Key Points
Lenalidomide is effective in refractory advanced cutaneous T-cell lymphoma, with an overall response rate of 28%.
Patients demonstrate a transient flare reaction in skin, blood, and/or lymph nodes that may be associated with improvement in disease burden.
Abstract
A phase 2 multicenter trial was performed to evaluate single-agent lenalidomide in advanced, refractory mycosis fungoides/Sézary syndrome. Thirty-two patients were enrolled with a median of 6 prior treatment regimens, including a median of 4 systemic therapies. Patients achieved an overall response rate of 28% (9 patients), and all were partial responses. Median overall survival was 43 months, median progression-free survival was 8 months, and median duration of response was 10 months. No grade 4 toxicities occurred. Grade 3 adverse events included fatigue (22%), infection (9%), and leukopenia (3%). Patients were frequently unable to tolerate the 25-mg starting dose of lenalidomide used in other hematologic malignancies due to fatigue, pain, and transient flare reaction (TFR) as a contributory factor. TFR appeared to correlate with clinical response, but the small sample size limited definitive conclusions, and the underlying mechanisms of this reaction are not known. Data from correlative studies on peripheral blood samples suggest that the effects of lenalidomide could be associated with decreased circulating CD25+ T cells and CD4+ T-cell numbers. Skin lesions showed a trend for increased CD8, CD25, and FoxP3 expression with decreased CD4:CD8 ratio. In conclusion, lenalidomide monotherapy demonstrated activity in refractory cutaneous T-cell lymphomas, along with acceptable toxicity. This trial was registered at www.clinicaltrials.gov as #NCT00466921.
Introduction
Cutaneous T-cell lymphomas (CTCLs) represent a subset of non-Hodgkin lymphomas (NHLs) and constitute a very rare (<10 cases per million) heterogeneous group derived from malignant, skin-homing mature T-helper cells.1-3 Mycosis fungoides (MFs), ranging from an indolent early stage to advanced stage disease, and Sézary syndrome (SS), an aggressive, erythrodermic and leukemic variant, are the 2 most common subtypes, accounting for approximately half of all CTCL cases. These entities are not curable with standard therapy. Patients with advanced-stage MF/SS frequently relapse and often develop disease refractory to treatment with an unfavorable prognosis.4
Overall survival (OS) has not been shown to benefit from aggressive intervention with cytotoxic agents.5 Historically, patients treated with cytotoxic agents have experienced serious treatment-associated toxicities due to prolonged myelosuppression, without durable benefit. Increased understanding of the pathogenesis of MF/SS with identification of new targets has led to new therapeutic approaches with biological agents to reduce treatment-related toxicity and improve outcomes. Reported efficacious single-agent therapies, including denileukin diftitox, bexarotene, deacetylase inhibitors (vorinostat, romidepsin, and panobinostat), and monoclonal antibodies for relapsed/refractory CTCL patients, have produced overall response rates (ORRs) of 24% to 45% in pivotal clinical trials.6-13
Decreased cell-mediated immunity with dominant Th2 cytokine profile is observed in advanced stages of MF/SS.14 These changes correlate with disease progression and host immunosuppression. The number of reactive cytotoxic CD8+ T cells and dendritic cells, which are characteristic in early patch stage cutaneous lesions, tend to decrease following the increase of neoplastic CD4+ T cells.15-17 Lenalidomide (Revlimid), an oral immunomodulatory drug and analog of thalidomide, is currently approved by the US Food and Drug Administration for the treatment of myelodysplastic syndrome, refractory/relapsed multiple myeloma, and mantle cell lymphoma.18-20 The immunomodulatory properties such as natural killer (NK)- and T-cell activation with induction of Th1 cytokine production and cytotoxic activity along with alteration of the tumor microenvironment through antiangiogenic, antiproliferative, and proapoptotic properties provided the rationale to use this agent in patients with CTCL.21
Lenalidomide inhibits growth of many hematologic cell lines21 and is currently being used in clinical trials to treat various hematologic malignancies and solid tumors.22-25 In particular, lenalidomide has shown promise in several studies in patients with NHL (not including CTCL), including in patients with relapsed/refractory disease.26-29 Despite the rationale for using lenalidomide in patients with CTCL, data on this treatment approach are lacking. We report final results from a phase 2 trial that evaluated the efficacy and safety of single-agent lenalidomide in patients with advanced, refractory CTCL. The study was also conducted to provide insight into the immunomodulatory effects of this treatment in CTCL.
Methods
Study participants
The study was conducted between 2005 and 2010 at 3 academic centers (Northwestern University, MD Anderson Cancer Center, and Stanford University) in the United States. Patients aged ≥18 years with stage IB to IVB disease of histologically proven MF/SS, including large-cell–transformed cases who had failed ≥1 prior skin-directed or systemic therapy, were considered eligible. A minimum of 4 weeks since completing skin-directed and/or systemic therapy was required and patients must have recovered from any toxic effects. Other eligibility criteria included Eastern Cooperative Oncology Group/World Health Organization performance status of ≤2, creatinine ≤2 mg/dL, and bilirubin ≤2.2 mg/dL, leukocyte count ≥3000/mm3 with absolute neutrophil count ≥1500/mm3, and platelet count ≥100 000/mm3. Pregnant or lactating women, women of childbearing age who were not using a reliable method of contraception, and patients with active infections and history of previous or concurrent neoplasm were excluded.
The current study was approved by the local institutional review board of each of the 3 participating US centers, and all patients gave written informed consent prior to enrollment in accordance with the Declaration of Helsinki.
Study design and interventions
This was an open-label, single-arm, multicenter phase 2 study of lenalidomide monotherapy. The first 19 patients enrolled received single-agent lenalidomide at a daily dose of 25 mg orally for 21 days of a 28-day cycle. Due to a frequently observed combination of debilitating fatigue and transient flare reactions (TFRs), the study was amended and subsequent patients initiated treatment at a dose of 10 mg daily. The dose was then increased by 5 mg every 28 days to a maximum of 25 mg daily, based on patient tolerability and response. Thromboembolic prophylaxis was not given with lenalidomide. Concomitant or intermittent use of topical or systemic steroids was not permitted to rule out any impact on lenalidomide efficacy.
Treatment was discontinued if disease progression was observed. Patients who experienced stable disease (SD) or partial response (PR) continued treatment of up to 2 years or until evidence of disease progression. Patients with a complete response (CR) were allowed to continue for an additional 2 cycles. All patients who discontinued lenalidomide were followed at 3-month intervals for a maximum of 12 months.
The disease status of all patients was monitored every 2 weeks during the first cycle and every 4 weeks of each subsequent cycle with appropriate physical examination, monitoring of Eastern Cooperative Oncology Group performance status, adverse event (AE) recording, laboratory assessments including Sézary cell counts, and computed tomography scans (at 16 weeks, on clinical response or end of treatment). Toxicity was assessed by the National Cancer Institute Common Terminology Criteria for AEs (NCI CTCAE) version 3.0.
The primary objectives of the study were to determine the efficacy, duration of response (DOR), and progression-free survival (PFS) in patients with refractory/relapsed MF/SS. Secondary objectives were to determine the toxicity associated with lenalidomide therapy in MF/SS and (Northwestern University site only) to investigate the effects of lenalidomide on the immune cell repertoire in lesional skin biopsy specimens and peripheral blood samples of patients with MF/SS at baseline and after the first cycle.
Assessment of response
Patients had to complete 1 cycle (28 days) of lenalidomide to be evaluable for response. The Physician’s Global Assessment and Composite Assessment (CA) of Index Lesion Disease Severity (CAILS) as previously published30 and standard at the time of study development were used to quantify skin disease burden with imaging and flow cytometry studies added to quantify nodal and blood disease. The presence of circulating malignant T cells (Sézary cells by CD4+/CD7− and/or CD+/CD26− immunophenotype) in the peripheral blood was assessed by flow cytometry with use of morphology in many cases. Criteria for response were defined as follows: CR, complete resolution of cutaneous, extracutaneous (lymph node and visceral) disease, and circulating Sézary cells for at least 4 weeks; PR, ≥50% reduction of cutaneous, extracutaneous disease, and circulating Sézary cells with no evidence of new lesions, for at least 4 weeks; SD, <50% improvement or <25% worsening from baseline in skin, nodes, and/or blood and with no new lesions; and progressive disease (PD), a >25% increase in cutaneous or extracutaneous disease, and/or Sézary cell count. A Sézary cell count of <5% was considered not significant.
DOR was defined as time of initial documentation of response (CR and PR) to the time of documentation of progression. OS was defined as the time from the start of treatment to the last follow-up or death. PFS was calculated from the first day of treatment until PD was documented, the course of treatment changed, or the patient died.
Correlative studies and immunophenotypic analysis
Evaluation of specific immune effector cell repertoire at baseline and day 1 of cycle 2 was assessed on skin and blood samples from consenting patients at 1 institution. Paraffin sections from 11 patients were stained for routine histopathology and CD4, CD8, CD25, forkhead box P3 (FoxP3), CD117 (cKit), and Langerin (CD207) according to standard protocols with appropriate positive controls. A semiquantitative scoring method was employed using a 4-point scale based on the percentage of positively staining tumor cells (<10%: 1+, >0%-20%: 2+, >20%-40%: 3+, >40%: 4+). For analysis, groups of negative and weak staining (scores 1 and 2) or moderate and strong staining (scores 3 and 4) were combined. Peripheral blood mononuclear cells from 6 patients were examined by fluorescence-activated cell sorter analysis for various T-cell, NK-cell, and myeloid and plasmacytoid dendritic cell markers such as CD4, CD8, CD25, CD56, CD158, CD11c, CD14, and CD123.
Statistical methods
The statistical analyses were performed on data from all enrolled patients. Sample size was based on a Simon 2-stage design with a type 1 error rate of 5% and an 80% power.31 A target number of 30 to 35 patients was required to yield 29 evaluable patients assuming a 20% response rate was adequate. Graphical methods and descriptive statistics, including relative frequencies and medians along with 95% binomial confidence intervals (CIs) were used to describe the study population and the response to treatment. OS, DOR, and PFS were estimated using the Kaplan-Meier method. A scatterplot was performed to determine differences of the immunologic components between 2 time points (baseline and after 1 cycle) and included a fitted regression line to present the trend in the data. All data analyses were performed using Stata v12.1 (Stata Corporation; College Station, TX).
Results
Patient characteristics
Thirty-two patients were enrolled. The majority of patients were white and female, with a median age of 64 years (range: 25-83 years; Table 1). Clinical stages were 7 (22%) stage IB, 2 (6%) stage IIA, 6 (19%) stage IIB, 7 (22%) stage III, 8 (25%) stage IVA, and 2 (6%) stage IVB. Eleven patients were diagnosed with SS and 3 with erythrodermic MF (confirmed by the International Society for Cutaneous Lymphomas criteria32,33 ). All patients were pretreated with a median of 6 treatments (range: 1-14), including a median of 4 (0-12) systemic treatments.
Baseline characteristics of patients treated with lenalidomide
| Characteristic . | Lenalidomide (n = 32) . |
|---|---|
| Age (y) | |
| Median (range) | 64 (25-83) |
| Gender | |
| Male | 15 |
| Female | 17 |
| Race | |
| White | 24 |
| Black | 7 |
| Hispanic | 1 |
| TNMB stage | |
| IB | 7 |
| IIA | 2 |
| IIB | 6 |
| III | 7 |
| IVA | 8 |
| IVB | 2 |
| Erythrodermic MF | 3 |
| Sézary syndrome | 11 |
| Prior treatments | |
| Median (range) | 6 (1-14) |
| Systemic | 4 (0-12) |
| Skin directed | 2 (0-6) |
| Characteristic . | Lenalidomide (n = 32) . |
|---|---|
| Age (y) | |
| Median (range) | 64 (25-83) |
| Gender | |
| Male | 15 |
| Female | 17 |
| Race | |
| White | 24 |
| Black | 7 |
| Hispanic | 1 |
| TNMB stage | |
| IB | 7 |
| IIA | 2 |
| IIB | 6 |
| III | 7 |
| IVA | 8 |
| IVB | 2 |
| Erythrodermic MF | 3 |
| Sézary syndrome | 11 |
| Prior treatments | |
| Median (range) | 6 (1-14) |
| Systemic | 4 (0-12) |
| Skin directed | 2 (0-6) |
TNMB, tumor, node, metastasis, blood.
All patients were off study at time of analysis. Seventeen patients discontinued the study as a result of recurrent disease or PD, 13 as a result of AEs including 8 patients secondary to personal preference as a result of fatigue and/or TFRs. One patient died due to disseminated encephalomyelitis 3 months after lenalidomide was discontinued, which was found to be unrelated to the drug. Nineteen patients (59%) received 1 to 3 cycles of lenalidomide. Eight patients (25%) completed 6 cycles, with 5 of these patients continuing treatment up to 14 cycles.
Response
Thirty-two patients were enrolled (intent-to-treat population).Three patients discontinued therapy early within the first cycle without response assessment, leaving 29 patients for response evaluation (Table 2). Eighteen patients were started on 25 mg lenalidomide daily. The subsequently 11 enrolled patients received 10 mg daily with 5-mg dose increments every 28 days. Five patients were able to escalate dose to 15 mg, 1 patient to 20 mg, and 5 patients to 25 mg daily.
Results of treatment with lenalidomide
| Outcome . | Lenalidomide (n = 32) . |
|---|---|
| Best response | |
| PR | 9 (28%) |
| SD | 17 (53%) |
| PD | 3 (9%) |
| Not evaluated* | 3 (9%) |
| Response duration (mo)† | |
| Median (95% CI) | 10 (1.1-11.0) |
| Progression-free survival (mo) | |
| Median (95% CI) | 8 (3.7-26.1) |
| OS (mo) | |
| Median (95% CI) | 43 (21.6-45.2) |
| Status at last follow-up | |
| Alive | 14 (44%) |
| Dead | 18 (56%) |
| Outcome . | Lenalidomide (n = 32) . |
|---|---|
| Best response | |
| PR | 9 (28%) |
| SD | 17 (53%) |
| PD | 3 (9%) |
| Not evaluated* | 3 (9%) |
| Response duration (mo)† | |
| Median (95% CI) | 10 (1.1-11.0) |
| Progression-free survival (mo) | |
| Median (95% CI) | 8 (3.7-26.1) |
| OS (mo) | |
| Median (95% CI) | 43 (21.6-45.2) |
| Status at last follow-up | |
| Alive | 14 (44%) |
| Dead | 18 (56%) |
Discontinued study during first cycle without response assessment.
Patients with PR.
Median follow-up time was 38 months. The ORR was 28% (9/32 patients), all with PRs. The median DOR was 10 months (95% CI: 1.1-11.0). Response to lenalidomide was observed in early and advanced stages of disease, including 5 patients with stage IB, 1 with stage IIA, 1 with stage IIB, and 2 with stage IVA. Six PRs were seen in the 25-mg starting dose group. Three PRs were seen in the 10-mg starting dose group, with responses documented at an escalated dose of 20 mg in 1 patient and 25 mg in 2 patients, respectively. The median time to response in the 25-mg starting group was 2 months and 4 months in patients with the 10-mg starting dose.
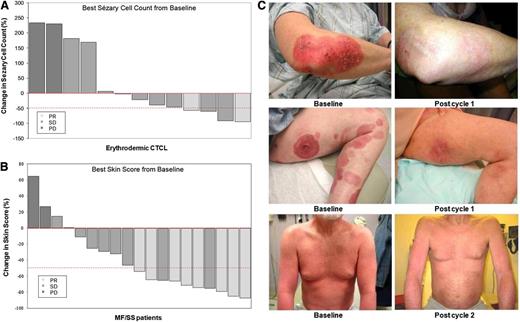
Lenalidomide was active in all compartments of disease, including skin, blood, and lymph nodes. Of 13 patients with erythrodermic MF/SS, 5 (38%) had a response to lenalidomide with >50% improvement in Sézary cell count, including 2 PRs when assessed for global response (Figure 1A). Of the 19 patients with clinical stage IB-IVB MF assessed for skin disease, improvement in baseline skin disease was seen in 14 patients; 10 (53%) had >50% improvement of skin disease burden by the CA, including seven PRs when assessed for global response (Figure 1B). Clinical photographs of patients with clinical stage IB, IIB, and erythrodermic MF (stage IVA) before and during treatment are shown in Figure 1C.
Clinical responses. Maximum percent improvements from baseline in (A) circulating Sézary cell counts (in 13 patients with erythrodermic CTCL and Sézary cell counts at baseline) and in (B) skin scores (in 19 patients with MFs/Sézary Syndrome). Partial responders (PR) are depicted by light gray bars, SD by medium gray bars, and PD by dark gray bars. The dashed line at −50% represents the threshold for PR. (C) Clinical presentations of patients with plaques (stage IB), tumors/plaques (stage IIB), and erythrodermic MFs (stage IVA) before and during treatment with near-complete clearance of plaques, tumors, and erythroderma.
Clinical responses. Maximum percent improvements from baseline in (A) circulating Sézary cell counts (in 13 patients with erythrodermic CTCL and Sézary cell counts at baseline) and in (B) skin scores (in 19 patients with MFs/Sézary Syndrome). Partial responders (PR) are depicted by light gray bars, SD by medium gray bars, and PD by dark gray bars. The dashed line at −50% represents the threshold for PR. (C) Clinical presentations of patients with plaques (stage IB), tumors/plaques (stage IIB), and erythrodermic MFs (stage IVA) before and during treatment with near-complete clearance of plaques, tumors, and erythroderma.
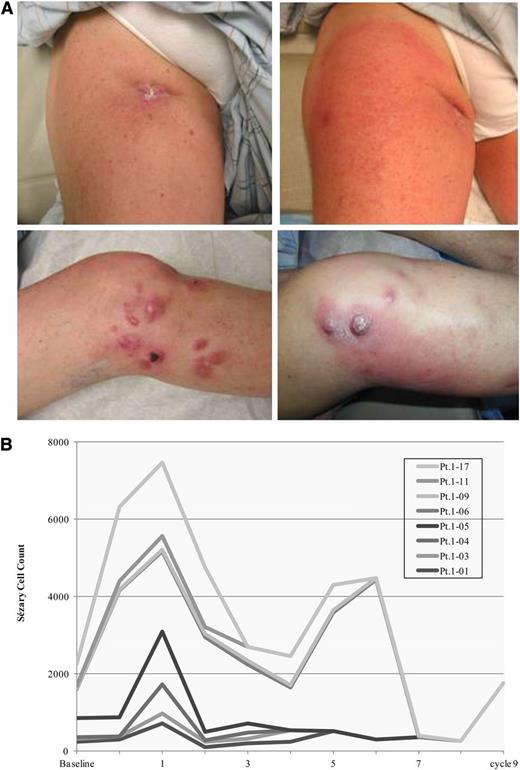
Nine patients had a documented TFR with pain, erythema often localized to lesional skin, lymph node swelling, and/or increased circulating Sézary cells during first cycle and subsequent cycles. All TFRs occurred at the 25-mg starting dose. Among the patients experiencing a TFR, 4 achieved an objective PR with >50% improvement in skin disease and/or Sézary cell count and 4 remained in SD. Interestingly, all 9 patients (including 4 with SS) developed an increase in circulating Sézary cells that eventually decreased or resolved with continued treatment. Clinical characteristics of a typical TFR are shown in Figure 2. TFR was not documented in patients at the 10-mg starting dose.
Tumor flare reaction. (A) Documented tumor flare reactions presenting as erythema around lymph node and cutaneous tumor lesions in a patient with tumor-stage MFs. (B) Increasing Sézary cell counts in patients with or without Sézary cell counts at baseline that eventually decreased or resolved with continued treatment. Pt., patient.
Tumor flare reaction. (A) Documented tumor flare reactions presenting as erythema around lymph node and cutaneous tumor lesions in a patient with tumor-stage MFs. (B) Increasing Sézary cell counts in patients with or without Sézary cell counts at baseline that eventually decreased or resolved with continued treatment. Pt., patient.
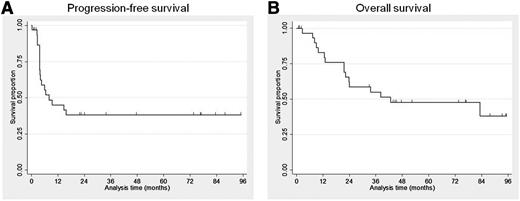
Survival
The median PFS was 8 months (95% CI: 3.7-26.1) and the median OS for all patients was 43 months (95% CI: 21.6-45.2) (Figure 3A-B). Kaplan-Meier OS estimates were 83% at 1 year, 62% at 2 years, and 48% at 5 years. At the time of the last follow-up, 18 patients (56%) had died, and 14 patients (44%) were alive. Twelve (67%) of the deaths were attributable to CTCL. Other causes of death included complication after allogeneic bone marrow transplant/umbilical cord transplant (n = 2), acute encephalomyelitis (n = 1), natural causes/age (n = 2), and unknown condition (n = 1). Among the 9 responders, 2 patients eventually died due to PD/complication after transplant.
Survival outcome. Progression-free survival (A) and overall survival (B) among patients receiving lenalidomide monotherapy.
Survival outcome. Progression-free survival (A) and overall survival (B) among patients receiving lenalidomide monotherapy.
Safety and tolerability
Data from all enrolled patients were included in the safety analysis. All patients received at least 1 dose of lenalidomide. The most common treatment-related side effects were fatigue (59%), lower leg edema (47%), and anemia (41%) (Table 3). Most AEs were mild to moderate in severity. The grade 3 AEs of any cause occurring during the trial were fatigue (22%), infection (9%), and leukopenia (3%). The grade 3 infections were pneumonia, leg cellulitis, and skin infection with Staphylococcus aureus/Pseudomonas in 1 patient each. No grade 4 toxicity occurred. Nine patients (28%) had documented TFR after starting treatment with lenalidomide at 25-mg daily dose. The rate of TFR was not witnessed in patients who received lenalidomide dose escalation, although fatigue (grade 1-2) still occurred. Thirteen patients discontinued treatment as a result of AEs, including 8 patients secondary to personal preference as a result of fatigue and/or TFRs. Although not a treatment-related effect, pruritus was one of the most common symptoms (75%) given by patients. Thromboembolic events or second malignancies were not seen.
Treatment-related AEs associated with lenalidomide that occurred in more than 10% of patients (n = 32)
| Adverse event . | Toxicity grade* . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | Total . | |
| Hematologic | |||||
| Anemia | 7 (22) | 6 (19) | 13 (41) | ||
| Leukopenia | 2 (6) | 4 (13) | 1 (3) | 7 (22) | |
| Constitutional | |||||
| Fatigue | 8 (25) | 4 (13) | 7 (22) | 18 (59) | |
| Pain/burn | 2 (6) | 9 (28) | 11 (34) | ||
| Skin | |||||
| Flares/erythema | 2 (6) | 7 (22) | 8 (25) | ||
| Infection | 1 (3) | 7 (22) | 3 (9) | 11 (34) | |
| Gastrointestinal | |||||
| Nausea/vomiting | 4 (13) | 4 (13) | |||
| Anorexia/weight loss | 3 (9) | 2 (6) | 5 (16) | ||
| Constipation/diarrhea | 6 (19) | 5 (16) | 11 (34) | ||
| Neurologic | |||||
| Neuropathy (sensory/motor) | 4 (13) | 2 (6) | 6 (19) | ||
| Edema | 7 (22) | 8 (25) | 15 (47) | ||
| Hypoalbuminemia | 5 (16) | 4 (13) | 9 (28) | ||
| Adverse event . | Toxicity grade* . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | Total . | |
| Hematologic | |||||
| Anemia | 7 (22) | 6 (19) | 13 (41) | ||
| Leukopenia | 2 (6) | 4 (13) | 1 (3) | 7 (22) | |
| Constitutional | |||||
| Fatigue | 8 (25) | 4 (13) | 7 (22) | 18 (59) | |
| Pain/burn | 2 (6) | 9 (28) | 11 (34) | ||
| Skin | |||||
| Flares/erythema | 2 (6) | 7 (22) | 8 (25) | ||
| Infection | 1 (3) | 7 (22) | 3 (9) | 11 (34) | |
| Gastrointestinal | |||||
| Nausea/vomiting | 4 (13) | 4 (13) | |||
| Anorexia/weight loss | 3 (9) | 2 (6) | 5 (16) | ||
| Constipation/diarrhea | 6 (19) | 5 (16) | 11 (34) | ||
| Neurologic | |||||
| Neuropathy (sensory/motor) | 4 (13) | 2 (6) | 6 (19) | ||
| Edema | 7 (22) | 8 (25) | 15 (47) | ||
| Hypoalbuminemia | 5 (16) | 4 (13) | 9 (28) | ||
Data are n (%) of patients.
National Cancer Institute Common Toxicity Criteria version 3.0.
Immunophenotypic analysis
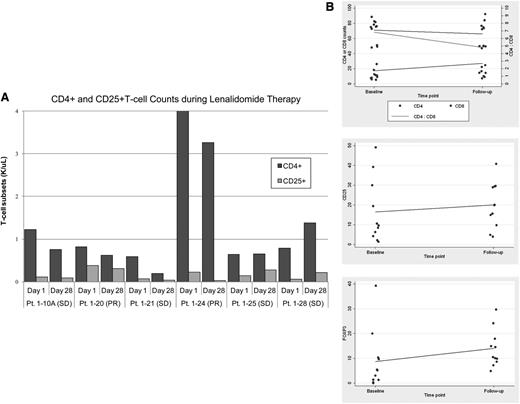
When compared with baseline levels, 4/6 patients with erythrodermic MF/SS screened for immunomodulatory changes in peripheral blood samples had a decrease in CD4+ T cells (range: 24%-68%) with a concomitant decrease in CD4+CD25+ T cells (range: 18%-87%) (Figure 4A). When compared with clinical response, 2 of these patients achieved PR and 2 achieved SD. The remaining 2 patients achieved SD, with 1 patient showing no or minimal change in CD4+ and CD4+ CD25+ T-cell counts, respectively, and 1 patient showing an increase in both CD4+ T cells (Δ 43%) and CD4+CD25+ T cells (Δ 50%). Due to low cell numbers, there was no trend in changes of NK-cell and myeloid and plasmacytoid dendritic cell populations seen.
Lenalidomide-induced immunophenotypic changes. (A) Effects of lenalidomide measured by CD4+ and CD25+ T-cell counts in peripheral blood mononuclear cells at day 28 compared with baseline (day 1). (B) Skin biopsy specimens analyzed for CD4+, CD8+, CD25+, and FoxP3+ T cells at day 28 compared with baseline (day 1). Each dot indicates single specimen at baseline and then after 1 cycle. A scatterplot was performed to determine differences of the immunologic components between 2 time points. The fitted regression line represents the trend in the data. Pt., patient.
Lenalidomide-induced immunophenotypic changes. (A) Effects of lenalidomide measured by CD4+ and CD25+ T-cell counts in peripheral blood mononuclear cells at day 28 compared with baseline (day 1). (B) Skin biopsy specimens analyzed for CD4+, CD8+, CD25+, and FoxP3+ T cells at day 28 compared with baseline (day 1). Each dot indicates single specimen at baseline and then after 1 cycle. A scatterplot was performed to determine differences of the immunologic components between 2 time points. The fitted regression line represents the trend in the data. Pt., patient.
The skin lesions of MF/SS patients showed a trend for increased CD25, FoxP3, and CD8 expression, with decreased CD4:CD8 ratio within lymphoid infiltrates (Figure 4B). There was no significant change in other immunologic cells such as mast cells, Langerhans cells, or dendritic cell subsets. Sections were also scored for atypia and density of dermal infiltrates based on a histologic grading system.34 Overall, infiltrates showed less epidermotropism with less atypia in responding patients compared with baseline.
Discussion
We conducted a phase 2 multicenter study to evaluate the efficacy and tolerability of lenalidomide in heavily pretreated patients with relapsed/refractory CTCL. This is the first study, to our knowledge, of lenalidomide in patients with CTCL. Lenalidomide monotherapy demonstrated activity with approximately one-third (28%) of patients achieving a PR. Of note, responses in skin manifestations were observed in 14/32 patients with MF/SS and in 8/14 patients with erythrodermic MF/SS. The data also show that lenalidomide induces durable responses with a median DOR of 10 months and a median PFS of 8 months, respectively.
Pivotal trials with biological therapies such as vorinostat, romidepsin, panobinostat, bexarotene, denileukin diftitox, and pralatrexate demonstrated ORRs between 17% and 45%.6-10,12,30,35 It is difficult to directly compare our results due to inherent differences in study design, assessment of response, and patient characteristics. Our patient population was similar, with 72% of patients in advanced stages, but relatively small compared with other trials in refractory/advanced CTCL. Although the ORR may compare with previously published data, most of the objective responses (PR in 6/9 patients) in our current trial were seen in stage IB/IIA. Only recently have guidelines for clinical trials in CTCL been published that standardize clinical end points and response criteria that might better allow for cross-trial comparison.36 The modified severity weighted assessment tool (mSWAT) for skin scoring was not established at the time our trial was designed. We used CAILS, adopted from previously published CTCL trials, and the physician’s global assessment.30 Despite responses in index lesions, new skin disease was documented as disease progression and resulted in early discontinuation from treatment in some patients. This may have underestimated the therapeutic efficacy of lenalidomide.
In the current trial, the majority of side effects were grade 1 or 2 in severity. No patients had a grade 4 AE. Only 1 patient experienced a grade 3 hematologic AE (leukopenia), which is in contrast with the higher grade 3/4 neutropenias and thrombocytopenias observed in patients with myelodysplastic syndromes,32 multiple myeloma,33 and mantle cell lymphoma.28 However, constitutional symptoms such as fatigue, pain, and TFR that mimicked worsening of patient’s erythroderma/disease remained a concern and were the most common reason for discontinuation of treatment. After the trial was amended, later-enrolled patients with a 10-mg starting dose and slow dose escalation resulted in improved tolerability.
A TFR has also been observed in chronic lymphocytic leukemia (CLL) patients that occurred mainly during the first cycle.22,37 In a phase 2 trial, lenalidomide-induced TFR correlated with clinical response in a small subset of CLL patients, suggesting that mechanisms underlying this reaction may represent an antitumor response.37 In our study, 9/18 patients at the 25-mg starting dose had documented TFR with subsequent improvement of skin, nodes, and/or Sézary cell counts; an objective PR was seen in 4 patients. This flare reaction was not witnessed at the 10-mg starting dose. In this limited sample, our findings suggest that the development of TFR may correlate with the clinical activity of lenalidomide in CTCL; still, a TFR was not documented in all responding patients.
TFR provides challenges for the use of lenalidomide for the management of CTCL. Our data support that the 25-mg daily dose schedule induces objective responses in CTCL, but this may be at the expense of greater flare reaction. Steroid prophylaxis was not permitted in our trial due to potential impact on lenalidomide efficacy and increased toxicity (thromboembolic events), with the latter seen in myeloma patients. Studies clarifying the mechanism and potential benefits of TFR vs the toxicity may help define the optimal starting dose of lenalidomide in this population. If TFR is associated with response, there may be advantages to starting at the 25-mg dose. In addition, the addition of corticosteroids for TFR prophylaxis may help to minimize the symptomatic effects of TFR but may also blunt the potential immunologic stimulation that is hypothesized to be of benefit, and it may lead to other adverse effects such as thromboembolic events.
We investigated the effects of lenalidomide on the specific immune-cell repertoire in blood and skin samples in a small subset of patients. Overall, a decline in both circulating CD4+ and CD25+ T-cell counts was detected. Sections from lesional skin biopsy specimens showed a trend for increased CD8, CD25, and FoxP3 expression with a decreased CD4 to CD8 ratio when compared with baseline. Whereas the effects on peripheral blood samples could be explained at least in part by direct cytotoxic effects on the malignant T cells, the findings on skin sections suggest immune activation through expansion of reactive CD8+ T cells and regulatory T cells. High numbers of CD8+ cytotoxic T cells and regulatory T cells have been correlated with improved survival.15,17 The exact mechanisms of action of lenalidomide in CTCL remain unclear. Effects may reflect different targets such as augmentation of antitumor activity and effects on microenvironment. Unfortunately, no patient who experienced a TFR was sampled for these laboratory investigations.
To date, no published data are available relative to the in vitro effects on CTCL cells. In CLL, lenalidomide is thought to alter the cytokine and cellular microenvironment by upregulating costimulatory molecules on B-CLL cells. This upregulation may promote TFR via a T-cell activation–dependent cytokine release.38 Future investigations will focus on costimulatory molecules on CTCL cells, which may help explain the immune phenomena.
In summary, our results indicate that lenalidomide is effective in CTCL with response duration that provides meaningful clinical benefit. However, the constitutional symptoms and flare reactions in our patients may warrant schedule modifications and/or TFR prophylaxis, despite the otherwise low toxicity profile. Patients frequently relapse and require repeat treatment courses for disease control. Consequently, a better understanding of the underlying mechanisms of disease is necessary to develop more effective therapeutic approaches. The multiple potential antineoplastic effects of lenalidomide make it an ideal candidate for combination therapy. Lenalidomide in combination with other agents has been proven to be effective with manageable toxicity in the treatment of CLL, multiple myeloma, and myelodysplastic syndromes, and similar approaches would be rational in CTCL. A phase 1 trial of romidepsin and lenalidomide in NHL including CTCL has been initiated.
Presented in part at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, December 10-13, 2011, and at the Second World Congress of Cutaneous Lymphomas, Berlin, Germany, February 6-9, 2013.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, medical staff, and physicians who participated in this study. C.Q. is a Ted Schwartz Scholar.
This study was sponsored by Celgene. Medical writing support was provided by Ryan Woodrow and Baxter Jeffs, Investigator-Initiated Research Writing Group of KnowledgePoint 360 Group, with whom Celgene contracted for technical writing.
Authorship
Contribution: C.Q. contributed to conceiving and designing the study; collecting, analyzing, and interpreting the data; and writing and approving the manuscript; S.T.R. contributed to conceiving and designing the study; collecting, analyzing, and interpreting the data; and writing/commenting and approving the manuscript; J.G. contributed to collecting, analyzing, and interpreting the data, writing/commenting, and approving of the manuscript; M.D. contributed to collecting and interpreting the data and writing/commenting and approving the manuscript; Y.H.K. contributed to collecting and interpreting the data and writing/commenting and approving the manuscript; S.W.D. contributed to interpreting the data, performing statistical analysis, and writing/commenting and approving the manuscript; and T.M.K. contributed to conceiving and designing the study; collecting, analyzing, and interpreting the data; and writing/commenting and approving the manuscript.
Conflict-of-interest disclosure: C.Q. has served as compensated advisor for Celgene; S.T.R. has served as a compensated consultant, advisor, and speaker for Celgene; J.G. declares no competing financial interests; M.D. has served as compensated consultant and advisor for Celgene; Y.H.K. has served as a compensated consultant and advisor for Celgene; S.W.D. declares no competing financial interests; and T.M.K. has served as compensated advisor and speaker for Celgene.
Correspondence: Christiane Querfeld, Department of Medicine/Dermatology Service, Memorial Sloan-Kettering Cancer Center, 160 E 53rd St, Suite 234, New York, NY 10022; e-mail: querfelc@mskcc.org.