In this issue of Blood, Boggio et al demonstrate that interleukin-17 (IL-17) can inhibit Fas-induced apoptosis in normal T lymphocytes.1 More importantly, they show that blocking IL-17 may be an effective strategy to treat two diseases, autoimmune lymphoproliferative syndrome (ALPS) and Diazani autoimmune lymphoproliferative disease (DALD), which are characterized by defects in the Fas apoptotic pathway.
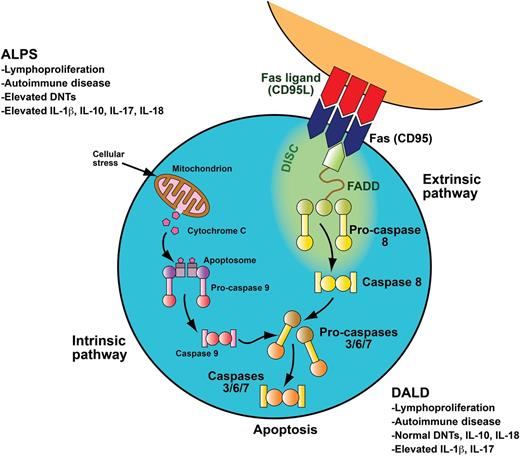
To downregulate the immune system after systemic insult, activated B and T lymphocytes upregulate Fas, and activated T lymphocytes upregulate Fas ligand. These two interact through the Fas-activating death domain (FADD) to trigger the caspase cascade, leading to cellular apoptosis. Apoptosis mediated through the FAS death receptor is part of the extrinsic apoptotic pathway. In contrast, apoptosis initiated in the mitochondria is part of the intrinsic apoptotic pathway. Patients with ALPS and DALD have a defect in the FAS apoptotic pathway. ALPS and DALD patients develop lymphoproliferation and autoimmune disease. ALPS patients have elevated peripheral blood DNTs, a hallmark of the disease, whereas DALD patients do not. Patients with ALPS typically have elevated serum levels of IL-10 and IL-18. Boggio et al demonstrate that DALD patients do not have elevated IL-10 or IL-18 levels. They also make the novel observations that both ALPS and DALD patients have elevated IL-17A and IL-17F levels as well as elevated IL-1β levels, providing a number of potential therapeutic targets for future investigations. Adapted with permission from a professional illustration by Sue Seif.
To downregulate the immune system after systemic insult, activated B and T lymphocytes upregulate Fas, and activated T lymphocytes upregulate Fas ligand. These two interact through the Fas-activating death domain (FADD) to trigger the caspase cascade, leading to cellular apoptosis. Apoptosis mediated through the FAS death receptor is part of the extrinsic apoptotic pathway. In contrast, apoptosis initiated in the mitochondria is part of the intrinsic apoptotic pathway. Patients with ALPS and DALD have a defect in the FAS apoptotic pathway. ALPS and DALD patients develop lymphoproliferation and autoimmune disease. ALPS patients have elevated peripheral blood DNTs, a hallmark of the disease, whereas DALD patients do not. Patients with ALPS typically have elevated serum levels of IL-10 and IL-18. Boggio et al demonstrate that DALD patients do not have elevated IL-10 or IL-18 levels. They also make the novel observations that both ALPS and DALD patients have elevated IL-17A and IL-17F levels as well as elevated IL-1β levels, providing a number of potential therapeutic targets for future investigations. Adapted with permission from a professional illustration by Sue Seif.
IL-17 is a member of a family of proinflammatory cytokines, including IL-17A and IL-17F, that are primarily produced by a subset of CD4+ T cells known as Th17 cells.2 IL-17 and Th17 cells have recently been shown to be important in the pathogenesis of a number of autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosis, psoriasis, and Crohn’s disease. In addition, they have important roles in cancer immune surveillance and in allograft rejection after organ transplant. Accordingly, a number of drugs that target IL-17 are in clinical development in early-phase clinical trials.
ALPS and DALD are similar disorders characterized by defective Fas-mediated apoptosis, leading to abnormal lymphocyte survival, autoimmune disease, and an increased risk of cancer (see figure).3 ALPS is diagnosed by a combination of (1) nonmalignant chronic lymphoproliferation; (2) elevated peripheral blood double-negative T cells (DNTs; cell phenotype CD3+, CD4–, CD8–, TCRα/β+); and (3) either a genetic defect in an ALPS causative gene (FAS, FASL, CASP10), in vitro evidence of defective Fas function, or abnormal biomarker elevation, such as vitamin B12, IL-10, IL-18, or soluble FAS ligand.3 Approximately 70% of patients with ALPS develop autoimmune disease, most commonly with autoimmune cytopenias. DALD is a similar disorder that also has chronic lymphoproliferation, autoimmune disease, and defective Fas function. DALD does not have elevated DNTs, but it does have high serum levels of osteopontin.
While many cytokines have been investigated in ALPS and DALD, surprisingly, there is no published work prior to the article by Boggio et al1 investigating the role of IL-17. Boggio et al make a number of important novel observations. They demonstrate that IL-17 is important in regulating Fas-mediated apoptosis in normal T cells, showing that recombinant IL-17A or IL-17F can block Fas-induced cell death in T cells collected from healthy donors. They also found that IL-17A and IL-17F were abnormally elevated in the sera of ALPS and DALD patients compared with healthy controls. The authors measured additional cytokines previously shown to be elevated in ALPS as controls, including IL-10 and IL-18, finding that both were elevated in their patients. Interestingly, they made the novel observation that IL-10 and IL-18 levels were not elevated in patients with DALD, providing further evidence that ALPS and DALD are distinct diseases. They also found that IL-1β was elevated in both ALPS and DALD patients. This is an important observation because IL-1β is currently targetable in the clinic with anakinra.4
Finally, they investigated whether targeting IL-17 could be of therapeutic benefit in ALPS and DALD. They demonstrated that IL-17A neutralization could increase Fas-induced cell death in T cells collected from ALPS and DALD patients. More importantly, they demonstrated that passive immunization with anti–IL-17A antibodies was effective at improving autoimmune disease in a mouse model of ALPS, MRL-lpr. Similar to work targeting other cytokines in MRL-lpr mice, the effects of IL-17A inhibition were not as robust against the lymphoproliferative manifestations of the disease. Most patients with ALPS and DALD who need treatment require therapy for autoimmune manifestations and not the lymphoproliferative features. Accordingly, IL-17 blockade may be an effective therapy for ALPS and DALD patients, and clinical trials are needed.
Currently, patients with ALPS and DALD are treated with immune suppressive agents, most commonly with corticosteroids, mycophenolate mofetil, or sirolimus.3 Many patients with ALPS and DALD need life-long treatment, and the use of long-term immune suppression in premalignant conditions could be problematic. In addition, not all patients respond to each available therapy, and some patients cannot tolerate the medicines. Accordingly, new treatment strategies are needed, and cytokine inhibition is a promising potential therapeutic approach.
Over the past decade, the concept of targeting cytokines to treat autoimmune, rheumatologic, and malignant diseases has evolved at a rapid pace. Currently, there are medicines approved by the US Food and Drug Administration and/or the European Union that target IL-1β, IL-2, IL-5, IL-6, tumor necrosis factor α (TNF-α), interferon gamma (INF-γ), and INF-α.4 Dozens of medicines that target additional cytokines are in preclinical development and early-phase clinical trials. In some circumstances, these medications are less effective than nonspecific immune suppressants; however, in other situations, they have shown considerable promise. TNF-α inhibitors are very active in rheumatoid arthritis and inflammatory bowel disease.4 The IL-6 inhibitor tocilizumab may be effective in reversing the cytokine release syndrome seen after novel T-cell activating anticancer therapies, including bispecific T-cell engaging single-chain antibody constructs and chimeric antigen receptor–modified T cells.5,6 Care is needed and preclinical studies are important before cytokine blockade is used in the clinic, because the cytokine cascade is very complex with positive and negative feedback. For example, IL-10 is markedly elevated in ALPS, leading to the hypothesis that IL-10 inhibition may be effective in ALPS. Yet, in animal models, inhibition of IL-10 caused a worsening of disease.7
Further studies are needed to determine the mechanisms underlying the elevated IL-17 levels in ALPS and DALD and to understand the downstream effects of those elevations on different signaling pathways. Signaling through the PI3K/Akt/mTOR pathway can positively regulate Th17 differentiation, and blockade of this pathway by using the mTOR inhibitor sirolimus can downregulate production of proinflammatory cytokines, including IL-17A by Th17 cells in other diseases.8 Sirolimus is a very active drug in murine and human ALPS, which raises the question: Is part of the benefit of sirolimus in ALPS through inhibition of Th17 and IL-17A? Recent studies have demonstrated that targeting Jak/Stat signaling may be effective in preclinical models of ALPS.9 Targeting Jak/Stat can impair proinflammatory IL-17 cytokine production, raising another question: Is part of the benefit of Jak/Stat inhibition in ALPS through inhibition of Th17 and IL-17?10
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal