in vivo, opening the door to countless opportunities to target malignant hematopoietic cells or the associated microenvironment. Second, inspection of the survival curves of leukemic mice show that the animals treated with MSC-MV fared better than those treated with MV alone without anti-MV antibodies (P = .02). Thus, MSCs seem to enhance the targeting of MV to ALL compared with the naked virus, suggesting that MSC virotherapy may be superior to systematic infusions of oncolytic viruses, despite the propensity of these viruses to infect and lyse malignant cells. Given the susceptibility of MSCs to viral infection in vitro and the relative ease of genetically engineering MSCs, the work of Castleton et al will likely spawn a wave of new studies of MSC-based biologic therapy of hematologic malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal