In this issue of Blood, Byrd et al present first-in-man data in chronic lymphocytic leukemia (CLL) using otlertuzumab (TRU-016), a fully humanized anti-CD37 protein therapeutic. Although this novel immunotherapeutic shows modest single-agent activity, combinations with phosphatidylinositol-3-kinase (PI3K) inhibitors might open new future perspectives.1
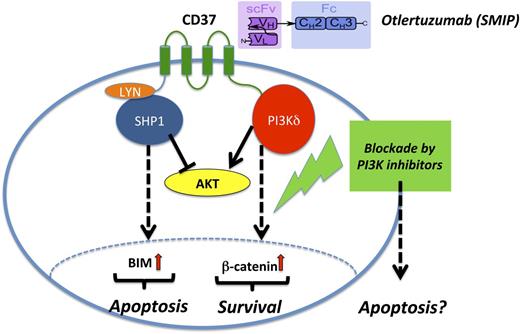
Mode of action of otlertuzumab (TRU-016) in CLL. Otlertuzumab represents a SMIP with an antibody-derived scFv specific for CD37 linked to an immunoglobulin constant domain (Fc). After ligation of CD37 by otlertuzumab, proapoptotic SHP1 molecules are recruited that finally result in B-cell lymphoma 2 interacting mediator of cell death upregulation and cell death. In contrast, CD37 ligation also results in prosurvival signals via the PI3K pathway via activation of β-catenin. Therefore, PI3K blockade by specific inhibitor, for example, idelalisib, might foster proapoptotic effects in the CLL cell.
Mode of action of otlertuzumab (TRU-016) in CLL. Otlertuzumab represents a SMIP with an antibody-derived scFv specific for CD37 linked to an immunoglobulin constant domain (Fc). After ligation of CD37 by otlertuzumab, proapoptotic SHP1 molecules are recruited that finally result in B-cell lymphoma 2 interacting mediator of cell death upregulation and cell death. In contrast, CD37 ligation also results in prosurvival signals via the PI3K pathway via activation of β-catenin. Therefore, PI3K blockade by specific inhibitor, for example, idelalisib, might foster proapoptotic effects in the CLL cell.
CD37 is almost exclusively expressed on mature human B cells and B-cell–derived lymphoid malignancies, arguing for its role as a prime target molecule for CLL immunotherapy.2 Otlertuzumab, formerly known as TRU-016, is a CD37-specific single-chain, homodimeric therapeutic protein based on modular protein technology: antibody-derived single-chain variable fragments (scFv) specific for CD37 are linked to immunoglobulin constant domains. This small modular immunopharmaceutical (SMIP) is smaller in size compared with classic antibodies and shows higher antibody-dependent cellular toxicity than most recombinant antibodies. Otlertuzumab was developed by humanizing SMIP-016, a mouse/human chimeric protein that demonstrated antitumor activity against lymphoid malignancies in preclinical studies, including in human B-cell tumor mouse xenograft models.3
In this phase 1 study, treatment-naive and pretreated CLL patients received otlertuzumab IV up to a dose of 30 mg/kg per week.1 Including the dose-escalation and expansion phase of this trial, all together 83 patients were treated. Besides dose-limiting toxicity–relevant hematologic toxicities, including grade 4 neutropenia and thrombocytopenia observed in 4 patients, the safety profile was quite acceptable. Adverse events included mostly fatigue, nausea, diarrhea, chills, and pyrexia as expected from immunotherapeutics. Although the treatment with otlertuzumab seems promising in treatment-naive patients with an 86% response rate, data are quite disappointing in pretreated patients: here, only 17% of them showed an objective response. The low overall response rate of 23% (National Cancer Institute 96 [NCI-96] criteria) or of 20% (International Workshop on Chronic Lymphocytic Leukemia 2008 [IWCLL-2008] criteria including computed tomography imaging), respectively, can be partially explained by the fact that this trial included many high-risk patients (n = 46) harboring a 17p or an 11q deletion or even both.
However, given the fact that frontline therapies in CLL become more and more effective, not only in the younger and fit ones, but also in the elderly and comorbid population, we will probably face more high-risk patients with unfavorable molecular and cytogenetic risk profiles in the relapsed setting in the future.4,5 And to be honest, the competition is overwhelming: small molecules blocking signaling pathways through the B-cell receptor (BCR), that is, Bruton tyrosine kinase (BTK) and PI3K inhibitors, seem to be much more powerful compared with otlertuzumab. These small molecules seem to work regardless of the numbers of pretreatments and irrespective of the genetic or molecular risk parameters. The BTK inhibitor ibrutinib and the PI3Kδ inhibitor idelalisib easily induce remissions in roughly three-quarters of relapsed/refractory patients with CLL.6,7 As if that is not enough, inhibitors of the BCL-2 protein family also broaden the spectrum of weapons in the attack against CLL cells. ABT-199, a highly potent, orally bioavailable, and BCL-2–selective inhibitor has shown promising data in CLL patients with high-risk cytogenetic aberrations and/or fludarabine-refractory disease.8 Nowadays, modern trials try to answer the question of whether a combination of these classes of drugs, eventually also combined with monoclonal antibodies, might be even more beneficial for the patient, not only in the relapse setting. And just to make the picture more complete and to mention other competitors in the field of immunotherapeutics: we all have followed up on innovations like chimeric antigen receptors with specificity for the B-cell antigen, coupled with sophisticated CD137 and CD3-ζ signaling domains.9
Given these amazing developments in the last few years in the field of CLL, otlertuzumab (TRU-016) seems to be more a true toddler, at least in the monotherapy setting. But a closer look on the mode of action might open a window of opportunity for this second-generation immunotherapeutic. Upon ligation by SMIPs like otlertuzumab, the CD37 molecule initiates events resulting as a net effect in apoptosis, but in this context, 2 opposing mechanisms by different tyrosine residues of CD37 have recently been elucidated: on the one hand, an immunoreceptor tyrosine-based inhibitory–like motif fosters recruitment of the proapoptotic phosphatase SHP1, and on the other hand, an immunoreceptor tyrosine-based activation motif enhances counteracting prosurvival signals by mediating PI3K-dependent survival.10 Therefore, a combination of otlertuzumab with blockade of PI3K signaling could shift this balance and enhance the proapoptotic axis (see figure). This seems much more rational than just combining an immunotherapeutic with classic cytotoxic drugs or just another monoclonal antibody. Whether this indeed would result in synergistic clinical outcomes in favor of the individual patient with CLL has to be tested, but rather soon. This could further pave the road for a chemo-free treatment of CLL thus combining modern small molecules like BCR inhibitors and next-generation immunotherapeutics like SMIPs.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal