In this issue of Blood, Shim et al report that monoclonal B-cell lymphocytosis (MBL), an essential precursor to chronic lymphocytic leukemia (CLL), is a surprisingly common finding in healthy blood donors, thus raising concerns about the potential risk infusion of these blood products may present to recipients.1
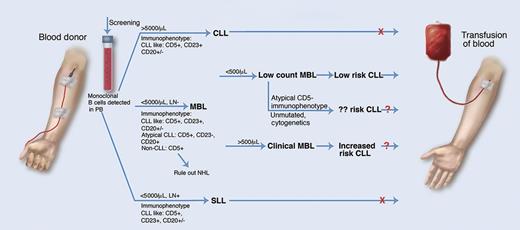
MBL in blood transfusion. Monoclonal B-cell lymphocytes are common in healthy blood donors and their detection may affect donor eligibility. MBL is defined as <5000 monoclonal B cells per μL of blood and no lymph node involvement. Low-count MBL (low risk of development of CLL, although risk may be affected by other factors) has <500 cells per μL and clinical MBL (increased risk of CLL) has >500 cells per μL. SLL, small lymphocytic leukemia. Professional illustration by Marie Dauenheimer.
MBL in blood transfusion. Monoclonal B-cell lymphocytes are common in healthy blood donors and their detection may affect donor eligibility. MBL is defined as <5000 monoclonal B cells per μL of blood and no lymph node involvement. Low-count MBL (low risk of development of CLL, although risk may be affected by other factors) has <500 cells per μL and clinical MBL (increased risk of CLL) has >500 cells per μL. SLL, small lymphocytic leukemia. Professional illustration by Marie Dauenheimer.
What does this mean for these blood donors and recipients? In the early days of flow cytometry, detection of circulating monoclonal B cells was considered diagnostic of B-cell neoplasia. However, studies emerged demonstrating that CLL-like monoclonal B-cell populations could be detected in the peripheral blood2 and possibly even in the lymph nodes3 of healthy adults, revolutionizing our understanding of the diagnostic interpretation of such a finding. MBL is not a homogeneous condition and the clinical significance of this finding is still not completely understood in every case. Although in low-count MBL (<500 monoclonal B cells per μL), the risk of evolution into CLL is low, in clinical MBL (>500/μL), the risk is significant, with 1% to 2% of patients per year progressing to CLL requiring therapy.4 Additional factors, such as IGHV gene usage, mutational status, ZAP-70 expression, and specific cytogenetic abnormalities (eg, 13q14 deletion), may affect risk of progression to CLL.5 Thus, MBL is similar to monoclonal gammapathy of uncertain significance and can be viewed as a premalignant condition with risk stratification based upon multiple factors.6
MBL is often detected during a clinical evaluation of an elevated peripheral blood lymphocyte count, although it can be an incidental finding in evaluation of other clinical symptoms or findings.2 It has also been described in directed studies of nonaffected members of familial CLL pedigrees and populations with environmental exposure to toxic waste.2 The incidence of MBL ranges from 1% to 18% in these decidedly nonnormal groups. The study by Shim et al, however, is of normal healthy blood donors and not individuals with abnormal findings or a familial or environmental exposure history placing them at increased risk of CLL. Their observation of 7.1% MBL is a better indication of the incidence of MBL in the general population and is consistent with the 7.4% observed in healthy volunteers in Italy. In the current study, gender (male predominance) and age were independent risk factors for MBL with a 1.4-fold increase in MBL per 10 years’ age among men. The incidence of MBL is known to increase with age; a study by Almeida et al indicates that low-count MBL may be present in the vast majority of individuals >70 years old7 and may be considered a normal finding in this age group. In >96% of the cases, low-count MBL was detected, with the majority having a CLL-like low-count MBL (but 14.8% were atypical [or CD5-negative low-count MBL] which may have a different prognosis). A total of 3.4% of the MBL cases were clinical MBL with the associated increased risk of progression to CLL. In addition, 18% of the MBL cases had germline, nonmutated status which may have increased risk of progression to CLL.
The transfer of MBL from donor to recipient has been reported in the setting of allogeneic stem cell transplantation,8 although this is not comparable to blood transfusion in an immune-competent individual. Furthermore, MBL has not been reported posttransfusion, although there is an increased risk for development of B-cell neoplasia, and in particular CLL, with blood transfusions.9 In view of the surprisingly common finding of MBL in normal healthy donors, one has to wonder whether there is a potential risk of transfer of a premalignant condition to recipients of blood transfusions, especially in cases of clinical MBL. In addition, the clinical implications of nonmutated status as well as CD5-negative or atypical CLL immunophenotype in low-count MBL are not fully understood and need further clarification. Therefore, the recommendation of Shim et al for a conservative approach to blood transfusions is warranted.
The implications of this study also raise questions concerning screening of donated blood (see figure). Because low-count MBL has minimal or negligible risk of progression, blood from such donors is likely acceptable, but what about clinical MBL? Should donors with a low-level lymphocytosis be screened for MBL, and how would this affect criterion for rejection of donated blood product? Further studies into the possible transference of MBL to blood transfusion recipients are indicated before these questions can be answered. A look-back study at donors of CLL or MBL recipients would be of interest, and the formation of a blood donor clinical MBL cohort for longitudinal study would be equally valuable.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal