Key Points
In vitro, IL-17 inhibits Fas-induced cell death and IL-17 neutralization improves lymphocyte apoptosis in patients with ALPS and DALD.
Treatment of MRLlpr/lpr mice with anti–IL-17A antibodies decreases the severity of autoimmune/lymphoproliferative disease.
Abstract
In autoimmune/lymphoproliferative syndrome (ALPS), defective Fas death receptor function causes lymphadenomegaly/splenomegaly, the expansion of T-cell receptor αβ+ CD4/CD8 double-negative T cells, and frequent development of hematologic autoimmunity. Dianzani autoimmune lymphoproliferative disease (DALD) has a similar phenotype but lacks the expansion of double-negative T cells. This work shows that patients with ALPS and DALD have high serum levels of interleukin 17A (IL-17A), IL-17F, and IL-17AF, which are involved in several autoimmune diseases, and that their T cells show increased secretion of these cytokines upon activation in vitro. The following data indicate that these cytokines may contribute to ALPS and DALD: (1) recombinant IL-17A and IL-17F significantly inhibit Fas-induced cell death in Fas-sensitive T cells from healthy donors; (2) this inhibitory effect is also induced by the patients’ serum and is reversed by anti–IL-17A antibodies; (3) IL-17A neutralization substantially increases Fas-induced cell death in T cells from ALPS and DALD patients in vitro; and (4) treatment with anti–IL-17A antibodies ameliorates the autoimmune manifestations and, at a lesser extent, the lymphoproliferative phenotype and prolongs survival in MRLlpr/lpr mice, which are an animal model of ALPS. These data suggest that IL-17A and IL-17F could be targeted therapeutically to improve Fas function in ALPS and DALD.
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) is a human genetic disorder of lymphocyte apoptosis resulting in the accumulation of polyclonal lymphocytes in the lymph nodes and in the spleen with expansion of T-cell receptor αβ (TCRαβ)-positive CD4/CD8 double-negative (DN) T cells and frequent development of autoimmune manifestations, mainly hemocytopenias. The disease is caused by genetic mutations that affect Fas-mediated apoptosis and that have an autosomal dominant inheritance pattern with incomplete penetrance.1,2 Most patients carry a heterozygous mutation in the FAS gene (ALPS-FAS), whereas very few patients carry mutations in the FAS LIGAND (ALPS-FASLG) or CASPASE10 (ALPS-CASP10) genes; however, the causal mutation is not known in a substantial proportion of patients (ALPS-UND). Moreover, a substantial number of patients carry somatic mutations in FAS in the DN T-cell population (ALPS-sFAS).3 We have also described patients with lymphadenomegaly/splenomegaly, autoimmune manifestations, and defective Fas function, but without expansion of DN T cells. This disease has been named Dianzani autoimmune lymphoproliferative disease (DALD) (OMIM reference #605233),3 and several features indicate that it may involve genes in the Fas pathway.4,5
In previous work, we showed that ALPS and DALD patients have high serum levels of osteopontin (OPN)6 and tissue inhibitor of metalloproteinase-1 (TIMP-1).7 An increase in OPN was partly due to polymorphisms in its gene and might cause the observed increase in TIMP-1 because OPN induces TIMP-1 secretion in monocytes. Because both OPN and TIMP-1 inhibit lymphocyte apoptosis in vitro, we speculated that their high levels may contribute to ALPS and DALD development in subjects with a hypofunctional Fas system.6,7 Moreover, the role of risk factor may be also played by gene variations decreasing function of perforin, involved in the function of cytotoxic T cells and natural killer cells.8-10
OPN is a proinflammatory cytokine that is also involved in the development of T helper 17 (Th17) cells.11 These are proinflammatory Th cells characterized by the secretion of interleukin 17A (IL-17A) and IL-17F, which can be secreted either as homodimers or as IL-17AF heterodimers.12 IL-17A and IL-17F can be also produced by other cell types, including CD8+ T cells, γδ T cells, natural killer cells, and neutrophils.13 They share about 50% sequence identity and several biological activities, including neutrophil recruitment and induction of proinflammatory cytokines, chemokines, and metalloproteinases.14 However, their receptor usage is partly different: their secretion is independently regulated in Th cells, they display different proinflammatory activity, and they are differently expressed in several autoimmune and allergic diseases.15-17
Increasing evidence indicates that Th17 cells play an important role in several autoimmune diseases, including multiple sclerosis, psoriasis, rheumatoid arthritis (RA), inflammatory bowel disease, anti-neutrophil cytoplasmic antibody–associated vasculitis, and systemic lupus erythematosus (SLE).18-22 SLE patients, in particular, have high serum levels of IL-17A and IL-23 and increased numbers of IL-17–producing T cells in the peripheral blood, and these high levels correlate with disease activity. Moreover, in vitro experiments have shown that IL-17A increases autoantibody production (anti–double-stranded [dsDNA], antinuclear) in the peripheral blood mononuclear cells (PBMCs) of patients with lupus nephritis, protects B cells from activation-induced cell death (AICD), and supports their proliferation and differentiation into immunoglobulin-secreting cells.23-26
Intriguingly, some SLE patients show expansion of DN T-cell populations, a hallmark of ALPS, and secrete high levels of IL-17A. Moreover, high levels of IL-17A are produced by DN T cells that infiltrate the nephritic kidneys in MRLlpr/lpr mice. These are used as an animal model of ALPS because they carry mutations in the FAS gene and have a typical ALPS-like phenotype with lymphadenomegaly/splenomegaly and expansion of DN T cells, but they also have features that are atypical of ALPS patients, such as lupus-like nephritis and anti-dsDNA autoantibodies.25,26
These observations prompted the present work, in which we investigate the roles of IL-17A and IL-17F in ALPS and DALD. Our results show that the levels of IL-17A, IL-17F, and IL-17AF are increased in ALPS and DALD patients and that IL-17A and IL-17F inhibit Fas-induced cell death (FICD) in vitro. Moreover, treatment of MRLlpr/lpr mice with anti–IL-17A antibodies decreases the severity of autoimmune/lymphoproliferative disease, decreases renal involvement, and prolongs survival.
Methods
Patients
We analyzed 18 ALPS (n = 9 ALPS-FAS, n = 1 ALPS-sFAS, and n = 8 ALPS-UND) and 18 DALD patients who were followed at the Pediatric Department, University of Turin, Italy, and age-matched healthy controls (n = 50). ALPS and DALD were diagnosed according to the criteria indicated in the 2009 ALPS National Institutes of Health International Workshop.3 Clinical and laboratory data are reported in supplemental Table 1 on the Blood website. Most patients had received corticosteroid therapy but analyses were always performed at least 4 weeks from the last treatment.
Written informed consent was obtained from patients and controls. This study was conducted in accordance with the Declaration of Helsinki. The study was planned according to the guidelines of the local ethical committee.
Cytokine secretion
Cytokine serum levels were evaluated by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN; eBioscience, San Diego, CA; MBL International, Woburn, MA).
PBMCs (1 × 105) were cultured for 5 days in round-bottomed 96-well plates in the presence of anti-CD3 (10 µg/mL) and an anti-CD28 (1 µg/mL; Ancell, Bayport, MN) monoclonal antibody (mAb) and in the presence of recombinant (r) IL-23 (rIL-23 50 ng/mL; R&D Systems). Then, IL-17A and IL17F were measured by ELISA on the supernatant; absorbance was evaluated using a microplate reader (Bio-Rad, Hercules, CA), and the I-smart program was used to calculate the standard curve. Cells were stained with a phycoerythrin-conjugated anti-CCR6 mAb and an allophycocyanin–conjugated anti-CD4 mAb (eBioscience). Alternatively, they were restimulated with Phorbol 12-myristate 13-acetate (50 ng/mL; Sigma, Saint Louis, MO) plus Ionomycin (500 ng/mL; Sigma) for 5 hours in the presence of Brefeldin-A (10 μg/mL; Sigma), permeabilized, and stained with a fluorescein isothiocyanate–conjugated anti-TCRαβ+ mAb and an Alexa Fluo 647-conjugated anti–IL-17A mAb (eBioscience). Then, they were analyzed by flow cytometry.
FICD assay
PBMCs were separated by density-gradient centrifugation. FICD was evaluated as previously reported4,5 in activated PBMCs using a soluble anti-Fas mAb (0.5 µg/mL CH11; UPSTATE Waltham, MA) in the presence and absence of rIL-17A or rIL-17F (5 ng/mL; Peprotech, Rocky Hill, NJ). In some experiments, FICD was also induced in the presence of a neutralizing anti–IL-17A antibody (10 µg/mL; R&D Systems). After 16 hours, cell survival was assessed by counting the live cells in each well using the trypan blue exclusion test. The results are expressed as relative cell survival % calculated as follows: (total live cell count in the assay well/total live cell count in the respective control well) × 100.
In some experiments, cells were harvested at the end of the FIDC assay and lysed in 150 mM NaCl, 20 mM Tris-HCl pH 8, 0.5% Nonidet P40 Substitute, 5 mM EDTA, aprotinin 1 µg/mL, leupeptin 1 µg/mL, pepstatin A 1 µg/mL, and PMSF 100 µg/mL for 30 min. Lysates were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to Hybond-C extra membranes (GE Healthcare, Piscataway, NJ), blotted with antibodies to FLIP (Alexis Axxora, San Diego, CA), XIAP (Alexis), Bcl-2 (Stressgen, Victoria, BC, Canada), and β-actin (Sigma) and a peroxidase-conjugated anti-mouse immunoglobulin secondary antibody (Ge Healthcare) and revealed by chemiluminescence.
Mice
Female MRLlpr/lpr mice (stock no. 000485) were purchased from The Jackson Laboratory. Eight-week-old MRLlpr/lpr females were randomized into 3 groups of 8 mice each and were treated with 4 intraperitoneal injections (1 every 4 days) of phosphate-buffered saline, anti–IL-17A antibody (100 µg/100 µL, R&D Systems), or an isotype-matched control (immunoglobulin-G2A [IgG2A], R&D Systems). The concentration of anti-dsDNA antibodies was evaluated by ELISA (α Diagnostic International, San Antonio, TX) in mouse sera (Low NSB Sample Diluent) at a 1:500 dilution. Proteinuria was evaluated using reagent strips (A. Menarini Diagnostic, Berkshire, UK) and was ranked as follows: 0 (negative); Pro.1+ (30 mg/dL); Pro.2+ (100 mg/dL); Pro.3+ (300 mg/dL); and Pro.4+ (1000 mg/dL). The lymph node and spleen sizes were expressed as the ratio between the organ wet weight (grams) and the body weight (grams) × 100. The experiments were approved by the local ethical committee for animal experimentation.
Statistical analysis
The Mann-Whitney U test was used to compare unpaired data from different groups, Wilcoxon’s signed rank test was used for the analysis of paired data, and Fisher’s exact test was used for the comparison of high IL-17 groups.
A Kaplan-Meier survival analysis with a log-rank test (Mantel-Cox) of the statistics was used to assess the survival rate and to compare the differences between the survival curves. All P values are 2-tailed, and the significance cutoff is P < .05. The statistical analyses were performed with GraphPad Instat software (GraphPad Software, San Diego, CA).
Results
Increased serum levels of IL-17A, IL-17F, and IL-17AF in ALPS and DALD patients
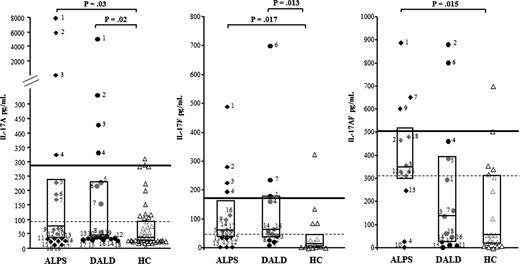
We measured IL-17A, IL-17F, and IL-17AF (IL-17s) by ELISA in the sera of 18 ALPS and 18 DALD patients and of 50 healthy matched controls. The levels of both IL-17A (ALPS: median 60 pg/mL, interquartile range, 37-221; DALD: 44 pg/mL, 37-229) and IL-17F (ALPS: 59 pg/mL, 37-156; DALD: 61 pg/mL, 37-172) were significantly higher in the ALPS and DALD patients than in the controls (IL-17A: 37 pg/mL, 22-93; IL-17F: 12 pg/mL, 4-46) (Figure 1), whereas the levels of IL-17AF were significantly higher in the ALPS (346 pg/mL, 298-514) but not in the DALD (140 pg/mL, 30-389) patients than in the controls (56 pg/mL, 19-311) (Figure 1). By setting the thresholds at the 75th (93 pg/mL) and 95th (286 pg/mL) percentiles of the control values, single patient analysis showed that the levels of at least 1 of these cytokines was over the 75th percentile in 83% of the ALPS (P < .0001 vs controls, Fisher exact test) and 56% of the DALD patients (P = .002) and greater than the 95th percentile in 33% of the ALPS and 33% of the DALD patients (P = .02).
Increased IL-17A, IL-17F, and IL-17AF serum levels in ALPS and DALD patients. Black diamonds indicate ALPS (n = 18), black circles indicate DALD (n = 18), and white triangles indicate the healthy controls (HC, n = 50). The horizontal bars are the medians, the boxes indicate the interquartile range, the dashed lines indicate the 75th, and the thick line indicates the 95th percentile of the HC (Mann-Whitney U test).
Increased IL-17A, IL-17F, and IL-17AF serum levels in ALPS and DALD patients. Black diamonds indicate ALPS (n = 18), black circles indicate DALD (n = 18), and white triangles indicate the healthy controls (HC, n = 50). The horizontal bars are the medians, the boxes indicate the interquartile range, the dashed lines indicate the 75th, and the thick line indicates the 95th percentile of the HC (Mann-Whitney U test).
