Key Points
Hypoxia induces altered platelet proteome/reactivity, which correlates with a prothrombotic phenotype.
CAPNS1-dependent calpain activity in platelet activation cascade is associated with hypoxia-induced thrombogenesis.
Abstract
Oxygen-compromised environments, such as high altitude, air travel, and sports, and pathological conditions, such as solid tumors, have been suggested to be prothrombotic. Despite the indispensable role of platelets in thrombus formation, the studies linking hypoxia, platelet reactivity, and thrombus formation are limited. In the present study, platelet proteome/reactivity was analyzed to elucidate the acute hypoxia-induced prothrombotic phenotype. Rats exposed to acute simulated hypoxia (282 torr/8% oxygen) demonstrated a decreased bleeding propensity and increased platelet reactivity. Proteomic analysis of hypoxic platelets revealed 27 differentially expressed proteins, including those involved in coagulation. Among these proteins, calpain small subunit 1, a 28-kDa regulatory component for calpain function, was significantly upregulated under hypoxic conditions. Moreover, intraplatelet Ca2+ level and platelet calpain activity were also found to be in accordance with calpain small subunit 1 expression. The inhibition of calpain activity demonstrated reversal of hypoxia-induced platelet hyperreactivity. The prothrombotic role for calpain was further confirmed by an in vivo model of hypoxia-induced thrombosis. Interestingly, patients who developed thrombosis while at extreme altitude had elevated plasma calpain activities and increased soluble P-selectin level. In summary, this study suggests that augmented calpain activity is associated with increased incidence of thrombosis under hypoxic environments.
Introduction
Hypoxia, experienced either during physical activities such as ascent to mountains, air travel, or sports activities or with pathological conditions such as solid tumors has been suggested to be associated with thrombotic episodes.1-5 In the case of high-altitude hypoxic exposure, both venous as well as arterial thrombotic events can occur, which include pulmonary thromboembolism, cerebral venous thrombosis, portal vein thrombosis, aortic thrombosis, stroke, and transient ischemic attack.6-9 At extreme altitude, adverse environmental conditions including hypobaric hypoxia and cold may facilitate the development of the corresponding prothrombotic phenotype.
In the past, various studies have been reported, focusing on hematological factors and on proteins involved in thrombin generation and fibrinolysis, to understand altitude-induced thrombotic events.10-13 Although platelets play an indispensable role in thrombogenesis, the involvement of platelets in hypoxia-induced thrombotic events has not been adequately explored. Most of the studies were focused on changes in platelet numbers; only a few reports focused on platelet reactivity at high altitude.14-17 In fact, in chronic obstructive pulmonary disease and sleep apnea, hypoxia has been associated with increased platelet reactivity.18,19 Platelet hyperreactivity, reflected by enhanced platelet adhesion, activation, and aggregation, is a sum of finely coordinated cell signaling events involving a shift in platelet proteome/secretome and structural proteins. In conjunction with this, the tightly controlled cytosolic Ca2+ also acts as an important secondary messenger to regulate the fundamental platelet reactivity via the key downstream signaling cascades.20
The proteome analysis, which is used for identifying novel proteins and pathways, has become an ideal tool to study anucleated cells such as platelets. Previously, platelet proteomic analysis revealed differential regulation of proteins in response to agonists and in diseases such as acute coronary syndrome.21,22 The cellular functions in platelets are regulated primarily by changes in protein expression and their modifications. In the present study, exposure of Sprague-Dawley rats to acute high-altitude hypoxia resulted in platelet hyperreactivity, leading to a prothrombotic phenotype. The proteome analysis of these phenotypically altered platelets revealed differential expression of proteins, which are involved in coagulation, calcium homeostasis, signal transduction, acute phase response, and cytoskeletal reorganization. The disturbed calcium homeostasis was further explored by quantifying cytosolic Ca2+ levels and calpain activity in platelets of hypoxic animals. Calpain small subunit 1 (CAPNS1), which functions like a chaperone for calpain proteases23 and controls cell spreading and migration,24 was found to be upregulated in hypoxia-exposed animals. Taking into consideration that calpains are activated by elevated cytosolic Ca2+ and are involved in platelet reactivity,25,26 the prothrombotic role for calpain under hypoxia was further confirmed by in vivo model of hypoxia-induced thrombosis. To investigate the potential of these preclinical investigations for clinical relevance, the calpain activity and soluble P-selectin (sP-selectin) level were analyzed in plasma samples of patients who developed deep vein thrombosis (DVT) at high altitudes (>3648 meters). The results from these studies for the first time suggest that a hypoxic environment alters the platelet proteome and induces platelet hyperreactivity, leading to a prothrombotic phenotype that is mainly mediated by activation of calpain.
Materials and methods
Materials
α-thrombin, adenosine diphosphate (ADP), and chronolume luciferin-luciferase reagents were purchased from Chrono-log. Anti-CD41 and anti-αIIbβ3 antibodies were purchased from Abcam. Immobilized pH gradient gel strips, ampholytes, and mineral oil for 2-dimensional electrophoresis were products of GE Healthcare, and trypsin was from Promega. Rat-specific enzyme-linked immunosorbent assay (ELISA) kits were purchased from Bmassay and USCN Life Sciences Inc. PD150606 was from Tocris Biosciences. The reverse transcription-polymerase chain reaction (RT-PCR) kit for platelet RNA analysis and all other reagents were from Sigma-Aldrich.
Animal exposure to simulated high-altitude conditions
All experiments were conducted in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India. Male Sprague-Dawley rats weighing 150 to 250 g were kept under standard laboratory conditions. The animals were exposed to simulated high-altitude (hypobaric hypoxia) conditions in a specially designed animal decompression chamber, which was maintained at pressure of 282 torr (equivalent to an altitude of 7620 meters with 8% oxygen) and at 10°C for various durations. Animals were exposed to simulated hypobaric hypoxia with cold surrounding, as at high-altitude regions, the associated low temperatures may also affect the biological systems. After optimization of the temperature range, 10°C was selected as a suitable temperature and was used for further hypoxic exposure studies. On the basis of initial hematological and coagulation screening data and previous studies, platelet studies were conducted after 6 hours of exposure to simulated altitude.
Flow-restriction animal model
To model the localized hypoxia-induced thrombosis, thrombus was induced in rats by proximal ligation of the inferior vena cava (IVC) just below the renal veins and ligation of lateral tributaries, as previously described27 with some modifications (see the supplemental Methods on the Blood Web site for details).
Human studies
Young (<40 years) male patients with lower limb DVT evacuated to either Western Command Hospital Chandimandir, Chandigarh, or Army Research and Referral Hospital, Delhi, India (tertiary care hospitals), were approached for consent to participate in the study. All patients (n = 10) had onset of DVT while placed at high altitudes (>3648 meters). Patients with preexisting systemic diseases, malignancy, any prior surgery, or vasculitis were excluded. All patients had their diagnosis confirmed by objective imaging methods. A complete thrombophilia screening assessment, composed of protein C and S deficiency, antithrombin III deficiency, factor V Leiden, and prothrombin 20210G/A polymorphism screening, was also performed; laboratory investigations details are provided in the supplemental Methods. Equal numbers of healthy, age-matched male participants were taken as controls, with no prior history for risk factors. Informed consent was obtained according to the Declaration of Helsinki.
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM). The statistical significance between 2 groups was tested by nonparametric unpaired 2-tailed Student t test, using Prism 5 software, and for multiple-group comparison, 1-way analysis of variance was applied, followed by Dunnett’s test. A P value less than .05 was considered significant.
Results
Hypoxic exposure results in hypercoagulative state
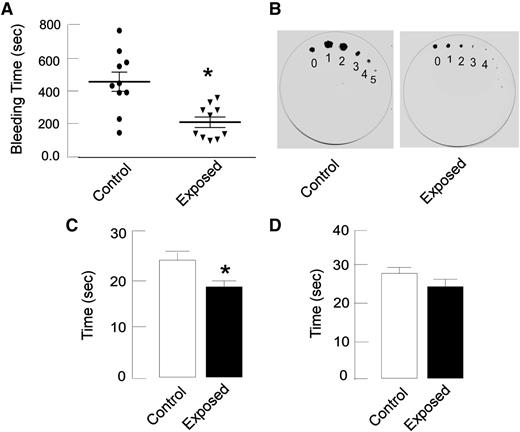
To investigate whether hypoxic exposure results in a prothrombotic phenotype in rats, bleeding time (by tail immersion and filter paper methods) and coagulation assays (prothrombin time, aPTT) were performed after hypoxic exposure. A significant shortening of bleeding times (202 ± 29 seconds) was observed in exposed animals compared with controls (440 ± 54 seconds; P < .05; n = 10; Figure 1A). The visual inspection of filter paper indicates the thickening of blood in exposed animals (Figure 1B). Similarly, coagulation assays demonstrated a hypercoagulative state under hypoxic environment, as reflected by a significantly decreased PT value and a similar trend in aPTT (Figure 1C-D). No significant change was observed in hemoglobin level, hematocrit levels, or platelet count in hypoxia-exposed animals, with the exception of a decrease in white blood cell count (supplemental Table 1).
Hypoxia exposure results in hypercoagulation. Rats were exposed to simulated hypoxic conditions as described in Materials and methods; tail vein bleeding assay, PT, and activated partial thromboplastin time (aPTT) assays were performed. (A) Rat tail was transected 4 mm from the tip and immersed in warm normal saline (37°C), and the time taken for complete cessation of blood flow was noted (it was significantly lower in hypoxia-exposed rats than in controls). (B) In the filter paper method, the tail tip was blotted gently onto Whatman paper at 1-minute intervals. The diameter of blood spots and the duration of bleeding were significantly lower in exposed rats compared with control rats. (C-D) PT (C) and aPTT (D) were measured in citrated plasma and reflected a similar trend as bleeding time. Data are presented as mean ± SEM (n = 10). *P < .05 vs control.
Hypoxia exposure results in hypercoagulation. Rats were exposed to simulated hypoxic conditions as described in Materials and methods; tail vein bleeding assay, PT, and activated partial thromboplastin time (aPTT) assays were performed. (A) Rat tail was transected 4 mm from the tip and immersed in warm normal saline (37°C), and the time taken for complete cessation of blood flow was noted (it was significantly lower in hypoxia-exposed rats than in controls). (B) In the filter paper method, the tail tip was blotted gently onto Whatman paper at 1-minute intervals. The diameter of blood spots and the duration of bleeding were significantly lower in exposed rats compared with control rats. (C-D) PT (C) and aPTT (D) were measured in citrated plasma and reflected a similar trend as bleeding time. Data are presented as mean ± SEM (n = 10). *P < .05 vs control.
As hypoxia exposure to rats also involved cold surroundings (chamber temperature of 10°C), a bleeding time assay was also performed in rats exposed to hypoxia under normothermic surrounding (chamber temperature of 28°C) to determine the possible effect (if any) of moderate cold surroundings on hypercoagulative tendency. Exposure under normothermic conditions resulted in a significant reduction in bleeding time, as under hypothermic conditions (supplemental Figure 1A). Collectively, these results support the view that altitude hypoxic conditions induce a prothrombotic phenotype.
Exposure to hypoxia induces platelet hyperreactivity
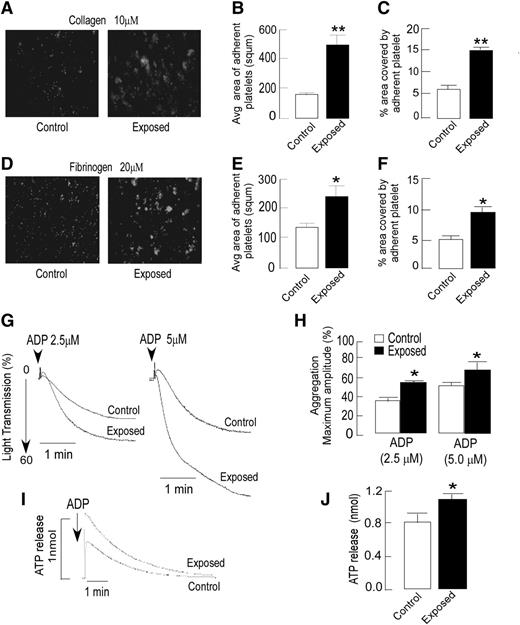
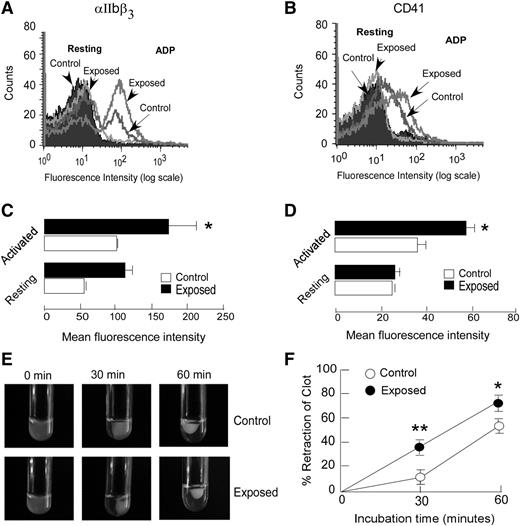
The effect of hypoxic exposure on platelets’ tendency for adhering to extracellular matrix proteins was investigated in vitro by platelet adhesion assay. Platelets isolated from exposed animals demonstrated an enhanced adhesion to both collagen- and fibrinogen-coated surfaces. The average size of adhered platelets and percentage area covered by the same were significantly higher in the exposed group compared with the controls (Figure 2A-F); the increase was greater on the collagen surface. Next, we tested whether platelet aggregation and dense granule release were affected by hypoxic exposure. Platelets from exposed animals showed significantly increased aggregation in response to ADP in a dose-dependent manner (Figure 2G-H). Both rate and extent of platelet aggregation were higher in the exposed group compared with the response to ADP (2.5 and 5 µM; P < .05), whereas only a modest difference was observed with thrombin (data not shown). The dense granule release, as evaluated by luminescence-based ATP release, was also found to be significantly higher (P < .05) in platelets from exposed animals compared with those from controls (Figure 2I-J). Activated platelets bear increased numbers of various transmembrane proteins, which serve as platelet activation markers. A flow cytometry-based approach was used for analyzing the surface expression of CD41 and αIIbβ3. The anti-αIIbβ3 antibody that interacts with the αIIbβ3 receptor complex28 demonstrated an enhanced surface expression of this complex that reflects greater platelet activation on induction with ADP in exposed animals than that seen in controls (Figure 3A,C). CD41, another platelet activation marker, also exhibited a similar response (Figure 3B,D). Because platelet aggregation requires the binding of fibrinogen to its receptor αIIbβ3 on the platelet surface,29 we further evaluated platelet–fibrinogen interactions, using an in vitro clot retraction assay. As shown in Figure 3E-F, hypoxic exposure resulted in higher reduction in clot size or increased the clot retraction. The clot retraction in exposed groups was 30% and 20% more than that of control groups at 30 and 60 minutes, respectively. These observations reflected higher platelet reactivity and greater platelet–fibrinogen interactions in exposed animals, which corroborates with the other platelet assays such as aggregation, activation, and adhesion.
Agonist-dependent increase in platelet adhesion, aggregation, and ATP release in exposed animals. (A-F) Platelets were isolated from both control and hypoxia-exposed rats, fluorescently labeled, and allowed to adhere to collagen or fibrinogen precoated plates for up to 60 minutes at 37°C. (A,D) Representative images of fluorescence microscopy-based platelet adhesion on type 1 collagen-coated (10 µM) and fibrinogen-coated (20 µM) plates, using fluorogenic dye calcein. Images were captured using a fluorescein isothiocyanate filter on a Motic Inverted Microscope AE31(×200 original magnification). (B-C,E-F) Quantitation of platelet adhesion data was expressed as average area or size of adhered platelet clumps and percentage area covered by adhered platelets on the collagen-coated plate (top) and the fibrinogen-coated plate (bottom) after a 60-minute incubation. Quantitation was performed by Motic ImagePlus 2.0 software. (G) For the platelet aggregation assay, platelet-rich plasma from rats of indicated groups, incubated at 37°C for at least 3 minutes, was induced by ADP with stirring at 1200 rpm and optically monitored. The rate and extent of ADP-induced platelet aggregation was higher in hypoxia-exposed animals compared with control animals. Representative aggregation curves are shown in response to ADP (2.5 and 5 µM). (H) A bar graph shows aggregation results expressed as maximal amplitude of aggregation. (I) Representative ATP release curve in response to ADP analyzed using a luciferase assay. (J) Quantitation of aggregation and ATP release were expressed as maximum amplitude. Data are presented (mean ± SEM) as average results of at least 3 independent experiments (n = 6). *P < .05, **P < .01 vs control. See the supplemental Methods for details.
Agonist-dependent increase in platelet adhesion, aggregation, and ATP release in exposed animals. (A-F) Platelets were isolated from both control and hypoxia-exposed rats, fluorescently labeled, and allowed to adhere to collagen or fibrinogen precoated plates for up to 60 minutes at 37°C. (A,D) Representative images of fluorescence microscopy-based platelet adhesion on type 1 collagen-coated (10 µM) and fibrinogen-coated (20 µM) plates, using fluorogenic dye calcein. Images were captured using a fluorescein isothiocyanate filter on a Motic Inverted Microscope AE31(×200 original magnification). (B-C,E-F) Quantitation of platelet adhesion data was expressed as average area or size of adhered platelet clumps and percentage area covered by adhered platelets on the collagen-coated plate (top) and the fibrinogen-coated plate (bottom) after a 60-minute incubation. Quantitation was performed by Motic ImagePlus 2.0 software. (G) For the platelet aggregation assay, platelet-rich plasma from rats of indicated groups, incubated at 37°C for at least 3 minutes, was induced by ADP with stirring at 1200 rpm and optically monitored. The rate and extent of ADP-induced platelet aggregation was higher in hypoxia-exposed animals compared with control animals. Representative aggregation curves are shown in response to ADP (2.5 and 5 µM). (H) A bar graph shows aggregation results expressed as maximal amplitude of aggregation. (I) Representative ATP release curve in response to ADP analyzed using a luciferase assay. (J) Quantitation of aggregation and ATP release were expressed as maximum amplitude. Data are presented (mean ± SEM) as average results of at least 3 independent experiments (n = 6). *P < .05, **P < .01 vs control. See the supplemental Methods for details.
Exposure to hypoxia induces higher surface expression of platelet activation markers and increased clot retraction. (A-D) Washed platelets from hypoxia-exposed and control animals were either stimulated with ADP (activated) or left unstimulated (resting). The αIIbβ3 and CD41 surface expressions were determined by flow cytometric analysis, using fluorescein isothiocyanate-conjugated antibodies. Flow cytometry histograms from representative experiments (A-B) and quantitation of fluorescence expressed as mean fluorescence intensities (C-D) are shown. Data are presented as mean ± SEM, a typical result of at least 3 independent experiments (n ≥ 6; *P < .05 vs activated control). (E-F) Clot retraction assay was performed in platelet rich plasma (PRP) isolated from control and exposed animals, as described in the supplemental Methods. The clot retraction in exposed animals was found to be significantly greater than in control animals. Shown are representative images of the clot retraction assay for different incubation periods; the clot size was quantified using ImageJ software and expressed as percent retraction of clot (mean ± SEM; n = 6). *P < .05, **P < .01 vs control. See the supplemental Methods for details.
Exposure to hypoxia induces higher surface expression of platelet activation markers and increased clot retraction. (A-D) Washed platelets from hypoxia-exposed and control animals were either stimulated with ADP (activated) or left unstimulated (resting). The αIIbβ3 and CD41 surface expressions were determined by flow cytometric analysis, using fluorescein isothiocyanate-conjugated antibodies. Flow cytometry histograms from representative experiments (A-B) and quantitation of fluorescence expressed as mean fluorescence intensities (C-D) are shown. Data are presented as mean ± SEM, a typical result of at least 3 independent experiments (n ≥ 6; *P < .05 vs activated control). (E-F) Clot retraction assay was performed in platelet rich plasma (PRP) isolated from control and exposed animals, as described in the supplemental Methods. The clot retraction in exposed animals was found to be significantly greater than in control animals. Shown are representative images of the clot retraction assay for different incubation periods; the clot size was quantified using ImageJ software and expressed as percent retraction of clot (mean ± SEM; n = 6). *P < .05, **P < .01 vs control. See the supplemental Methods for details.
Platelet function was also tested in rats exposed to hypoxia under normothermic surroundings (chamber temperature of 28°C) to determine whether moderate hypothermic surroundings (chamber temperature of 10°C) are contributing to platelet hyperreactivity under hypoxic environment. The hypoxia exposure under normothermic conditions resulted in a significant enhancement of rate and extent of platelet aggregation, as under hypothermic conditions (supplemental Figure 1B-D). In summary, all platelet functional assays demonstrated significantly enhanced platelet reactivity in response to hypoxic exposure.
Hypoxic exposure modulates platelet proteome
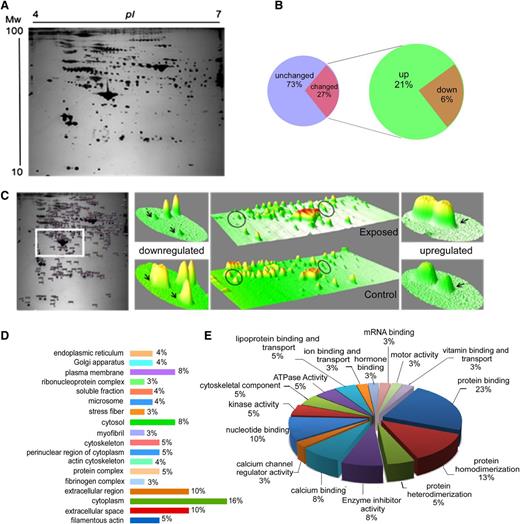
To obtain an insight into the molecular events underlying platelet hyperreactivity in response to hypoxic exposure, the platelet proteome was analyzed using 2-dimensional gel electrophoresis, followed by identification of differentially expressed proteins by matrix-assisted laser desorption-ionization time-of-flight tandem mass spectrometry (MS/MS) (Figure 4A-C). Initial optimizations suggested that a pH range of 4 to 7 was ideal for 2-dimensional-polyacrylamide gel electrophoresis on platelet samples, as this range provided the detection of the maximum number of protein features and improved resolution compared with other narrow pH ranges.
Platelet proteome analysis from control and exposed rats. Platelets were isolated from both control and hypoxia-exposed animals, and total platelet protein was prepared for platelet proteome analysis. Each protein lysate was subjected first to isoelectric focusing, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the second dimension, as described in the supplemental Methods. The differentially expressed proteins were identified using matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. (A) Representative 2-dimensional gel electrophoresis (2DE) gel image of platelet proteome (4-7 pI range, 13 cm). (B) The pie chart of platelet protein features from 2DE gel after differential analysis of gel images by Progenesis SameSpots software shows that 27% of platelet protein features in range of pH 4 to 7 were altered in hypoxia-exposed platelets. (C) A typical heat map of a selected portion from 2DE gels showing representative differential spots in 3 dimensions, prepared using ImageJ software. (D-E) Bioinformatic analysis of identified proteins was performed with the GeneCodis Web tool (http://genecodis.cnb.csic.es). (D) Gene Ontology cellular compartment analysis and (E) Gene Ontology molecular function analysis. See the supplemental Methods for details.
Platelet proteome analysis from control and exposed rats. Platelets were isolated from both control and hypoxia-exposed animals, and total platelet protein was prepared for platelet proteome analysis. Each protein lysate was subjected first to isoelectric focusing, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the second dimension, as described in the supplemental Methods. The differentially expressed proteins were identified using matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. (A) Representative 2-dimensional gel electrophoresis (2DE) gel image of platelet proteome (4-7 pI range, 13 cm). (B) The pie chart of platelet protein features from 2DE gel after differential analysis of gel images by Progenesis SameSpots software shows that 27% of platelet protein features in range of pH 4 to 7 were altered in hypoxia-exposed platelets. (C) A typical heat map of a selected portion from 2DE gels showing representative differential spots in 3 dimensions, prepared using ImageJ software. (D-E) Bioinformatic analysis of identified proteins was performed with the GeneCodis Web tool (http://genecodis.cnb.csic.es). (D) Gene Ontology cellular compartment analysis and (E) Gene Ontology molecular function analysis. See the supplemental Methods for details.
Software analysis of gel images resulted in the detection of more than 700 spots per gel, from which 258 spots were chosen for differential expression analysis. The MS/MS data analysis resulted in identification of 27 differentially expressed proteins in platelet samples obtained from exposed animals. From 27 identified proteins, 21 proteins had a MascotMowse score equal to or greater than 50 (Table 1), and 19 were upregulated, whereas 8 proteins were suppressed.
Platelet proteins differentially expressed in hypoxia-exposed animals
| Protein description . | Accession . | Mol wt . | Fold change . | Mowse score . | General function . |
|---|---|---|---|---|---|
| Apolipoprotein E | APOE_RAT | 35.7 | −4.2 | 214 | Mediates the binding, internalization, and catabolism of lipoprotein particles |
| Actin related Protein 2 | ARP2_RAT | 44.7 | −4.2 | 101 | Regulation of actin polymerization |
| Calpain small subunit 1 | CSS1_RAT | 28.5 | 5.3 | 38 | Regulation of calpain activity, cell migration |
| Calreticulin | CALR_RAT | 48 | −6.0 | 347 | Molecular calcium-binding chaperone, quality control in the endoplasmic reticulum, vascular regulatory antithrombotic role |
| Calumenin | CALU_RAT | 37 | −6.2 | 60 | Vascular regulatory, regulation of vitamin K-dependent carboxylation of multiple amino-terminal glutamate residues |
| Complement C3 precursor | CO3_RAT | 186 | −2.5 | 178 | Activation of the complement system |
| Coronin1A | COR1A_RAT | 51 | −2.9 | 74 | A crucial component of the cytoskeleton of highly motile cells |
| Elongation factor 1 γ | EF1G_RAT | 50 | −2.2 | 92 | Protein synthesis |
| α Enolase | ENOA_RAT | 47.1 | 3.0 | 68 | Activator of the complement system and a mediator of the local inflammatory response glycolysis, plays a part in growth control, hypoxia tolerance, and allergic responses |
| Fibrinogen α | FIBA_RAT | 86.6 | 2.7 | 90 | Yield monomers that polymerize into fibrin and acts as a cofactor in platelet aggregation |
| Fibrinogen γ | FIBG_RAT | 50.6 | 3.3 | 357 | Yield monomers that polymerize into fibrin and acts as a cofactor in platelet aggregation |
| Guanine nucleotide binding protein 2 | GBB2_RAT | 37.3 | −3.0 | 63 | Glycoproteins bound to plasma membrane, involved as a modulator or transducer in various transmembrane signaling systems |
| TF precursor | TF_RAT | 33.4 | 2.1 | 20 | Key component of extrinsic coagulation pathway |
| Haptoglobin precursor | HPT_RAT | 38.5 | 2.5 | 71 | Combines with free plasma hemoglobin, preventing loss of iron through the kidneys, secreted in response to hypoxia as well as acute inflammation |
| Heat shock cognate 71 | HSP7C_RAT | 70.8 | 2.3 | 150 | Chaperon, regulation of transcription, response to stress |
| T kininogen 1 | KNT1_RAT | 47.7 | 3.6 | 117 | Acute-phase protein |
| Myosin light chain PP 6 | MYL6_RAT | 16.9 | 5.9 | 87 | Motor protein, also involved in collagen-induced platelet activation |
| Tropomyosin α 1 | TPM1_RAT | 32.6 | 4.2 | 50 | Binds to actin filaments, cytoskeletal reorganization |
| Tropomyosin β | TPM2_RAT | 32.8 | 4.6 | 96 | Binds to actin filaments, cytoskeletal reorganization |
| Tropomyosin α 4 | TPM4_RAT | 28.5 | 2.0 | 64 | Binds to actin filaments, cytoskeletal reorganization |
| Serrotransferrin precursor | TRFE_RAT | 76.3 | 2.4 | 108 | Precursor to macromolecular activators of phagocytosis which enhance leukocyte phagocytosis |
| Janus Kinase 3 | JAK3_RAT | 122 | 3.6 | 26 | Cytokine-mediated signaling and regulation of cytosolic calcium. |
| α-1-antitrypsin | A1AT_RAT | 46.1 | 4.2 | 50 | Inhibitor of serine proteases; platelet isoform secreted in α granules |
| ρ Guanine nucleotide exchange factor 7 | ARHG7_RAT | 73 | 4.2 | 19 | Involved in ras-related C3 botulinum toxin substrate 1-dependent signaling, cell migration, attachment, and cell spreading. |
| Vitamin D binding Protein | VTDB_RAT | 53.5 | 2.0 | 99 | Carries vitamin D in plasma, has T lymphocyte surface association |
| α-2-macroglobulin receptor-associated protein precursor | AMRP_RAT | 42 | 5.7 | 33 | Binds to members of low-density lipoprotein receptor family and inhibits binding of their ligands |
| Nonmuscle caldesmon (L- caldesmon) | CALD1_RAT | 60.5 | 3.1 | 49 | Actin and myosin binding protein implicated in regulation of actomyosin interactions |
| Protein description . | Accession . | Mol wt . | Fold change . | Mowse score . | General function . |
|---|---|---|---|---|---|
| Apolipoprotein E | APOE_RAT | 35.7 | −4.2 | 214 | Mediates the binding, internalization, and catabolism of lipoprotein particles |
| Actin related Protein 2 | ARP2_RAT | 44.7 | −4.2 | 101 | Regulation of actin polymerization |
| Calpain small subunit 1 | CSS1_RAT | 28.5 | 5.3 | 38 | Regulation of calpain activity, cell migration |
| Calreticulin | CALR_RAT | 48 | −6.0 | 347 | Molecular calcium-binding chaperone, quality control in the endoplasmic reticulum, vascular regulatory antithrombotic role |
| Calumenin | CALU_RAT | 37 | −6.2 | 60 | Vascular regulatory, regulation of vitamin K-dependent carboxylation of multiple amino-terminal glutamate residues |
| Complement C3 precursor | CO3_RAT | 186 | −2.5 | 178 | Activation of the complement system |
| Coronin1A | COR1A_RAT | 51 | −2.9 | 74 | A crucial component of the cytoskeleton of highly motile cells |
| Elongation factor 1 γ | EF1G_RAT | 50 | −2.2 | 92 | Protein synthesis |
| α Enolase | ENOA_RAT | 47.1 | 3.0 | 68 | Activator of the complement system and a mediator of the local inflammatory response glycolysis, plays a part in growth control, hypoxia tolerance, and allergic responses |
| Fibrinogen α | FIBA_RAT | 86.6 | 2.7 | 90 | Yield monomers that polymerize into fibrin and acts as a cofactor in platelet aggregation |
| Fibrinogen γ | FIBG_RAT | 50.6 | 3.3 | 357 | Yield monomers that polymerize into fibrin and acts as a cofactor in platelet aggregation |
| Guanine nucleotide binding protein 2 | GBB2_RAT | 37.3 | −3.0 | 63 | Glycoproteins bound to plasma membrane, involved as a modulator or transducer in various transmembrane signaling systems |
| TF precursor | TF_RAT | 33.4 | 2.1 | 20 | Key component of extrinsic coagulation pathway |
| Haptoglobin precursor | HPT_RAT | 38.5 | 2.5 | 71 | Combines with free plasma hemoglobin, preventing loss of iron through the kidneys, secreted in response to hypoxia as well as acute inflammation |
| Heat shock cognate 71 | HSP7C_RAT | 70.8 | 2.3 | 150 | Chaperon, regulation of transcription, response to stress |
| T kininogen 1 | KNT1_RAT | 47.7 | 3.6 | 117 | Acute-phase protein |
| Myosin light chain PP 6 | MYL6_RAT | 16.9 | 5.9 | 87 | Motor protein, also involved in collagen-induced platelet activation |
| Tropomyosin α 1 | TPM1_RAT | 32.6 | 4.2 | 50 | Binds to actin filaments, cytoskeletal reorganization |
| Tropomyosin β | TPM2_RAT | 32.8 | 4.6 | 96 | Binds to actin filaments, cytoskeletal reorganization |
| Tropomyosin α 4 | TPM4_RAT | 28.5 | 2.0 | 64 | Binds to actin filaments, cytoskeletal reorganization |
| Serrotransferrin precursor | TRFE_RAT | 76.3 | 2.4 | 108 | Precursor to macromolecular activators of phagocytosis which enhance leukocyte phagocytosis |
| Janus Kinase 3 | JAK3_RAT | 122 | 3.6 | 26 | Cytokine-mediated signaling and regulation of cytosolic calcium. |
| α-1-antitrypsin | A1AT_RAT | 46.1 | 4.2 | 50 | Inhibitor of serine proteases; platelet isoform secreted in α granules |
| ρ Guanine nucleotide exchange factor 7 | ARHG7_RAT | 73 | 4.2 | 19 | Involved in ras-related C3 botulinum toxin substrate 1-dependent signaling, cell migration, attachment, and cell spreading. |
| Vitamin D binding Protein | VTDB_RAT | 53.5 | 2.0 | 99 | Carries vitamin D in plasma, has T lymphocyte surface association |
| α-2-macroglobulin receptor-associated protein precursor | AMRP_RAT | 42 | 5.7 | 33 | Binds to members of low-density lipoprotein receptor family and inhibits binding of their ligands |
| Nonmuscle caldesmon (L- caldesmon) | CALD1_RAT | 60.5 | 3.1 | 49 | Actin and myosin binding protein implicated in regulation of actomyosin interactions |
Bioinformatic analysis of identified differentially expressed proteins revealed that all these proteins belong to different cellular locations, such as the cell membrane, cytoskeletal, mitochondrial, endoplasmic reticulum, vesicular, and so on (Figure 4D), and were found to be involved in various important biological processes, including acute phase response, blood coagulation and complement activation, oxidative stress response, platelet activation, lipoprotein metabolism, lipid transport, vasodilation, and so on (Table 1). Furthermore, these proteins correspond to diverse molecular functions, including nucleotide binding, hormone binding, calcium channel regulation, ATPase activity, calcium binding, enzyme inhibition, and response to ions such as Fe3+, Mg2+, Ca2+, and so on (Figure 4E).
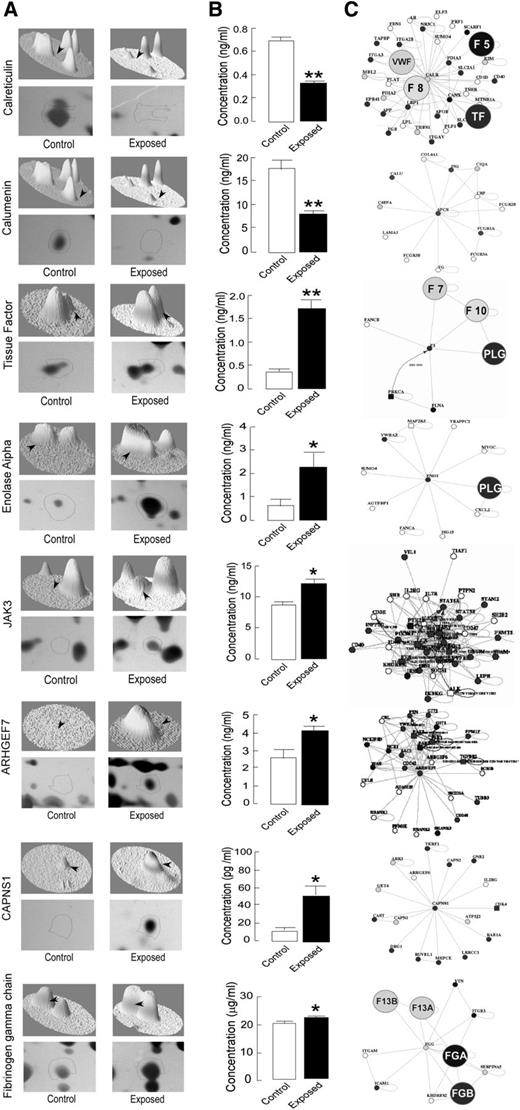
On the basis of the cutoff score, fold induction, and functional priorities, a protein list was prepared for analysis, validation, and follow-up experiments. To validate proteomic results, the levels of selective proteins, which included fibrinogen γ, calreticulin, calumenin, CAPNS1, nonneural α enolase, Janus kinase 3, and ρ guanine exchange factor 7, were quantitated by rat-specific ELISA kits (Figure 5A-B). The platelet-specific protein–protein interaction analysis, using Web tool PlateletWeb (http://plateletweb.bioapps.biozentrum.uni-wuerzburg.de), suggested the involvement of these proteins in key regulatory events (Figure 5C). ELISA results for 3 of the proteins including fibrinogen β, fibrinogen α, and coronin 1A were not in accordance with the proteomic findings, and thus were not followed-up further (data not shown).
ELISA-based validation and protein–protein interaction analysis of identified differential proteins. Selective differential proteins identified by MS/MS were used for heat map generation and 3-dimensional view analysis. The differentially expressed proteins were quantified in platelet samples from control and exposed rats by ELISA, and the platelet-specific protein–protein interaction analysis was performed with an open Web source PlateletWeb (http://plateletweb.bioapps.biozentrum.uni-wuerzburg.de). (A) Representative protein spots of identified proteins on 2DE gel with heat map in 3-dimensional view. (B) ELISA-based validation of protein levels in platelet samples expressed as mean ± SEM (n = 6; *P < .05, **P < .01). (C) Interaction maps of differentially expressed proteins showing their interacting partners, using the PlateletWeb database. The proteins directly involved in coagulation cascade are highlighted.
ELISA-based validation and protein–protein interaction analysis of identified differential proteins. Selective differential proteins identified by MS/MS were used for heat map generation and 3-dimensional view analysis. The differentially expressed proteins were quantified in platelet samples from control and exposed rats by ELISA, and the platelet-specific protein–protein interaction analysis was performed with an open Web source PlateletWeb (http://plateletweb.bioapps.biozentrum.uni-wuerzburg.de). (A) Representative protein spots of identified proteins on 2DE gel with heat map in 3-dimensional view. (B) ELISA-based validation of protein levels in platelet samples expressed as mean ± SEM (n = 6; *P < .05, **P < .01). (C) Interaction maps of differentially expressed proteins showing their interacting partners, using the PlateletWeb database. The proteins directly involved in coagulation cascade are highlighted.
Proteins with prothrombotic nature, which includes important coagulation factors, tissue factor (TF), and fibrinogen, were upregulated, whereas those having antithrombotic activity, including calcium-binding proteins calreticulin and calumenin,30,31 were suppressed under hypoxic conditions. The identified proteins also included some upregulated proteins playing major roles in the platelet activation process. A recent study observed TF expression in human platelets, but not in platelets from mice.32 Also, there is conflicting information regarding TF expression in platelets; thus, we have also examined the TF mRNA to verify its expression in platelets from control and hypoxia-exposed animals, using RT-PCR (supplemental Figure 2). The RT-PCR data demonstrated the presence of TF transcripts in platelets, and TF expression was found to be enhanced in hypoxia-exposed rats. These results strongly supported the proteomic and ELISA data and suggest that differential TF expression regulation in rat platelets may be a result of signal-dependent splicing, as demonstrated in human platelets earlier.33
Collectively, the hypoxia-induced changes in the expression pattern of platelet proteins indicate that hypoxic exposure shifts the platelet proteome toward the prothrombotic state.
Calpains play a vital role in hypoxia-induced prothrombotic phenotype
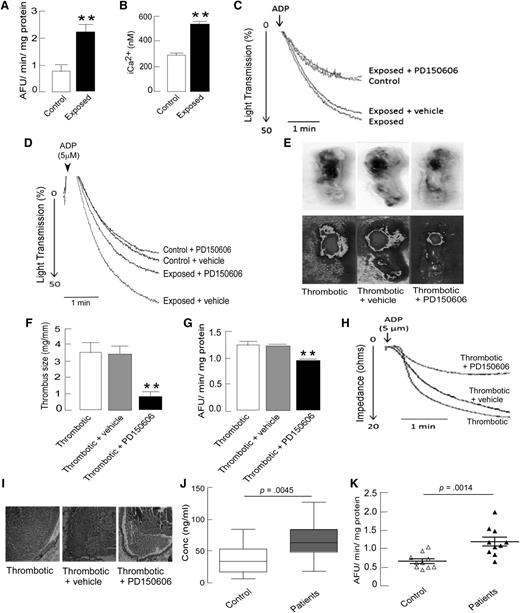
The analysis of hypoxia-induced differentially expressed proteins directed our focus toward calcium-triggered events. Calcium-based regulation of intracellular events is known to be central in platelet activation. Calpain, a thiol protease, has been found to be regulated by calcium influx and oxidative stress.34,35 Upregulation of CAPNS1 in platelets from exposed animals was evident from the proteomic and ELISA results (Figures 4-5). To confirm the activation of platelet calpain, platelet intracellular calcium and calpain activity were measured in PRP and were found to be significantly higher in exposed animals compared with in controls (Figure 6A-B). Thus, differential regulation of calcium-binding proteins, elevated platelet intracellular Ca2+, and higher calpain activity in hypoxic animals suggested that a disturbed Ca2+ homeostasis and activated calpain might contribute to hyperreactive platelets and, ultimately, a prothrombotic phenotype under hypoxia.
Increased calpain activity after hypoxia exposure in rats and human DVT patients, and the antithrombotic effect of calpain inhibition in vivo and ex vivo. Platelets were isolated from indicated groups and processed for either florescence-based calpain activity or intracellular calcium assays, as described in the supplemental Methods. (A) Quantitation of calpain activity in platelets demonstrating higher proteolytic activity of calpain in exposed animals. (B) Intraplatelet calcium levels measured by Fura-2 based fluorogenic assay showed increased platelet iCa2+ in exposed animals. (C-D) Representative aggregation curves showing reversal of hypoxia-induced platelet aggregation by preincubation of PRP with calpain inhibitor PD150606 (50 µM) ex vivo (C) and by preinfusion of PD150606 (1 mg/kg body weight) in vivo (D). (E-I) The antithrombotic and platelet inhibitory effects of preinfusion of PD150606 via tail vein in rat model of stasis-induced thrombosis, as described in the supplemental Methods. (E) Representative images of extracted portions of IVC with thrombus (top) from thrombotic animals with their heat maps (bottom), showing smaller thrombus in the case of PD150606 preinfusion. (F) Quantitations of the size of the thrombus isolated from the IVC portions of thrombotic animals (n = 8). (G) Decreased calpain activity in platelets isolated from thrombotic animals preinfused with PD150606. (H) Representative platelet aggregation curves showing a strong negative effect of PD150606 preinfusion in thrombotic rats compared with vehicle control or no infusion. Data are presented as mean ± SEM (n = 8) and analyzed by unpaired t test. *P < .05, **P < .01 vs thrombotic + vehicle. (I) Hematoxylin-eosin-stained sections of thrombus with vessel wall, showing morphological differences (×200 original magnification). (J-K) The plasma samples from human DVT patients from high-altitude regions and from control individuals were analyzed for soluble P selectin levels and calpain activity, (J) Higher sP-selectin levels in plasma samples (data presented as box and whiskers plot) indicated hyperactive platelets in high-altitude-induced DVT patients. (K) Calpain activity in human plasma samples of high-altitude-induced DVT patients compared with age- and sex-matched healthy controls, shown as a scatter plot (n = 10). The activity in patients was significantly (P = .0014) higher in comparison with controls. Data are presented as mean ± SEM (n = 10 in each group) and analyzed by t-test compared with respective controls.
Increased calpain activity after hypoxia exposure in rats and human DVT patients, and the antithrombotic effect of calpain inhibition in vivo and ex vivo. Platelets were isolated from indicated groups and processed for either florescence-based calpain activity or intracellular calcium assays, as described in the supplemental Methods. (A) Quantitation of calpain activity in platelets demonstrating higher proteolytic activity of calpain in exposed animals. (B) Intraplatelet calcium levels measured by Fura-2 based fluorogenic assay showed increased platelet iCa2+ in exposed animals. (C-D) Representative aggregation curves showing reversal of hypoxia-induced platelet aggregation by preincubation of PRP with calpain inhibitor PD150606 (50 µM) ex vivo (C) and by preinfusion of PD150606 (1 mg/kg body weight) in vivo (D). (E-I) The antithrombotic and platelet inhibitory effects of preinfusion of PD150606 via tail vein in rat model of stasis-induced thrombosis, as described in the supplemental Methods. (E) Representative images of extracted portions of IVC with thrombus (top) from thrombotic animals with their heat maps (bottom), showing smaller thrombus in the case of PD150606 preinfusion. (F) Quantitations of the size of the thrombus isolated from the IVC portions of thrombotic animals (n = 8). (G) Decreased calpain activity in platelets isolated from thrombotic animals preinfused with PD150606. (H) Representative platelet aggregation curves showing a strong negative effect of PD150606 preinfusion in thrombotic rats compared with vehicle control or no infusion. Data are presented as mean ± SEM (n = 8) and analyzed by unpaired t test. *P < .05, **P < .01 vs thrombotic + vehicle. (I) Hematoxylin-eosin-stained sections of thrombus with vessel wall, showing morphological differences (×200 original magnification). (J-K) The plasma samples from human DVT patients from high-altitude regions and from control individuals were analyzed for soluble P selectin levels and calpain activity, (J) Higher sP-selectin levels in plasma samples (data presented as box and whiskers plot) indicated hyperactive platelets in high-altitude-induced DVT patients. (K) Calpain activity in human plasma samples of high-altitude-induced DVT patients compared with age- and sex-matched healthy controls, shown as a scatter plot (n = 10). The activity in patients was significantly (P = .0014) higher in comparison with controls. Data are presented as mean ± SEM (n = 10 in each group) and analyzed by t-test compared with respective controls.
To confirm the role of calpain in hypoxia-induced platelet hyperreactivity, a cell-permeable, calpain-specific inhibitor, 3-(4-iodophenyl)-2-mercapto-(Z)-2-propenoic acid (PD150606), which binds to CAPNS1,36 was used for further animal studies. Preincubation of PRP with PD150606 (50 µM) for 10 minutes at 37°C resulted in the reversal of hypoxia-induced platelet hyperreactivity (Figure 6C). Further, the preinfusion of animals with PD150606 (1 mg/kg body weight) via tail vein before hypoxic exposure resulted in partial reversal of hypoxia-induced platelet hyperreactivity (Figure 6D) and partial restoration of reduced bleeding time in exposed animals (360 ± 45 seconds with PD150606 vs 212 ± 22 seconds with vehicle). These in vitro and in vivo observations indicated that CAPNS1-regulated calpain might be playing an important role during hypoxia-induced thrombogenesis.
To further investigate the role of CAPNS1-dependent calpain regulation in hypoxia-induced thrombosis in vivo, a flow-restriction animal model was used that resulted in the generation of thrombus as a result of ligation (Figure 6E). In this model, the thrombus formation was induced by stasis of blood flow at the site of ligation, which creates a hypoxic microenvironment.27,37 Animals preinfused with calpain inhibitor, PD150606 (1 mg/kg body weight), demonstrated significantly reduced thrombus size (0.80 ± 0.33 mg/mm) compared with those preinfused with vehicle (3.41 ± 0.51 mg/mm; P < .01) (Figure 6E-F). Similarly, calpain activity and platelet aggregation were found to be significantly lower in animals preinfused with PD150606 compared with vehicle controls (Figure 6G-H). Histological investigations also suggested the reduction in thrombus formation (Figure 6I). Moreover, to demonstrate the direct effect of hypoxia on thrombus formation, the IVC-ligated animals were exposed to simulated environmental hypoxia (using a decompression chamber). Thrombus formation was found to be accelerated after 6 hours of hypoxic exposure, as reflected by a significantly enhanced thrombus size in these animals compared with in unexposed ligated animals (supplemental Figure 3). Furthermore, the effect of preinfusion with PD150606 was more pronounced, as indicated by thrombus size in exposed ligated animals compared with in their unexposed counterparts (supplemental Figure 3). To exclude the off-target effect (if any) of a single calpain inhibitor (PD150606), 2 additional known cell permeable inhibitors (calpeptin and MDL28170) of calpain activity were also tested. The preinfusion of animals with these inhibitors individually resulted in significant attenuation in thrombus size (supplemental Figure 4). However, PD150606 proved to be the most potent among these 3 inhibitors.
Next, for translational implications, we conducted a human study to investigate the calpain activity in patients who had developed DVT at high altitude. Consistent with in vivo animal studies, calpain activity was found to be significantly higher in human patients’ plasma samples compared with in the controls (1.213 ± 0.121 arbitrary fluorescent units/min/mg vs 0.70 ± 0.062 arbitrary fluorescent units/min/mg; P = .0014; n = 10; Figure 6K). All 10 patients selected for this study were negative for 2 common thrombophilia traits: factor V Leiden (1691G/A, rs6025) and prothrombin (20210G/A, rs1799963) mutations. Moreover, we did not find any association between thrombophilia factors and enhanced calpain activity in these patients (see supplemental Table 2 for details). There was no significant difference in the mean age of patients (35 years) and controls (32 years), and all were healthy with a normal body mass index range (<29.9 kg/m2). We also analyzed the levels of sP-selectin, a soluble marker of platelet activation in the plasma samples of patients and controls. Plasma sP-selectin was significantly higher in patients compared with controls, which is suggestive of increased platelet reactivity in the patients (Figure 6J). These results emphasize the translational implication of preclinical data and strongly support a prothrombotic role for calpain under hypoxic conditions.
Discussion
This study is the first attempt to analyze the platelet proteome under hypoxic conditions as well as to demonstrate the hypoxia-induced differential expression of platelet proteins. We found that the enhanced calpain activity regulated by CAPNS1 plays a major role in platelet hyperreactivity and thrombogenesis under hypoxic environment. Although in many studies hypoxia associated with high altitude has been suggested to be prothrombotic,10-12 certain studies have challenged these observations.38,39 Hence, in the current study, we tried to investigate and understand the effect of acute hypoxic exposure on coagulation and platelets.
We observed the prothrombotic phenotype in rats after hypoxia exposure, which was reflected by decreased bleeding and prothrombin times. The platelets from these experimental hypoxic rats showed higher reactivity, which was demonstrated by multiple parameters including platelet adhesion, aggregation, and activation. The hyperreactivity of platelets was further supported by the enhanced surface expression of CD41 and αIIbβ3, as well as by increased clot retraction in exposed animals. Earlier studies on platelet reactivity under a hypoxic environment produced conflicting results.16,39 Rats were selected as the ideal animal model to perform this study because of 2 factors: first, rats are preferred over mice for conducting pathophysiological studies, and second, there have been significant similarities in rat and human platelet proteins, which encourages the use of rat models to study platelets and related prothrombotic events.40 The current animal model involves hypobaric hypoxic exposure at a surrounding temperature of 10°C to reproduce the hypoxic conditions at high-altitude regions, which have much lower temperatures than sea-level regions. To evaluate the effect of cold surroundings on hypoxia-induced prothrombotic tendency, animals were exposed to hypoxia under both normothermic (chamber temperature of 28°C) and hypothermic (chamber temperature of 10°C) conditions, and bleeding time as well as platelet aggregation assays were performed. The results were similar in both the groups; that is, the exposed animals experienced significantly decreased bleeding time and increased platelet aggregation compared with controls (supplemental Figure 1). These preliminary observations emphasize that hypoxia is the key factor affecting platelets at high altitude.
Until the 1990s, it was believed that platelets carried proteins synthesized from their parent cell megakaryocytes. However, with the evidence of de novo protein synthesis and the discovery of protein synthesis machinery in platelets, these were found to be active protein-synthesizing anucleated cells. The subsequent discovery of platelet spliceosome and alternative splicing revealed a posttranscriptional mode of regulation of protein expression in platelets, making the platelet biology more complex. Therefore, we analyzed the platelet proteome to study the effect of hypoxic exposure on platelet proteins, and thus made an attempt to understand the events underlying hypoxia-induced platelet hyperreactivity. The proteomic analysis of hypoxic platelets exhibited differential expression of many platelet proteins. These observations comprised important coagulation cascade proteins such as fibrinogen and TF, some key signaling proteins involved in calcium regulation and platelet activation, and CAPNS1, which is responsible for regulating calpain activity. The bioinformatic analysis of the data revealed that the altered proteins belonged to different cell locations with diverse molecular functions, which therefore was suggestive of the whole-platelet proteome alteration in the hypoxic environment. The increased levels of platelet TF in hypoxia supports our coagulation assay data, where PT was found to be decreased after hypoxia. Hypoxia not only resulted in upregulation of prothrombotic proteins but also conferred a decreased antithrombotic tendency by suppressing endoplasmic reticulum resident proteins calreticulin and calumenin, which have been reported to be antithrombotic in nature,30,31 and maintaining calcium homeostasis.20
The enhanced CAPNS1-regulated calpain activity, along with elevated Ca2+, may play an important role in hypoxia-induced thrombogenesis, as demonstrated by in vivo animal results. The in vivo hypoxic setting for thrombus formation was created by an IVC ligation approach that resulted in a hypoxic microenvironment due to stasis.27,37 The upregulation of hypoxia-inducible factor 1α in both exposed (but nonligated) and thrombotic animals strongly supported our approach (data not shown). The pretreatment with highly selective and potent calpain inhibitor PD150606 resulted in reversal of platelet hyperreactivity and reduced the thrombus formation in these animals. The effect of calpain inhibition on thrombus formation was also analyzed with 2 additional calpain inhibitors, MDL28170 and calpeptin, to rule out possible off-target effects.
On the basis of the current observations presented in this article, a few points about probable modes of action of calpain can be drawn. First, the increased proteolytic activity of calpain in platelets, which was observed without agonist-induced aggregation (Figure 6A), demonstrates that the calpain activation by hypoxia precedes agonist-induced platelet aggregation. Second, platelet hyperreactivity induced by hypoxia depends largely on calpain activity, as preinfusion of calpain inhibitor limits the hypoxia-induced platelet aggregation (Figure 6D). Third, calpain activity affects platelet hyperreactivity induced by hypoxia more than that of normoxic platelets (Figure 6D). It is known that calpain gets fully activated (and translocates to membrane) on agonist-induced platelet activation.41 Therefore, from the current observations, it appears that hypoxic exposure accelerates the rate of transformation of calpain from its resting state to a fully activated state and positively regulates hypoxia-induced platelet reactivity. However, it remains to be explored whether hypoxia induces the known calpain reactions in platelets or triggers an altogether different set of biomolecular reactions. These nascent findings about the platelet–calpain relationship under hypoxia may provide future directions for deciphering the exact mechanism by which calpain protease systems mediate hypoxia-induced prothrombotic effects via regulating platelet function, as well as drive the hunt for novel substrates of calpain in platelets under a hypoxic environment. Another interesting approach may be investigating the role of calpain in modulating platelet proteome under hypoxia, in view of recently reported stabilization of the platelet proteome of diabetic patients by calpain inhibition.42
In addition to platelet activation, the prothrombotic nature of calpain can also be attributed to the diverse nature of its substrates, which comprise cytoskeletal proteins, membrane proteins, kinases, phosphatases, and ATPases.43 More important, the human plasma samples of the rare type of lower limb DVT caused by hypoxic environment at high attitudes also had elevated calpain activity and sP-selectin levels. The sample size in the study was limited because of certain constraints, such as lower inhabitability and accessibility at extreme altitudes as well as complicated logistic issues. In spite of these constraints, the important findings in the present study are suggestive of its potential translational application.
In conclusion, this study for the first time reports that a hypoxic environment results in an altered platelet proteome inducing the platelet hyperreactivity. Further, CAPNS1-mediated regulation of calpain activity appears to play a major role in hypoxia-induced thrombogenesis, both in animals and humans, suggesting a potential link between oxygen-compromised status and thrombogenic index.
The online version of this article contains a data supplement.
Part of this study was presented as a poster at the 22nd International Congress on Thrombosis, Nice, France, October 6-9, 2012.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are extremely thankful to Directorate General Armed Forces Medical Sciences, India, for approving the human study and to Somnath Singh, Minakshi Basu, Manish Sharma, R. J. Tirpude, Karan Pal, and Havaldar Satish for their support during the study, and Emmanuel J. Favaloro, Manojkumar Valiyaveettil, and Manju Bala for editing the manuscript.
This work was funded by Defense Research and Development Organization, project SL-10 /DIP-255. T.T. is a senior research fellow of the Indian Council of Medical Research.
Authorship
Contribution: T.T. performed the experiments, analyzed data, and wrote manuscript; S.A., Y.A., and S.S. performed mass spectrometry experiments; N.G. performed in vivo rat thrombosis model experiments; A.S. performed the platelet RNA experiments, V.N., T.C., and N.B. participated in the human study; S.B.S. and L.G. edited the manuscript; and M.Z.A. designed the study, interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohammad Z. Ashraf, Genomics Division, Defense Institute of Physiology & Allied Sciences, Timarpur, Delhi, 110054 India; e-mail: mohammadzashraf@gmail.com.