Abstract
Target-specific oral anticoagulants (TSOACs) that directly inhibit thrombin (dabigatran) or factor Xa (rivaroxaban, apixaban) are effective and safe alternatives to vitamin K antagonists (VKAs) and low-molecular-weight heparin (LMWH). Although these agents have practical advantages compared with VKAs and LMWH, there are no antidotes that reverse their anticoagulant effect. Clinical evidence for the efficacy of nonspecific therapies that promote formation of fibrin (prothrombin complex concentrate [PCC], activated PCC [aPCC], and recombinant factor VIIa) in the setting of TSOAC-associated bleeding is lacking, and these prohemostatic products are associated with a risk of thrombosis. In the absence of specific antidotes, addition of PCC or aPCC to maximum supportive therapy may be reasonable for patients with severe or life-threatening TSOAC-associated bleeding. Targeted antidotes for these agents are in development.
Introduction
Target-specific oral anticoagulants (TSOACs) that target thrombin (dabigatran) or factor Xa (rivaroxaban and apixaban) offer practical advantages over vitamin K antagonists (VKAs) for long-term oral anticoagulant therapy, including rapid onset of action, short half-lives, fewer drug and food interactions, and predictable pharmacokinetics eliminating the need for routine monitoring of anticoagulant effect. In large clinical trials, these agents demonstrated similar or enhanced efficacy and/or safety compared with VKAs and low-molecular-weight heparin (LMWH) for prevention and treatment of thromboembolism. Table 1 outlines currently approved indications for clinical use of TSOACs.
Approved indications for use of novel oral anticoagulants
| Drug/region . | Prevention of stroke/systemic embolism in nonvalvular atrial fibrillation . | Prevention of VTE after hip/knee replacement surgery . | Treatment of acute VTE . | Prevention of VTE recurrence . |
|---|---|---|---|---|
| Dabigatran | ||||
| United States | ✓ | |||
| Canada | ✓ | ✓ | ||
| Europe | ✓ | ✓ | ||
| Rivaroxaban | ||||
| United States | ✓ | ✓ | ✓ | ✓ |
| Canada | ✓ | ✓ | ✓ | ✓ |
| Europe | ✓ | ✓ | ✓ | ✓ |
| Apixaban | ||||
| United States | ✓ | |||
| Canada | ✓ | ✓ | ||
| Europe | ✓ | ✓ |
| Drug/region . | Prevention of stroke/systemic embolism in nonvalvular atrial fibrillation . | Prevention of VTE after hip/knee replacement surgery . | Treatment of acute VTE . | Prevention of VTE recurrence . |
|---|---|---|---|---|
| Dabigatran | ||||
| United States | ✓ | |||
| Canada | ✓ | ✓ | ||
| Europe | ✓ | ✓ | ||
| Rivaroxaban | ||||
| United States | ✓ | ✓ | ✓ | ✓ |
| Canada | ✓ | ✓ | ✓ | ✓ |
| Europe | ✓ | ✓ | ✓ | ✓ |
| Apixaban | ||||
| United States | ✓ | |||
| Canada | ✓ | ✓ | ||
| Europe | ✓ | ✓ |
VTE, venous thromboembolism.
Unlike VKAs for which vitamin K and coagulation factor replacement (with either prothrombin complex concentrate [PCC] or plasma) can be used in case of bleeding, there are no therapies that specifically reverse the anticoagulant effect of the TSOACs. Management of TSOAC-associated bleeding is further complicated by the difficulty in determining the degree of anticoagulant effect present at the time of the bleed. Widely available tests of the integrity of the coagulation cascade are variably responsive to the effect of the TSOACs (and in many cases, may fall within the normal range even when therapeutic drug levels are present), and specific tests (although available) are largely limited to highly specialized coagulation laboratories.1,2 In this narrative review, we discuss available evidence for reversal strategies and provide a practical approach to the management of TSOAC-associated bleeding.
TSOAC mechanism of action and pharmacokinetics
Oral direct thrombin inhibitor (dabigatran)
Dabigatran is the only oral direct thrombin inhibitor currently available. It is administered as the pro-drug dabigatran etexilate, which is then converted to the active compound, dabigatran, in vivo. After oral administration, peak plasma levels are achieved within 1 to 3 hours.3 Dabigatran undergoes significant renal excretion (80-85%) and has a half-life of 7 to 17 hours in patients with normal renal function (Table 2).3,4 Dabigatran blocks the enzymatic activity of thrombin, thus preventing the conversion of fibrinogen to fibrin and thrombin-mediated platelet activation, along with thrombin’s other effects.5,6
Pharmacokinetics of novel oral anticoagulants
| Novel oral anticoagulant . | Time to peak concentration (hours) . | Half-life (hours) . | Extent of renal excretion . |
|---|---|---|---|
| Dabigatran3,4 | 1-3 | 7-9* | 80-85% |
| 7-17† | |||
| Rivaroxaban10,11,14 | 2-4 | 7-17* | 36% |
| 12-13‡ | |||
| 6-9† | |||
| Apixaban15,17,18 | 1-3 | 8-14* | 25% |
| Novel oral anticoagulant . | Time to peak concentration (hours) . | Half-life (hours) . | Extent of renal excretion . |
|---|---|---|---|
| Dabigatran3,4 | 1-3 | 7-9* | 80-85% |
| 7-17† | |||
| Rivaroxaban10,11,14 | 2-4 | 7-17* | 36% |
| 12-13‡ | |||
| 6-9† | |||
| Apixaban15,17,18 | 1-3 | 8-14* | 25% |
Healthy adults, single dose.
Healthy adults, multiple doses.
Healthy elderly, single dose.
Oral factor Xa inhibitors (rivaroxaban and apixaban)
Both rivaroxaban and apixaban inhibit free and clot-associated factor Xa through interactions with its active site.7-9 Plasma levels of rivaroxaban peak 2 to 4 hours following oral administration (Table 2).10,11 It is excreted by the kidneys (36%) and has a reported half-life of 6 to 13 hours depending on the dose and age of the subjects studied.10-14 Peak plasma levels of apixaban are achieved 1 to 3 hours after oral administration.8,12,15,16 It is excreted by the kidneys to a lesser extent (25%) than either rivaroxaban or dabigatran (Table 2).12,15,17,18
TSOAC-associated bleeding
Major or life-threatening bleeding (eg, gastrointestinal and intracranial) is the most feared complication of anticoagulant therapy, including TSOACs. In published clinical trials, rates of major bleeding among patients receiving TSOACs were comparable to, or lower than, those among patients receiving warfarin or LMWH for currently approved indications.19-28 Variation in estimates of major bleeding is likely influenced by interstudy differences in definitions of major bleeding, indications for use, drug dosage, treatment duration, and patient characteristics.29
Rates of bleeding seen in real world patients treated with dabigatran, are consistent with those seen in studies and less than those seen with warfarin. Postmarketing surveillance efforts are underway to define the bleeding risk outside the clinical trial setting.30,31 The US Food and Drug Administration is currently evaluating reports of gastrointestinal and intracranial hemorrhage in new users of dabigatran or warfarin through analysis of insurance claim and administrative data from the Mini-Sentinel database. In a recent analysis, there did not appear to be higher bleeding rates associated with dabigatran compared with warfarin.30,32
A nationwide Danish prospective cohort study assessed the safety and efficacy of dabigatran (n = 4978) compared with warfarin (n = 8936) for the treatment of anticoagulant-naïve patients with atrial fibrillation in everyday clinical practice.33 Rates of major bleeding (dabigatran, 110-mg dose: adjusted hazard ratio [aHR] 0.82, 95% confidence interval [CI] 0.59-1.12; dabigatran, 150-mg dose: aHR 0.77, 95% CI 0.51-1.13) and gastrointestinal bleeding (dabigatran, 110-mg dose: aHR 0.60, 95% CI 0.37-0.93; dabigatran, 150-mg dose: aHR 1.12, 95% CI 0.67-1.83) were similar between dabigatran- and warfarin-treated patients. Both doses of dabigatran were associated with reduced rates of intracranial hemorrhage compared with warfarin (dabigatran, 110-mg dose: aHR 0.24, 95% CI 0.08-0.56; dabigatran, 150-mg dose: aHR 0.08, 95% CI 0.01-0.40). Risk of death was also reduced with both doses of dabigatran compared with warfarin (dabigatran, 110-mg dose: aHR 0.79, 95% CI 0.65-0.95; dabigatran, 150-mg dose: aHR 0.57, 95% CI 0.50-0.80).
Monitoring coagulation status in TSOAC-associated bleeding
Although TSOACs do not require routine monitoring of the anticoagulant effect, emergency situations may necessitate assessment of coagulation status. Conventional coagulation assays have limited accuracy and reliability for determining the TSOAC anticoagulant effect and should be interpreted with caution in this setting.1,2 Modified conventional tests and alternative assays are more accurate and reliable but are not routinely available or standardized and lack established ranges of results that correspond to drug effect.1 Results of coagulation tests should be interpreted relative to known performance of the test with the agent in question, dosing interval, drug pharmacokinetics, and renal function (especially for dabigatran). The clinical value of coagulation test results is limited by a lack of outcome data in patients with TSOAC-associated bleeding; the relationship between test results and bleeding is currently unknown. Table 3 shows the effect of TSOACs on available coagulation tests.34
Effect of novel oral anticoagulants on coagulation tests
| Novel oral anticoagulant . | PT . | aPTT . | Thrombin clotting time . | Ecarin clotting time . | Hemoclot assay . | Anti–factor Xa activity . | |
|---|---|---|---|---|---|---|---|
| Clot based . | Chromogenic . | ||||||
| Dabigatran | ↑ or ↔ | ↑ | ↑ | ↑ | ↑* | — | — |
| Rivaroxaban | ↑ or ↔ | ↑ or ↔ | — | — | — | ↑ | ↑* |
| Apixaban | ↑ or ↔ | ↑ or ↔ | — | — | — | ↑* | ↑* |
| Novel oral anticoagulant . | PT . | aPTT . | Thrombin clotting time . | Ecarin clotting time . | Hemoclot assay . | Anti–factor Xa activity . | |
|---|---|---|---|---|---|---|---|
| Clot based . | Chromogenic . | ||||||
| Dabigatran | ↑ or ↔ | ↑ | ↑ | ↑ | ↑* | — | — |
| Rivaroxaban | ↑ or ↔ | ↑ or ↔ | — | — | — | ↑ | ↑* |
| Apixaban | ↑ or ↔ | ↑ or ↔ | — | — | — | ↑* | ↑* |
↑, simple increase; ↔, no change; —, not applicable.
Dabigatran prolongs the thrombin time (TT; also known as thrombin clotting time), even at low concentrations.35-37 It has a linear dose-dependent relationship with dabigatran concentration but shows dramatic prolongation with increasing concentration.3,38 A prolonged TT thus reliably indicates that dabigatran is present; however, it is not particularly useful as an indicator of drug concentration across the range of normally achieved levels. The activated partial thromboplastin time (aPTT) does not correlate well with plasma concentrations of dabigatran, shows significant interindividual variability, and is influenced by the reagents and instruments used for testing.36,37,39 The prothrombin time (PT), often reported as international normalized ratio, has low sensitivity and substantial test variability, making it unsuitable for measuring the effects of dabigatran.36
The PT is typically prolonged in the presence of clinically relevant concentrations of rivaroxaban, but its utility is limited by reagent-dependent variability and poor correlation between the degree of PT prolongation and the plasma concentration of rivaroxaban.40-45 Chromogenic anti–factor Xa assays (of the sort used for monitoring LMWH) correlate well with plasma rivaroxaban levels. They have reduced variability and improved sensitivity and specificity when measured using a rivaroxaban calibration curve.46,47 aPTT is less sensitive than PT for measuring the effect of rivaroxaban and shows significant variability depending on the reagents used.43
The PT also has limited utility in the presence of apixaban due to poor test sensitivity and reliability.1,47 The dilute PT (or modified PT), clot-based anti–factor Xa (HepTest; Sekisui Diagnostics, Stanford, CT), and chromogenic anti–factor Xa assays are superior to the conventional PT assay for determining the anticoagulant effect of apixaban.1,44,47,48 The aPTT is unsuitable for measuring the apixaban effect due to poor assay sensitivity.18,47
Reversal of TSOAC anticoagulant effect
Coagulation factor replacement
Prothrombin complex concentrate.
Prothrombin complex concentrates (PCCs) contain vitamin K–dependent coagulation factors II, IX, X (3-factor PCC) or II, VII, IX, and X (4-factor PCC) and varying amounts of proteins C and S. There is no high-quality evidence establishing the efficacy and safety of PCC for the reversal of the TSOAC anticoagulant effect in patients with bleeding complications. However, in healthy volunteers receiving rivaroxaban (20 mg daily for 2.5 days), infusion of 4-factor PCC (50 U/kg) corrected PT prolongation and abnormal endogenous thrombin potential.49 In the same study, PCC did not correct the prolonged aPTT, ecarin clotting time, or TT induced by dabigatran (150 mg twice daily for 2.5 days). Preliminary results from an open-label, parallel group study showed that PCC (3-factor and 4-factor formulations) administered to healthy volunteers treated with rivaroxaban (20 mg twice daily for 4 days) corrected PT prolongation and restored thrombin generation to baseline.50 However, the clinical relevance of laboratory test correction in the setting of TSOAC-associated bleeding has yet to be determined.
Use of PCC to ameliorate dabigatran-related bleeding in animal models has yielded conflicting results with reduced intracranial hematoma expansion and 24-hour mortality in mice, reduced bleeding following kidney incision in rabbits, but no effect on blood loss following mouse tail transaction.51-53 PCC had no effect on bleeding in rivaroxaban-treated rabbits despite partial correction of laboratory abnormalities.54 In in vitro models, PCC normalized some abnormal thrombin generation indices induced by dabigatran, rivaroxaban, and apixaban but not abnormal thromboelastometry parameters induced by rivaroxaban.55-63
In a meta-analysis, PCC was associated with a 1.4% risk of thromboembolic events when administered to VKA-treated patients with bleeding complications.64
Activated prothrombin complex concentrate.
Activated PCC (aPCC; factor 8 inhibitor bypassing activity; Baxter Bioscience, Vienna, Austria) contains factors II, VII, IX, and X, which are activated during the manufacturing process. It was developed as a prohemostatic agent to control bleeding in hemophiliacs with inhibitors to factors VIII or IX.65 Clinical data regarding the efficacy of aPCC in the setting of TSOAC-associated bleeding are lacking. aPCC corrected the anticoagulant effect of high-dose rivaroxaban in animal models.66,67 In vitro data suggest that aPCC corrects some abnormal clot-based coagulation tests, thromboelastometry parameters, and thrombin generation indices induced by dabigatran, rivaroxaban, and apixaban.55,58,61-63,68-70 A case report describes aPCC use in a dabigatran-treated patient with iatrogenic hemopericardium.71
Pharmacovigilance data in hemophilia patients demonstrate a low risk of thrombosis with aPCC use (4-8 events/105 infusions).72,73 The majority of these events (81%) occurred in patients with thrombotic risk factors. However, the likelihood that aPCC will cause thrombosis in a person with anticoagulant-related bleeding (who by definition has an enhanced the risk of thrombosis) is not known.
Recombinant factor VIIa.
Recombinant factor VIIa (rfVIIa) was also developed for the treatment of bleeding episodes in hemophilia patients with inhibitors. There are no clinical studies evaluating its efficacy for TSOAC-associated bleeding. In animal models, rfVIIa failed to ameliorate bleeding following treatment with dabigatran or rivaroxaban.51,52,54,74 In vitro studies suggest a variable effect on rivaroxaban- and apixaban-induced abnormalities in clot-based coagulation tests, thrombin generation indices, and thromboelastometry.54,55,61-63
Off-label use of rfVIIa is associated with an increased risk of arterial thromboembolic events compared with placebo (5.5% vs 3.2%, relative risk 1.68, 95% CI 1.20-2.36).75
Plasma.
To our knowledge, there are no clinical studies evaluating the efficacy and safety of plasma in the setting of TSOAC-associated bleeding. Although plasma may have contributed to resolution of gastrointestinal hemorrhage in a single patient treated with dabigatran, other reports fail to demonstrate its utility in this setting.76-78
Specific antidotes in development
Humanized monoclonal antibody fragment against dabigatran.
Early clinical development of a humanized monoclonal antibody fragment against dabigatran (aDabi-Fab) is currently underway. In animal models, aDabi-Fab reversed prolonged clot-based coagulation tests and reduced rat tail bleeding following administration of dabigatran.81-84
Inactive factor Xa derivatives.
Recombinant (andexanet α) and plasma-derived factor Xa derivatives lacking catalytic and membrane binding activity are currently under development as specific factor Xa antidotes. Following exposure to factor Xa inhibitors, andexanet α dose-dependently reversed factor Xa inhibition, corrected abnormal clot-based coagulation tests in vitro, and restored hemostasis in a rabbit model of bleeding.85 Preliminary results of a phase 2, double-blind, placebo-controlled trial showed that a bolus dose of andexanet α antagonized the anti-Xa activity of apixaban (5 mg twice daily, 11 doses) in healthy volunteers.86
Extracorporeal therapies
Dabigatran can be removed by hemodialysis, with 49% to 68% of active dabigatran removed after 4 hours of hemodialysis in patients with end-stage renal disease.87,88 Duration of dialysis appears to have the greatest impact on dabigatran plasma concentration.89 In a recent case series, plasma dabigatran levels were reduced by 52% to 77% in 5 dabigatran-treated patients with life-threatening bleeding following treatment with hemodialysis. Three of the 5 patients experienced rebound increases in dabigatran concentration following hemodialysis, likely related to drug redistribution from the extravascular compartment.
Rivaroxaban and apixaban are highly protein bound, which limits the degree to which they can be removed by dialysis.12,13,90 Charcoal hemoperfusion removes highly protein bound drugs, but, to our knowledge, there are no studies assessing the removal of rivaroxaban or apixaban with hemodialysis or charcoal hemoperfusion.
Adjunctive hemostatic therapies
Desmopressin
There are no studies evaluating the efficacy and safety of desmopressin (1-desamino-8-D-arginine vasopressin) in the context of TSOAC-associated bleeding. Desmopressin is traditionally used for patients with severe bleeding attributable to platelet dysfunction (eg, uremia) or type I von Willebrand disease. Because desmopressin promotes release of von Willebrand factor from endothelial cells, it is biologically plausible (although unproven) that desmopressin could be of benefit to a patient with TSOAC-associated bleeding. If repeated doses are given, close monitoring of serum electrolytes is required due to the potential for hyponatremia. In a meta-analysis of perioperative desmopressin, there was no increased risk of thromboembolic events compared with placebo.91 However, the prothrombotic potential of desmopressin in patients with increased thromboembolic risk, such as those receiving TSOAC therapy, is unclear.
Antifibrinolytic agents
Tranexamic acid and ε-aminocaproic acid stabilize fibrin clots by interfering with fibrinolysis and can also be used as adjunctive therapies for severe bleeding in a variety of circumstances. Their hemostatic efficacy in the setting of TSOAC-associated bleeding is unknown. There was no increased risk of thrombosis (myocardial infarction, stroke, deep vein thrombosis, pulmonary embolism) in a recent Cochrane review that evaluated tranexamic acid and ε-aminocaproic acid as a way to reduce perioperative blood loss.92 Another systematic review showed that tranexamic acid reduced perioperative blood loss in patients undergoing hip or knee replacement surgery with no increased risk of VTE.93
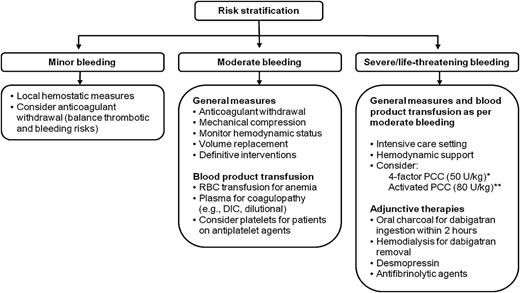
Suggested management strategy for TSOAC-associated bleeding
Patients presenting with TSOAC-associated bleeding should be risk-stratified based on hemodynamic stability, source of bleeding, and degree of blood loss. Supportive measures and urgent referral for definitive intervention remain the cornerstones of management given the lack of specific reversal agents and limited clinical outcome data for nonspecific therapies. Figure 1 shows a suggested algorithm for management. Attention should be paid to the time elapsed since last dose, renal function (particularly for dabigatran), and concomitant medications that may increase bleeding risk (eg, aspirin, clopidogrel) or interfere with TSOAC metabolism. Inhibitors of P-glycoprotein such as amiodarone, verapamil, ketoconazole, quinidine, clarithromycin, and grapefruit juice may increase plasma TSOAC concentrations.12,94 Coadministration of CYP3A4 enzyme inhibitors such as azole antimycotic agents and HIV protease inhibitors may increase concentrations of rivaroxaban and apixaban.90,95 In patients with normal renal function, the plasma concentration of any TSOAC will decline rapidly, usually reaching a nadir 12 to 24 hours after the last dose is taken. The patient’s indication for anticoagulant treatment and baseline thromboembolic risk are important considerations when assessing and communicating the relative benefits and risks of management strategies because these strategies are not supported by high-quality evidence and may be associated with an increased risk of thrombosis (eg, PCC, aPCC, and rVIIa).
Suggested strategy for management of TSOAC-associated bleeding. Adapted from previously published review article.34 *Preferred agent for rivaroxaban/apixaban. Possibility of benefit must be balanced against known risk of thrombosis. **Preferred agent for dabigatran. Possibility of benefit must be balanced against known risk of thrombosis. DIC, disseminated intravascular coagulation; RBC, red blood cell.
Suggested strategy for management of TSOAC-associated bleeding. Adapted from previously published review article.34 *Preferred agent for rivaroxaban/apixaban. Possibility of benefit must be balanced against known risk of thrombosis. **Preferred agent for dabigatran. Possibility of benefit must be balanced against known risk of thrombosis. DIC, disseminated intravascular coagulation; RBC, red blood cell.
Minor bleeding
Minor bleeding (eg, epistaxis, ecchymosis, and menorrhagia) can be managed conservatively with the application of local hemostatic measures. Brief drug discontinuation can be considered but should be balanced against the risk of thrombosis. Both their short half-life and rapid onset of anticoagulant effect may influence the decision to interrupt TSOACs. In particular, extended interruptions appropriate for warfarin, with its long elimination and reinitiation half-life, may be inappropriate for TSOACs.
Moderate bleeding
Anticoagulants should be discontinued in the event of moderate bleeding (eg, gastrointestinal bleeding). The duration of discontinuation depends on the patient’s clinical status and feasibility of local or mechanical hemostatic interventions. In patients at high thromboembolic risk, a low-dose parenteral anticoagulant can be considered during the period of TSOAC discontinuation. Transfusion support with red blood cells and plasma should be provided for patients with actual or expected severe anemia and coagulopathy (eg, disseminated intravascular coagulation and dilutional coagulopathy), respectively. Platelet transfusion may be considered for patients receiving concomitant antiplatelet therapies.
Severe/life-threatening bleeding
Severe/life-threatening bleeding should be managed with life-supporting therapies and early referral for procedural or surgical intervention. In addition to the maximum supportive measures described above, nonspecific reversal therapies may be considered based on available, but limited, evidence. aPCC (80 U/kg) is weakly preferred over PCC for dabigatran-associated bleeding, whereas 4-factor PCC (50 U/kg) is weakly preferred over aPCC for rivaroxaban- or apixaban-associated bleeding; however, use of either agent is based on poor-quality data and as such should not be considered a requirement in the setting of severe or life-threatening bleeding. Hemodialysis may be a useful adjunctive therapy for dabigatran removal, when feasible. Antifibrinolytic therapies and desmopressin may also be added.
Summary
Bleeding in patients receiving TSOACs occurs with a frequency similar to VKAs. Most bleeding is minor or moderate and does not require specific interventions other than local measures and temporary drug interruption. Major or life-threatening bleeding may require proceduralist-led interventions, life-sustaining therapies, and nonspecific procoagulant medications. Specific antidotes are under development but are not yet available. Transfusion therapy may ameliorate bleeding, but supporting evidence is poor. Management by experts with experience with TSOACs is recommended where possible.
Acknowledgment
M.A.C. holds a Career Investigator award from the Heart and Stroke Foundation of Canada and the Leo Pharma Chair in Thromboembolism research.
Authorship
Contribution: D.M.S., D.A.G., and M.A.C. conceived this article; D.M.S. performed the literature review and wrote the first draft; D.A.G. and M.A.C. reviewed and edited the final paper; and D.M.S., D.A.G., and M.A.C. provided sign off on the submitted version.
Conflict-of-interest disclosure: M.A.C. has sat on advisory boards for Leo Pharma, Pfizer, Bayer, Boehringer Ingelheim, Alexion, CSL Behring, Portola, Viropharm, and AKP America. M.A.C. has prepared educational materials for Pfizer, Octapharm, and CSL Behring. M.A.C. has provided expert testimony for Bayer and for Merck. M.A.C.’s institution has received funding for research projects from Boehringer Ingelheim, Octapharm, Pfizer, and Leo Pharma. M.A.C. has received funding for presentations from Leo Pharma, Bayer, Celgene, and CSL Behring. In the last 2 years, D.A.G. has chaired or served on advisory boards for CSL Behring, Janssen, Roche, Bristol Meyers Squibb, and Daiichi Sankyo. The remaining author declares no competing financial interests.
Correspondence: Deborah M. Siegal, c/o Mark Crowther, Rm L208, 50 Charlton Ave East, Hamilton, ON, Canada L8N 4A6; e-mail: deborah.siegal@medportal.ca.