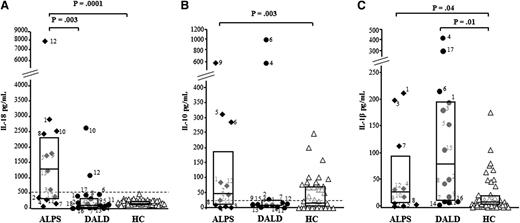
Then, we compared the serum levels of IL-17s with those of IL-10 and IL-18, which are frequently elevated in ALPS patients, and IL-1β, known to support production of IL-17s. As shown in Figure 2 and supplemental Table 1, IL-18 levels were higher in the ALPS (1262 pg/mL, 337-2356) patients than in the DALD (113 pg/mL, 54-381) patients and the controls (181 pg/mL, 134-252). IL-10 levels were higher in the ALPS (46 pg/mL, 10-187) patients but not in the DALD (11 pg/mL, 7-24) patients than in the controls (10 pg/mL, 2-64). IL-1β levels were higher in the ALPS (27 pg/mL, 7-94) and the DALD (78 pg/mL, 11-192) patients than in the controls (6 pg/mL, 3-18). However, no significant correlation was found between the levels of these cytokines and the levels of IL-17s (data not shown).
Serum levels of IL-18, IL-10, and IL-1β in ALPS and DALD patients. Levels of IL-18 (A), IL-10 (B), and IL-1β (C) in ALPS (black diamonds), DALD (black circles), and healthy controls (white triangles). The horizontal bars are the medians, the boxes indicate the interquartile range, and the dashed lines in A and B indicate the cutoff value for ALPS diagnosis. Differences were tested with the Mann-Whitney U test.
Serum levels of IL-18, IL-10, and IL-1β in ALPS and DALD patients. Levels of IL-18 (A), IL-10 (B), and IL-1β (C) in ALPS (black diamonds), DALD (black circles), and healthy controls (white triangles). The horizontal bars are the medians, the boxes indicate the interquartile range, and the dashed lines in A and B indicate the cutoff value for ALPS diagnosis. Differences were tested with the Mann-Whitney U test.
Peripheral blood Th17 cells are increased in ALPS and DALD patients
To assess whether Th17 cells are involved in the high serum levels of IL-17s, we evaluated IL-17A and IL-17F secretion by PBMCs from 15 patients (8 ALPS and 7 DALD) and 15 controls. PBMCs were activated by triggering CD3 + CD28 and were cultured for 5 days in the presence of rIL-23 to support Th17 cell expansion. At day 5, the proportions of CD4+CCR6+ cells and TCRαβ+IL-17A+ cells, comprising Th17 cells, were assessed by flow cytometry, and IL-17A and IL-17F secretion was assessed by ELISA in the supernatants.
The flow cytometry analysis showed that the proportions of CD4+CCR6+ and TCRαβ+IL-17A+ cells were higher in both patient groups than in the controls (mean ± standard error [SE], CD4+CCR6+: ALPS 4.6% ± 1.3%, DALD 3.1% ± 1%, and controls 1.4% ± 0.3%; TCRαβ+IL-17A+: ALPS 1.66% ± 0.65%, DALD 3.5% ± 1.73%, and controls 0.65% ± 0.15%) (Figure 3A-B).
Increased Th17 cells in ALPS and DALD patients. The proportion of CD4+CCR6+ cells (A) and TCRαβ+IL-17A+ (B) in PBMCs activated by triggering of CD3 + CD28 and cultured with rIL-23. The levels of IL-17A (C) and IL-17F (D) in the culture supernatants are shown. The mean ± SE from 15 patients and 15 healthy controls (HC) is shown; *P < .05 (Mann-Whitney U test).
Increased Th17 cells in ALPS and DALD patients. The proportion of CD4+CCR6+ cells (A) and TCRαβ+IL-17A+ (B) in PBMCs activated by triggering of CD3 + CD28 and cultured with rIL-23. The levels of IL-17A (C) and IL-17F (D) in the culture supernatants are shown. The mean ± SE from 15 patients and 15 healthy controls (HC) is shown; *P < .05 (Mann-Whitney U test).
The ELISA evaluations showed that IL-17A secretion was significantly higher in the cultures from both of the patient groups than in those from the healthy controls (mean ± SE: ALPS 905 ± 305 pg/mL, DALD 944 ± 344, controls 295 ± 97). In contrast, IL-17F secretion was higher in the DALD patients than in the controls, but not in the ALPS patients (ALPS 853 ± 662 pg/mL, DALD 2979 ± 770, controls 843 ± 146) (Figure 3C-D).
rIL-17A and rIL-17F inhibit T-cell apoptosis
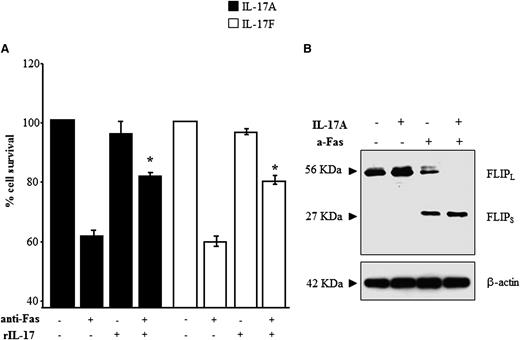
Because ALPS and DALD are caused by defective Fas-induced lymphocyte apoptosis, we investigated the effects of IL-17A and IL-17F on FICD in Fas-sensitive T cells obtained from the healthy controls (n = 7). The cells were treated with an anti-Fas mAb in the presence or absence of rIL-17A or rIL-17F and, after 16 hours, whole cell survival was assessed by the trypan blue exclusion test. The results show that both rIL-17A and rIL-17F significantly inhibited FICD, as detected by an increase in whole cell survival (Figure 4A), whereas these cytokines had no significant effect on the survival of cells not treated with the anti-Fas mAb. Moreover, titration experiments using rIL-17A and rIL-17F in the 2.5-0.1 ng/mL concentration range showed that both cytokines protected T cells from FICD even at the lowest dose, which is comparable to the doses detected in the patients’ sera (data not shown).
rIL-17s inhibit FICD and increase expression of cFLIPS. FICD was performed in T cells from the healthy controls in the presence or absence of each recombinant cytokine. The results are expressed as % cell survival (A). The mean ± SE from 7 experiments are presented, *P < .05 (Wilcoxon’s signed rank test). (B) Western blot analysis of FLIP expression in cells harvested from the FIDC assay (representative of 4 experiments).
rIL-17s inhibit FICD and increase expression of cFLIPS. FICD was performed in T cells from the healthy controls in the presence or absence of each recombinant cytokine. The results are expressed as % cell survival (A). The mean ± SE from 7 experiments are presented, *P < .05 (Wilcoxon’s signed rank test). (B) Western blot analysis of FLIP expression in cells harvested from the FIDC assay (representative of 4 experiments).
To investigate whether the antiapoptotic effect of IL-17s was mediated by modulation of cellular inhibitors of FICD, we evaluated expression of cellular FLIP (cFLIP), XIAP, and Bcl-2 at the end of the FICD assay. Western blotting with anti-FLIP antibodies detected 2 bands compatible with the isoforms cFLIPL and cFLIPS and showed that, in the absence of Fas stimulation, cells express high levels of cFLIPL, whereas cFLIPS is undetectable; this pattern is not influenced by addition of IL-17A. By contrast, after Fas stimulation, cells express intermediate levels of both cFLIPS and cFLIPL in basal conditions, whereas, in the presence of IL-17A, they express high levels of cFLIPS but cFLIPL is undetectable (Figure 4B). By contrast, no effect was found in XIAP and Bcl-2 expression (not shown).
Neutralization of IL-17A in the sera of ALPS and DALD patients partly rescues lymphocytes’ death
The titration experiments suggest that the IL-17A and IL-17F concentrations detected in the ALPS and DALD patients may contribute to the apoptotic defect in their lymphocytes. To confirm this possibility, we performed the FICD assay on Fas-sensitive T cells from healthy donors (n = 9) in the presence of either fetal bovine serum (FBS) or serum from patient ALPS-5 (ALPS-sFAS), with high levels of IL-17A, but not of IL-17F, and in the presence or absence of anti–IL-17A neutralizing antibodies. The results show that FICD was induced at significantly higher levels in FBS than in the patient’s serum. Moreover, the addition of anti–IL-17A antibodies had no substantial effect on FICD induced in the FBS-supplemented cultures, whereas it substantially increased the FICD induced in the patient’s serum (Figure 5A). These results suggest that the IL-17A in the ALPS patient serum can inhibit FICD in Fas-sensitive T cells.
Effects of ALPS serum and IL-17A neutralization in FICD. FICD was performed in T cells from healthy donors (A: n = 9) or patients (B: ALPS, n = 5; diamonds; DALD, n = 4; circles) in the presence of either FBS or an ALPS patient’s serum. The absolute control cell survival was similar in FBS and in the patient’s serum; *P < .05 (Wilcoxon’s signed rank test).
Effects of ALPS serum and IL-17A neutralization in FICD. FICD was performed in T cells from healthy donors (A: n = 9) or patients (B: ALPS, n = 5; diamonds; DALD, n = 4; circles) in the presence of either FBS or an ALPS patient’s serum. The absolute control cell survival was similar in FBS and in the patient’s serum; *P < .05 (Wilcoxon’s signed rank test).
We then repeated these experiments in the T cells from 5 patients with ALPS (2 ALPS-FAS, 1 ALPS-sFAS, and 2 ALPS-UND) and 4 with DALD by performing the FICD assay in either FBS or the autologous serum (AS) in the presence or absence of anti–IL-17A antibodies. Figure 5B shows that FICD was significantly higher in the presence of FBS than in the presence of AS. Moreover, the addition of the anti-IL-17A antibodies significantly increased FICD both in the presence of FBS and in the presence of AS. These data confirm that IL-17A contained in the serum of ALPS and DALD patients can further inhibit FICD in Fas-resistant cells and show that an inhibitory effect may also be exerted by the IL-17A endogenously secreted in vitro by the patients’ cells in the FICD assay.
Effects of passive immunization with anti–IL-17A antibodies in MLRlpr/lpr mice
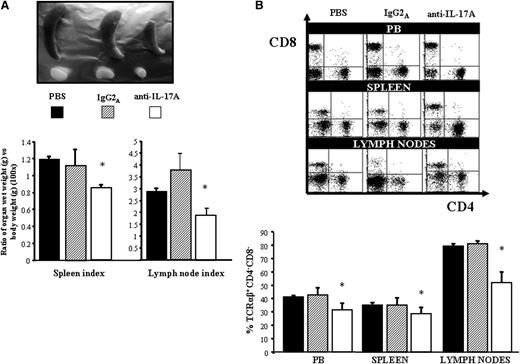
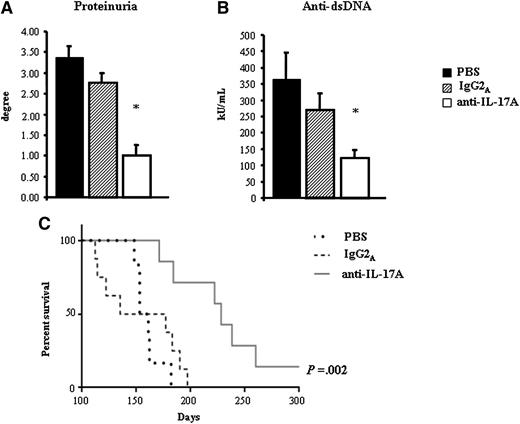
These results show that high levels of IL-17A and IL-17F may contribute to the apoptotic defect in the ALPS and DALD patients, and that neutralization of these cytokines may partly overcome the Fas function defect. To assess the role of IL-17A in vivo, we evaluated the effect of treatment with anti–IL-17A antibodies in the ALPS-like disease developed by MRLlpr/lpr mice. Eight-week-old MRLlpr/lpr female mice were treated with 4 intraperitoneal injections of an anti–IL-17A antibody (1 injection every 4 days), and signs of lymphoproliferation and autoimmunity were evaluated 15 days after the last injection. The control treatments were performed with either phosphate-buffered saline or isotype-matched IgG2A. Lymphoproliferation was evaluated in terms of the spleen and lymph node sizes and the expansion of DN T cells in the blood, lymph nodes, and spleen. Autoimmunity was evaluated in terms of serum levels of anti-dsDNA autoantibodies and proteinuria to assess the renal damage. The results showed that treatment with anti–IL-17A antibodies significantly decreased the spleen and lymph node sizes, assessed both as the absolute weight and as a ratio with the whole body weight, compared with the control treatments (Figure 6A). Moreover, the treatment significantly decreased the expansion of DN T cells in the peripheral blood, lymph nodes and spleen, as assessed by flow cytometry (Figure 6B). Finally, the treatment decreased the serum levels of anti-dsDNA autoantibodies, as assessed by ELISA, the proteinuria, and prolonged the overall survival (Figure 7).
Effect of IL-17A neutralization in the MRLlpr/lpr lymphoproliferative pattern. (A) Representative organs (top) and mean ± SE of organ volumes (bottom) from the differently treated mice (n = 8/group). (B) DN T-cell expansion is shown in representative cytofluorimetric plots (top) and the mean ± SE of the data (bottom) from each group are shown. *P < .05 (Mann-Whitney U test).
Effect of IL-17A neutralization in the MRLlpr/lpr lymphoproliferative pattern. (A) Representative organs (top) and mean ± SE of organ volumes (bottom) from the differently treated mice (n = 8/group). (B) DN T-cell expansion is shown in representative cytofluorimetric plots (top) and the mean ± SE of the data (bottom) from each group are shown. *P < .05 (Mann-Whitney U test).
Effect of IL-17A neutralization on the MRLlpr/lpr autoimmune phenotype and on lifespan. The mean ± SE of proteinuria (A) and serum anti-dsDNA autoantibodies (B) of the differently treated groups of mice are shown. (C) A Kaplan-Meier survival analysis is shown; anti–IL-17A vs IgG2A: P = .0039; anti–IL-17A vs vehicle: P = .0006.
Effect of IL-17A neutralization on the MRLlpr/lpr autoimmune phenotype and on lifespan. The mean ± SE of proteinuria (A) and serum anti-dsDNA autoantibodies (B) of the differently treated groups of mice are shown. (C) A Kaplan-Meier survival analysis is shown; anti–IL-17A vs IgG2A: P = .0039; anti–IL-17A vs vehicle: P = .0006.
Discussion
This work shows that levels of IL-17A, IL-17F, and IL-17AF are increased in ALPS and DALD patients, and the results suggest that these increased levels may contribute to the development of these diseases by increasing the lymphocyte apoptotic defect caused by defective Fas function.
The increased levels were detected in both the serum and in vitro activated PBMCs, and concentrations of IL-17A and IL-17F comparable to those detected in the patients’ sera were able to inhibit FICD in Fas-sensitive T cells from healthy donors. Moreover, the patients’ sera were able to inhibit FICD, and this effect was reversed by neutralization of IL-17A.
The ALPS and the DALD patients also displayed high levels of IL-1β, which is intriguing because IL-1β plays a key role in induction of IL-17 expression.17 By contrast, IL-10 and IL-18 were increased in the ALPS but not in the DALD patients, which marks a difference between these diseases. Moreover, both groups of patients displayed decreased levels of CD4+CD25+FoxP3+ cells, comprising regulatory T cells, which is in line with the frequent autoimmune manifestations of both diseases (controls median 1.12%, interquartile range 0.96-1.7; ALPS 0.2%, 0.15-0.4, P = .0003; DALD 0.3%, 0.1-0.38, P = .0007; supplemental Table 1).
The antiapoptotic effect of IL-17A and IL-17F has not previously been described in human T cells, but it is consistent with a report showing that IL-17A can inhibit AICD, which is triggered in B cells by crosslinking of the B cells receptor.24
An intriguing result was that neutralization of IL-17A substantially increased cell apoptosis in the standard FICD assay performed on cells from the patients with high levels of IL-17A, including either ALPS-FAS, ALPS-UND, or DALD patients, whereas it had no effect on cells from healthy donors. This result indicates that endogenous secretion of IL-17A and possibly of IL-17F and IL-17AF may play a role in the apoptotic defect detected by the FIDC assay, at least in this subgroup of patients.
Several studies in mice have compared the sensitivity of Th1, Th2, and Th17 cells with FICD and AICD and have shown that Th17 cells are more resistant to both FICD and AICD than Th1 cells but less than Th2 cells.27 Our data suggest that the relative resistance to apoptosis of Th17 cells may be ascribed, in part, to autocrine effects of IL-17s. In the ALPS and DALD patients, the defective function of Fas may further increase this resistance to apoptosis and may favor the expansion of this T-cell subset and the increased levels of IL-17s, which may in turn favor the development of autoimmunity. This mechanism may also play a role in other autoimmune diseases because high levels of IL-17 similar to that detected in our patients have been described in SLE,24,28 RA,29,30 and psoriasis.31-33 Moreover, decreased Fas function has been also detected in patients displaying autoimmune diseases different from ALPS and DALD.34-36
The antiapoptotic effect of IL-17s may be partly due to modulation of cFLIP expression since exposure to IL-17A substantially upmodulated cFLIPS and downmodulated cFLIPL in Fas-stimulated T cells. This is in line with works showing that cFLIPS displays higher antiapoptotic activity than cFLIPL and that the low sensitivity of Th17 cells to apoptosis may be ascribed to expression of high levels of cFLIPs.37,38
These data suggest that neutralization of IL-17s may be effective in improving lymphocyte apoptosis in patients with ALPS and DALD. This possibility is also supported by the in vivo experiments in MRLlpr/lpr mice, in which IL-17A neutralization had positive effects on the autoimmune features of the diseases and prolonged the animals’ lifespan. Moreover, it displayed positive effects on the lymphoproliferative features of the diseases that were slight but significant. These therapeutic effects were weaker than those obtained by treating MRLlpr/lpr mice with long-term chemotherapies, but stronger than those obtained with other immunotherapeutic approaches, such as neutralization of IL-23 or IL-18.39-41 In this light, it is intriguing that both IL-23 and IL-18 are involved in the IL-17s network, because IL-23 supports Th17 cell expansion and IL-18 potentiates secretion of IL-17s.39,42-44 These data may suggest that T cells producing IL-17s may play a pathogenic role in mild forms of the lpr disease.
Thus, IL-17s may play a role similar to that played by OPN, high levels of which in ALPS, DALD, and in MRLlpr/lpr mice, may favor the development of the disease by reinforcing the lymphocyte apoptotic defect through the direct inhibition of AICD and the induction of secretion of TIMP-1 that can inhibit both FICD and AICD. Moreover, OPN has been shown to induce the secretion of IL-17A and could theoretically play a role in the increased IL-17A secretion observed in our patients.11
IL-17s appear to play a role in several autoimmune diseases. In animal models, IL-17A is involved in collagen-induced arthritis45 and in experimental autoimmune encephalomyelitis,21 whereas IL-17F appears to exacerbate the intestinal inflammation observed in dextran sulfate sodium–induced colitis.46 In humans, a key role has been hypothesized for IL-17A in cell-mediated autoimmune diseases such as multiple sclerosis22 and in type 1 diabetes mellitus.47 More recently, however, a role has also been proposed for IL-17s in SLE, in which it could support both the humoral autoimmune response by increasing B-cell survival and the inflammation in lupus lesions, with a particular role in renal lesions, by increasing the recruitment of inflammatory cells.24 In SLE, high IL-17A production has been partly ascribed to DN T cells, which are abundant in kidney lesions and have been suggested to be terminally differentiated Th17 cells. Similarly, DN T cells infiltrating the kidneys have been shown to produce high levels of IL-17A in MRLlpr/lpr mice.48 In ALPS, the DN T cells have been hypothesized to be exhausted T cells and have been shown to produce high levels of IL-10. IL-10 may play a key role in ALPS, and its high serum levels are included as a minor criterion for ALPS diagnosis.3 Our experiments did not allow us to detect the production of IL-17s in DN T cells because these cells were lost in our culture conditions. Moreover, DN T-cell expansion did not correlate with the serum levels of IL-17s in ALPS patients and were absent in DALD patients, who nevertheless also had high levels of IL-17s. These data suggest that the production of high levels of IL-17s is not strictly dependent on DN T cells in ALPS and DALD patients. Finally, no correlation was found between serum levels of IL-17s and IL-10 or between the in vitro T-cell secretion of IL-17s and IL-10 in our patients.
Recently, the therapeutic use of IL-17A antagonists has been investigated in several autoimmune diseases. LY243982 was the first effective humanized anti–IL-17A mAb to be used in the treatment of RA.49 More recently, a phase III clinical trial reported the efficacy of a fully human anti–IL-17A antibody (AIN457) in psoriasis, RA, and uveitis.50 Furthermore, a study evaluating the safety, tolerability, and efficacy of AIN457 in patients with relapsing-remitting multiple sclerosis is in progress (www.clinicaltrials.gov, Novartis).
Patients with ALPS and DALD generally respond to high doses of corticosteroids; however, some of them are refractory and others do not tolerate the side effects of treatment. IL-17A neutralization has been proven to be efficient in other autoimmune diseases and may also offer a targeted and personalized therapeutic option for these patients. Moreover, in ALPS patients, the autoimmune manifestations are often attenuated in adulthood, but they maintain the lymphoproliferative phenotype and are predisposed to developing several types of lymphomas, which is a risk that may be targeted by anti–IL-17 therapy.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Associazione Italiana Ricerca sul Cancro (IG 10237, Milano), Regione Piemonte (IMMONC Piattaforme Innovative), Fondazione Italiana Sclerosi Multipla (Genova 2010/R/12-2011/R/11), Fondazione Cariplo (Milano), Fondazione Amici di Jean (Torino), Fondazione Cassa di risparmio di Cuneo (Cuneo), and Programmi di ricerca di rilevante interesse nazionale Project 2009 (Ministero dell'Istruzione, dell'Università e della Ricerca, Rome).
Authorship
Contribution: E.B., N.C., E.O., G.C., E.T., and C.L.G. performed the study; U.R. and A.M. recruited the patients; U.D. and A.C. designed the study and wrote the manuscript; and U.D., U.R., and A.C. analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Umberto Dianzani, Interdisciplinary Research Center of Autoimmune Diseases (IRCAD) and Department of Health Sciences, “A. Avogadro” University of Eastern Piedmont, via Solaroli 17, I-28100 Novara, Italy; e-mail: umberto.dianzani@med.unipmn.it.
References
Author notes
E.B and N.C. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal