In this issue of Blood, Cortés-Puch and colleagues investigate a strategy to improve the quality of stored red blood cells (RBCs) by washing the blood, thereby removing “noxious” substances, including cell-free hemoglobin, transferrin-bound iron, non–transferrin-bound iron (NTBI), and plasma labile iron, that accumulate during storage.1 Cortés-Puch and colleagues found that in a canine model of Staphylococcus aureus pneumonia, transfusion of “old” (42-day) blood was associated with impaired hemodynamics, greater lung injury and shock index, and reduced survival. These outcomes were improved upon when washing RBCs, which was associated with a reduction of NTBI in the circulation. Importantly, washing “fresh” (7-day) RBCs had the opposite effect, reversing the improved survival that was observed after exchange transfusion with younger RBCs; this effect was also related to an increased release of NTBI.
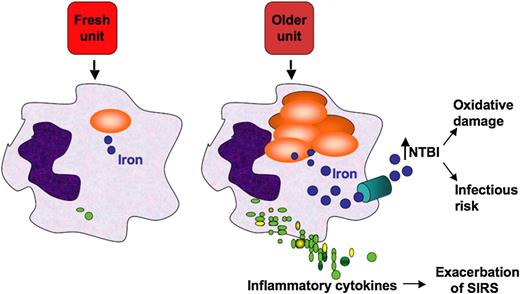
Proposed mechanistic pathway (the “iron hypothesis”) explaining how transfusion of older stored RBCs may induce adverse effects in patients. Transfusion of stored, but not fresh, RBCs delivers an acute bolus of RBCs and RBC-derived iron to the monocyte/macrophage system, resulting in oxidative stress and inflammatory cytokine secretion. Some of the macrophage-ingested iron is also released back into the circulation (ie, NTBI), where it can also cause oxidative damage and enhance bacterial proliferation. SIRS, systemic inflammatory response syndrome. Reprinted from Hod et al6 with permission.
Proposed mechanistic pathway (the “iron hypothesis”) explaining how transfusion of older stored RBCs may induce adverse effects in patients. Transfusion of stored, but not fresh, RBCs delivers an acute bolus of RBCs and RBC-derived iron to the monocyte/macrophage system, resulting in oxidative stress and inflammatory cytokine secretion. Some of the macrophage-ingested iron is also released back into the circulation (ie, NTBI), where it can also cause oxidative damage and enhance bacterial proliferation. SIRS, systemic inflammatory response syndrome. Reprinted from Hod et al6 with permission.
The American Medical Association has identified overuse of 5 medical treatments, including blood transfusions along with cardiac stents, ear tubes, antibiotics, and the induction of birth in pregnant women, and has highlighted the danger of unnecessary transfusion. Although blood transfusion has long been considered to be a safe and effective therapy for patients with anemia, increasing evidence has identified adverse patient outcomes that are associated with RBC transfusion.2 Rather than conferring a benefit, blood transfusions may in fact be injurious.3
Retrospective, observational studies have suggested that the etiology of a number of the adverse events associated with RBC therapy may be due to storage lesions in banked blood.4,5 Residual plasma and white blood cells present in stored blood contain inflammatory mediators, free radicals, macromolecule oxidation products, dead and broken cells and vesicles, deranged electrolytes, and other components contributing to “storage lesions.” Measures such as leukoreduction and improved storage solutions have generally been able to help with this situation, albeit to limited extents.
The possibility that an iron effect might be a risk of blood transfusion has been proposed and investigated previously. Hod and colleagues6 found that in a mouse model, transfusions with stored RBCs increased NTBI and initiated inflammation compared with mice transfused with fresh RBCs. They subsequently reported that in human volunteers reinfused with either fresh (3- to 7-day) or old (40- to 42-day) autologous blood, significant differences between the fresh and older transfusions were found only in iron parameters and markers of extravascular hemolysis.7 The mechanisms by which these observed effects were achieved remain elusive; nevertheless, circulating NTBI derived from rapid clearance of transfused, older RBCs may promote transfusion-related complications, including risk of infection (see figure).
It has long been known that a significant storage lesion is present in stored RBCs that impairs the intended benefit of a blood transfusion.8 The depletion of intracellular diphosphoglycerate (2,3 DPG) in stored RBCs results in a left shift of the oxygen dissociation for hemoglobin, so that although transfusion of RBCs increases oxygen carrying capacity acutely, the transfused RBCs give up oxygen reluctantly to the tissues in the peripheral circulation until after a time-dependent repletion of intracellular 2,3 DPG occurs in vivo in the transfused RBC. It is therefore likely that any perceived patient benefit observed acutely (within 6 hours or so) from a blood transfusion is due to an increase in volume rather than any increase in oxygen consumption as a consequence of an increase in oxygen delivery. On the risk side, definitive evidence for whether transfusion of stored RBCs is causal for increased morbidity and mortality, rather than being simply associated with adverse patient outcomes, awaits completion of an important ongoing, prospective, randomized trial in adult patients undergoing open heart surgery.9 The relative benefits/risks of blood are therefore important elements in any decision to transfuse, especially when alternative therapies are available for the management of anemia.10 In the meantime, changes in strategies of blood inventory management, such as preferential issuing of fresher RBCs or washing RBCs, must wait until such evidence is in hand.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal