Key Points
Bendamustine-bortezomib-dexamethasone is active and well tolerated in relapsed/refractory myeloma.
Bendamustine with bortezomib and dexamethasone was evaluated in 79 patients with relapsed/refractory multiple myeloma. Median age was 64 years, and patients had a median of 2 prior treatment lines (range, 1 to 6 lines). Bendamustine 70 mg/m2 days 1 and 4; bortezomib 1.3 mg/m2 intravenously days 1, 4, 8, and 11; and dexamethasone 20 mg days 1, 4, 8, and 11 once every 28 days was given for up to 8 cycles. Primary end point was overall response rate (ORR). Secondary end points were progression-free survival (PFS), overall survival, time to response, and toxicity. ORR was 60.8%, and when minor responses were included, 75.9%. Median time to response was 31 days. ORR rate was similar in patients previously exposed to bortezomib, lenalidomide, and bortezomib plus lenalidomide. PFS was 9.7 and OS was 25.6 months. Multivariate analysis showed high lactate dehydrogenase, ≥3 prior treatment lines, and low platelet counts correlating with short survival. Grade 3/4 thrombocytopenia was noted in 38%, and grade 3/4/5 infections were noted in 23%. Grade ≤2 polyneuropathy increased from 19% at baseline to 52% at cycle 8 and grade 4, from 0% to 7%. Bendamustine-bortezomib-dexamethasone is active and well tolerated in patients with relapsed/refractory myeloma. This trial was registered in the EudraCT database as No. 2008-006421-13.
Introduction
The introduction of thalidomide, bortezomib, and lenalidomide has markedly improved the outcome of patients with multiple myeloma. Still, the great majority of patients relapse after first-line therapy and need effective second-line and further therapies. Treatment selection for relapsed/refractory patients depends on various disease- and patient-related factors, on the results and tolerance of previous treatments, and on the availability of alternative treatment regimens. Bortezomib retreatment is an option for patients previously effectively treated with this drug yielding response rates of 40%,1 but combinations with other drugs, particularly those not used before, may improve outcome.
Bendamustine is an attractive combination partner for bortezomib because of its specific mode of activity and favorable toxicity profile. Bendamustine is a bifunctional mechlorethamine derivative with alkylator and antimetabolite activities.2 It lacks cross-reactivity with many other cancer drugs, inhibits mitotic checkpoints, deregulates DNA repair genes, activates proapoptotic genes, exerts activity in p53-deficient cell lines resistant to standard therapy and, compared with other frequently used alkylating agents, induces more DNA double-strand breaks when administered at equitoxic doses3,-5 resulting in no cross-resistance with melphalan and several other cytotoxic drugs.6 Accordingly, bendamustine overcomes resistance to melphalan7 and also to dexamethasone8 in myeloma cell lines.
Clinically, bendamustine has already been tested as single-agent therapy and in combination with bortezomib or immunomudulatory drugs and usually with corticosteroids, mostly in smaller series of patients with relapsed/refractory myeloma. On the basis of a study conducted several years ago, bendamustine-prednisone was approved for first-line therapy in Europe.9 Here, we aimed to evaluate the efficacy and tolerance of bendamustine-bortezomib-dexamethasone (BBD) in a phase 2 study in patients with relapsed/refractory multiple myeloma. In addition, we wanted to explore the activity of this regimen in patients pretreated with bortezomib or lenalidomide or with both drugs.
Patients and methods
Eighty patients with relapsed/refractory multiple myeloma were screened, and 79 were enrolled and constitute the intent-to-treat (ITT) population. Median age was 64 years (range, 40 to 86 years). Twenty-seven patients had International Staging System (ISS) stage I multiple myeloma, 31 stage II, and 21 stage III. Median number of prior treatment lines was 2 with 1 to 2 prior lines in 50, 3 to 4 in 23, and 5 to 6 in 6 patients. Further details are shown in Table 1. The treatment regimen consisted of bendamustine 70 mg/m2 days 1 and 4; bortezomib 1.3 mg/m2 intravenously days 1, 4, 8, and 11; and dexamethasone 20 mg days 1, 4, 8, and 11. Cycles were repeated every 4 weeks to a maximum of 8 cycles. In case of no response, treatment was discontinued at cycle 4. Major enrollment criteria included relapsed/refractory myeloma after autologous stem cell transplantation or after conventional therapy, life expectancy of ≥3 months, 1 or more prior treatment lines, Eastern Cooperative Oncology Group performance status ≤2, absolute neutrophil count ≥1.5 × 109/L, and platelets ≥75 × 109/L. The primary end point was response rate (RR), and secondary end points were progression-free survival (PFS), overall survival (OS), time to response, and toxicity. Fluorescent in situ hybridization (FISH) analysis was performed on isolated mononuclear cells stained with clonal-specific anti–light chain sera.
Patient characteristics
| Characteristic . | No. . | % . |
|---|---|---|
| No. of patients | 79 | |
| Age, y | ||
| Median | 64 | |
| Range | 40-86 | |
| Gender | ||
| Male | 37 | |
| Female | 42 | |
| ISS stage | ||
| I | 27 | 34.2 |
| II | 31 | 39.2 |
| III | 21 | 26.6 |
| ECOG performance status | ||
| 0-1 | 75 | |
| ≥2 | 4 | |
| β2 microglobulin, mg/L | ||
| Median | 3.8 | |
| Range | 1.2-36.7 | |
| IgG | 36 | 45.6 |
| IgA | 16 | 32.9 |
| Light chain myeloma | 18 | 22.8 |
| Oligosecretory myeloma | 9 | 11.4 |
| FISH-defined cytogenetic risk groups | ||
| High risk: t(4;14), t(14;16), del17p, 1q21 | 30 | 43.5* |
| Standard risk: all others | 39 | 56.5* |
| Prior treatment lines | ||
| Median | 2 | |
| Range | 1-6 | |
| Prior exposure to bortezomib | 50 | 63.3 |
| Prior exposure to lenalidomide | 42 | 53.2 |
| Prior exposure to both bortezomib and lenalidomide | 29 | 36.7 |
| Characteristic . | No. . | % . |
|---|---|---|
| No. of patients | 79 | |
| Age, y | ||
| Median | 64 | |
| Range | 40-86 | |
| Gender | ||
| Male | 37 | |
| Female | 42 | |
| ISS stage | ||
| I | 27 | 34.2 |
| II | 31 | 39.2 |
| III | 21 | 26.6 |
| ECOG performance status | ||
| 0-1 | 75 | |
| ≥2 | 4 | |
| β2 microglobulin, mg/L | ||
| Median | 3.8 | |
| Range | 1.2-36.7 | |
| IgG | 36 | 45.6 |
| IgA | 16 | 32.9 |
| Light chain myeloma | 18 | 22.8 |
| Oligosecretory myeloma | 9 | 11.4 |
| FISH-defined cytogenetic risk groups | ||
| High risk: t(4;14), t(14;16), del17p, 1q21 | 30 | 43.5* |
| Standard risk: all others | 39 | 56.5* |
| Prior treatment lines | ||
| Median | 2 | |
| Range | 1-6 | |
| Prior exposure to bortezomib | 50 | 63.3 |
| Prior exposure to lenalidomide | 42 | 53.2 |
| Prior exposure to both bortezomib and lenalidomide | 29 | 36.7 |
ECOG, Eastern Cooperative Oncology Group; IgG, immunoglobulin G.
Cytogenetic results available from 60 patients: t (4;14), 10; t(14;16), 1; del17p, 9; 1q21, 22 (20 patients had ≥2 aberrations).
Myeloma response was assessed according to the International Uniform Response Criteria for Multiple Myeloma10 with the addition of minor response (MR)11 and near complete response (nCR) (CR without confirmation by immunofixation or with positive immunofixation).
Patients were enrolled by 9 participating centers in Austria and the Czech Republic from July 2010 to September 2012 and were observed until April 2013. All patients gave written informed consent before entering the study, which was conducted in accordance with the Declaration of Helsinki. The study was approved by the ethical committees responsible for all participating study centers.
For sample size calculation, a true RR of 60% was considered to be promising enough for this regimen to be further explored (eg, in a phase 3 trial) with a probability of 5% for type I and 20% for type II error (corresponding to a power of 80%). Accordingly, 70 patients evaluable for response had to be recruited. Because it was estimated that 20% of patients would drop out before completion of the second cycle (nonevaluable patients with respect to the per-protocol population), a sample size of a total of 85 recruited patients was calculated to allow for 70 evaluable patients in the per-protocol population.
Response rates, toxicities, and survival have been calculated by ITT analysis. OS, time to disease progression, and time to response were estimated by the product limit method.12 Univariate comparisons of these end points were performed by using the log-rank test.13 The Cox proportional hazards model14 was applied for multivariate analyses of time-to-event type data, which included all univariate characteristics correlated at P < .3. For all analyses, the significance end point was set to 0.05, and all values reported are two-sided. SPSS, version 17, was used for all analyses. All analyses are descriptive and exploratory, and no adjustments for multiplicity were made.
The following risk factors were tested for possible correlations with response, PFS, and OS in univariate analysis: age >65 years, ISS stage III, albumin <35g/L, hemoglobin <9.0 g/dL, lactate dehydrogenase (LDH) >250 U/L, glomerular filtration rate <50 mL/min, time from diagnosis >46 months, number of previous treatment lines ≥3, baseline platelets <150 000/μL, baseline neutrophils <2800/μL, relapsing or refractory to the treatment line prior to start of the BBD regimen, and pretreatment with bortezomib, lenalidomide, or both.
Adverse events were graded by National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
All patients signed a written informed consent before inclusion. The trial is registered in the EudraCT database (No. 2008-006421-13), was approved by the health authorities and ethical committees in all participating countries, and was conducted in accordance with the 1964 Declaration of Helsinki and the Guidelines for Good Clinical Practice.
Results
Patient characteristics are shown in Table 1. Of the 79 patients enrolled, 71 had completed ≥2 cycles (per-protocol population). Median time of follow-up was 13.7 months, and median number of cycles administered was 6, in both the ITT and the per-protocol population. Sixty-three percent and 53% of patients were previously exposed to bortezomib or lenalidomide, respectively, and 37% to both drugs. CR, very good partial response (VGPR/near CR), and partial response (PR) were noted in 12 (15.2%), 16 (20.3%), and 20 patients (25.3%), respectively, yielding an overall response rate (ORR) of 60.8%. MR was observed in 12 patients (15.2%). If MR was added to PR or better, the ORR was 75.9%. Median time to response (PR or better) was 31 days, and median time to best response was 111 days. In the 71 patients evaluable per protocol, an ORR of 67.6% (84.5% when MR was included) was noted.
Table 2 shows RRs of different risk groups of the ITT population. ORR in patients with adverse cytogenetic features was 53% and 67% without (P = .323). The ORR was slightly but not significantly lower in patients previously exposed to bortezomib compared with bortezomib-naive patients (56% vs 69%; P = .340) and in those pretreated with lenalidomide compared with lenalidomide-naive patients (55.0% vs 67.6%; P = .260). Similar findings were observed in patients previously exposed to both bortezomib and lenalidomide compared with patients pretreated with neither of these novel drugs (46.9% vs 63.2%; P = .385) and in those who had 3 or more previous treatment lines or those who had fewer than 3 previous treatment lines (51.7% vs 66.0%; P = .239). Eight of the 50 patients with prior bortezomib and 9 of the 42 patients with prior lenalidomide treatment had 2 or 3 lines of previous bortezomib- or lenalidomide-based therapies. In bortezomib-pretreated patients, ORR rates were similar, independent of the number of pretreatments, while in patients with more than 1 prior lenalidomide treatment line, a slightly lower ORR rate was noted. Rates of VGPR or better tended to be lower in patients pretreated with novel drugs, particularly in those who had ≥2 prior lines of lenalidomide therapy. VGPR rates in patients pretreated with both lenalidomide and bortezomib were 18.8% compared with 47.4% in those without prior exposure to both drugs (P = .054). RRs were slightly lower, albeit not statistically significant, in patients refractory to the last treatment line before start of the BBD regimen compared with those who had relapsing disease.
ORR, PFS, and OS in the entire patient cohort in patients with different characteristics
| Patients . | No. of patients . | ≥VGPR (%)* . | ORR (%)* . | Median PFS (mo) . | Median OS (mo) . |
|---|---|---|---|---|---|
| All patients | 79 | 35.4 | 60.8 | 9.7 | 25.6 |
| FISH high-risk | 30 | 36.7 | 53.0 | 9.3 | 22.8 |
| FISH standard-risk | 39 | 35.9 | 67.0 | 11.1 | N/R |
| Pretreatment with bortezomib | 50 | 28.0 | 56.0 | 8.6 | 25.6 |
| None | 29 | 48.3 | 69.0 | 12.4 | N/R |
| 1 line | 42 | 28.6 | 54.8 | 8.6 | 25.6 |
| 2-3 lines | 8 | 25.0 | 62.5 | 4.4 | 9.8 |
| Pretreatment with lenalidomide | 42 | 26.2 | 55.0 | 8.3 | 20.6 |
| None | 37 | 45.9 | 67.6 | 12.3 | N/R |
| 1 line | 33 | 33.3 | 57.6 | 8.5 | 21.6 |
| 2-3 lines | 9 | 0.0 | 44.4 | 5.4 | 13.5 |
| Pretreatment with lenalidomide and bortezomib | 32 | 18.8 | 46.9 | 7.1 | 17.4 |
| Pretreatment with neither lenalidomide nor bortezomib | 19 | 47.4 | 63.2 | 12.0 | N/R |
| No. of prior treatment lines | |||||
| 1-2 | 50 | 42.0 | 66.0 | 12.0 | N/R |
| 3-6 | 29 | 24.1 | 51.7 | 7.8 | 19.9 |
| Relapse status at start of BBD | |||||
| Relapsing to the preceding treatment line* | 58 | 36.2 | 63.8 | 11.1 | 27.3 |
| Refractory to the preceding treatment line* | 21 | 28.6 | 52.4 | 8.0 | 23.7 |
| Patients . | No. of patients . | ≥VGPR (%)* . | ORR (%)* . | Median PFS (mo) . | Median OS (mo) . |
|---|---|---|---|---|---|
| All patients | 79 | 35.4 | 60.8 | 9.7 | 25.6 |
| FISH high-risk | 30 | 36.7 | 53.0 | 9.3 | 22.8 |
| FISH standard-risk | 39 | 35.9 | 67.0 | 11.1 | N/R |
| Pretreatment with bortezomib | 50 | 28.0 | 56.0 | 8.6 | 25.6 |
| None | 29 | 48.3 | 69.0 | 12.4 | N/R |
| 1 line | 42 | 28.6 | 54.8 | 8.6 | 25.6 |
| 2-3 lines | 8 | 25.0 | 62.5 | 4.4 | 9.8 |
| Pretreatment with lenalidomide | 42 | 26.2 | 55.0 | 8.3 | 20.6 |
| None | 37 | 45.9 | 67.6 | 12.3 | N/R |
| 1 line | 33 | 33.3 | 57.6 | 8.5 | 21.6 |
| 2-3 lines | 9 | 0.0 | 44.4 | 5.4 | 13.5 |
| Pretreatment with lenalidomide and bortezomib | 32 | 18.8 | 46.9 | 7.1 | 17.4 |
| Pretreatment with neither lenalidomide nor bortezomib | 19 | 47.4 | 63.2 | 12.0 | N/R |
| No. of prior treatment lines | |||||
| 1-2 | 50 | 42.0 | 66.0 | 12.0 | N/R |
| 3-6 | 29 | 24.1 | 51.7 | 7.8 | 19.9 |
| Relapse status at start of BBD | |||||
| Relapsing to the preceding treatment line* | 58 | 36.2 | 63.8 | 11.1 | 27.3 |
| Refractory to the preceding treatment line* | 21 | 28.6 | 52.4 | 8.0 | 23.7 |
N/R, not reached.
Last line before start of BBD.
Differences between groups not significant.
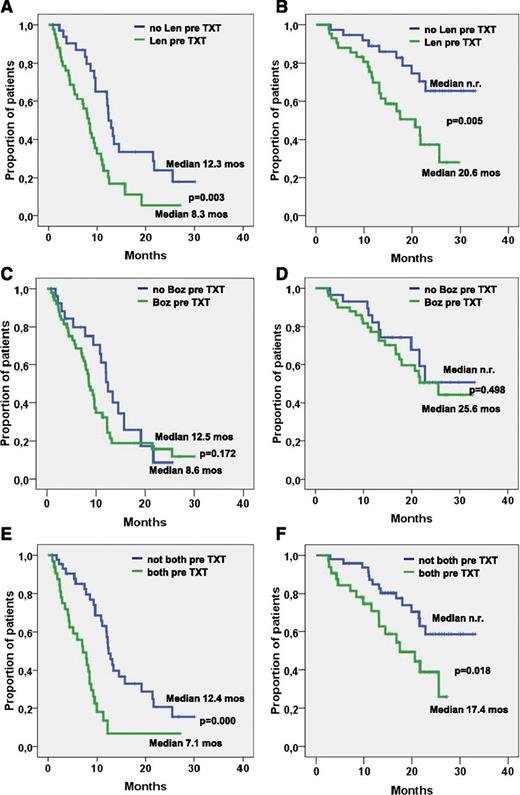
PFS was 9.7 months and did not differ significantly between patients with and without adverse cytogenetic features (9.3 vs 11.1 months; P = .567; Figure 1B), with stage I to II and stage III disease (10 vs 9.3 months: P = .792; Figure 1C), time from first-line treatment shorter or longer than 46 months (10.8 vs 8.9 months; P = .841), no pretreatment or pretreatment with bortezomib (12.5 vs 8.6 months; P = .172, Figure 2C). PFS was significantly longer in patients who had 1 to 2 compared with those who had 3 to 6 prior treatment lines (12.0 vs 7.8 months; P = .012; Figure 1E), in those not pretreated with lenalidomide (12.2 vs 8.3 months; P = .003; Figure 2A), and in those not pretreated with both bortezomib and lenalidomide (12.4 vs 7.1 months; P = .000; Figure 2E), while the difference did not reach statistical significance in those relapsing to the last line before start of BBD therapy compared with patients who were refractory to this treatment line (11.1 vs 8.0 months; P = .251).
PFS and OS in different patient cohorts. PFS and OS in (A) all patients and (B) patients with standard-risk and high-risk cytogenetics. (C) PFS and (D) OS in patients with International Staging System (ISS) stage I-II and stage III disease. (E) PFS and (F) OS in patients with fewer than 3 or more than 3 prior treatment lines. n.r., not reached; pre-TXT, pretreatment.
PFS and OS in different patient cohorts. PFS and OS in (A) all patients and (B) patients with standard-risk and high-risk cytogenetics. (C) PFS and (D) OS in patients with International Staging System (ISS) stage I-II and stage III disease. (E) PFS and (F) OS in patients with fewer than 3 or more than 3 prior treatment lines. n.r., not reached; pre-TXT, pretreatment.
PFS and OS related to pretreatment. (A) PFS and (B) OS in patients with and without lenalidomide (Len) pretreatment. (C) PFS and (D) OS in patients with and without bortezomib (Boz) pretreatment. (E) PFS and (F) OS in patients with or without pretreatment with both lenalidomide and bortezomib.
PFS and OS related to pretreatment. (A) PFS and (B) OS in patients with and without lenalidomide (Len) pretreatment. (C) PFS and (D) OS in patients with and without bortezomib (Boz) pretreatment. (E) PFS and (F) OS in patients with or without pretreatment with both lenalidomide and bortezomib.
The median OS was 25.6 months, and survival did not differ significantly in patients with and without adverse cytogenetic features (22.8 months vs median not reached; P = .261; Figure 1B), in those with time from first-line treatment shorter or longer than 46 months (median not reached vs 21.6 months; P = .184), and in those with or without pretreatment with bortezomib (25.6 months vs median not reached; P = .498; Figure 2D). OS was significantly longer in patients with stage I/II compared with those with stage III disease (median not reached vs 21.6 months; P = .030; Figure 1D) in patients with 1 to 2 compared with patients with 3 to 6 prior treatment lines (median not reached vs 19.9 months; P = .001; Figure 1F), in those not pretreated with lenalidomide (median not reached vs 20.6 months; P = .005; Figure 2B), and in those not pretreated with both bortezomib and lenalidomide (median not reached vs 17.4 months; P = .018; Figure 2F). Survival was slightly longer without reaching levels of significance (median 27.3 vs 23.7 months; P = .146) in patients relapsing after the last treatment line before the BBD regimen compared with those who were refractory to the treatment line before starting the BBD regimen. Univariate analysis of possible correlations between the prognostic factors cited in “Patients and methods” and ORR revealed only low baseline platelet counts and pretreatment with both bortezomib and lenalidomide to be significantly associated with a low RR. Short PFS was found to be significantly associated with a low baseline platelet count, 3 or more previous treatment lines, pretreatment with lenalidomide, and particularly treatment with both lenalidomide and bortezomib (Table 3). Significant correlations with OS were noted for ISS stage III, low hemoglobin and platelet count levels, pretreatment with lenalidomide, pretreatment with 3 or more previous treatment lines, and particularly pretreatment with both lenalidomide and bortezomib (Table 3).
Results of univariate and multivariate Cox regression analysis of possible risk factors and treatment objectives (only significant results shown)
| Parameter . | Response . | PFS . | OS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Univariate analysis | |||||||||
| ISS stage III | — | — | — | — | 2.379 | 1.182-4.788 | .015 | ||
| Platelets <150 000/μL | 0.310 | 0.105-0.921 | .035 | 1.971 | 1.066-3.644 | .030 | 2.288 | 1.079-4.849 | .031 |
| Hemoglobin <9 g/dL | — | — | 1.946 | 0.941-4.024 | .072 | 2.656 | 1.143-6.168 | .023 | |
| LDH ≥250 U/L | — | — | — | — | 0.521 | 1.127-5.637 | .024 | ||
| Lenalidomide pretreatment | — | — | 2.292 | 1.308-4.016 | .004 | 2.794 | 1.315-5.939 | .008 | |
| Bortezomib and lenalidomide pretreatment | 0.374 | 0.147-0.953 | .039 | 3.151 | 1.783-5.568 | .000 | 2.268 | 1.130-4.554 | .021 |
| >2 previous treatment lines | — | — | 1.978 | 1.153-3.394 | .013 | 3.261 | 1.607-6.620 | .001 | |
| Multivariate analysis* | |||||||||
| Platelets <150 000/μL | 1.930 | 1.036-3.596 | .038 | 2.961 | 1.345-6.520 | .007 | |||
| LDH ≥250 U/L | — | — | — | — | 2.878 | 1.238-6.690 | .014 | ||
| Lenalidomide pretreatment | — | — | 2.393 | 1.361-4.206 | .002 | — | — | ||
| Previous stem cell transplantation | — | — | 1.787 | 1.033-3.092 | .038 | — | — | ||
| >2 previous treatment lines | — | — | — | — | 3.030 | 1.482-6.195 | .002 | ||
| Parameter . | Response . | PFS . | OS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Univariate analysis | |||||||||
| ISS stage III | — | — | — | — | 2.379 | 1.182-4.788 | .015 | ||
| Platelets <150 000/μL | 0.310 | 0.105-0.921 | .035 | 1.971 | 1.066-3.644 | .030 | 2.288 | 1.079-4.849 | .031 |
| Hemoglobin <9 g/dL | — | — | 1.946 | 0.941-4.024 | .072 | 2.656 | 1.143-6.168 | .023 | |
| LDH ≥250 U/L | — | — | — | — | 0.521 | 1.127-5.637 | .024 | ||
| Lenalidomide pretreatment | — | — | 2.292 | 1.308-4.016 | .004 | 2.794 | 1.315-5.939 | .008 | |
| Bortezomib and lenalidomide pretreatment | 0.374 | 0.147-0.953 | .039 | 3.151 | 1.783-5.568 | .000 | 2.268 | 1.130-4.554 | .021 |
| >2 previous treatment lines | — | — | 1.978 | 1.153-3.394 | .013 | 3.261 | 1.607-6.620 | .001 | |
| Multivariate analysis* | |||||||||
| Platelets <150 000/μL | 1.930 | 1.036-3.596 | .038 | 2.961 | 1.345-6.520 | .007 | |||
| LDH ≥250 U/L | — | — | — | — | 2.878 | 1.238-6.690 | .014 | ||
| Lenalidomide pretreatment | — | — | 2.393 | 1.361-4.206 | .002 | — | — | ||
| Previous stem cell transplantation | — | — | 1.787 | 1.033-3.092 | .038 | — | — | ||
| >2 previous treatment lines | — | — | — | — | 3.030 | 1.482-6.195 | .002 | ||
HR, hazard ratio; OR, odds ratio.
Cutoff P < 0.3.
In multivariate stepwise Cox regression analysis, prior exposure to lenalidomide, low baseline platelet counts, and prior stem cell transplantation were found to correlate with shorter PFS, while higher baseline LDH, 3 or more prior treatment lines, and low platelet counts were shown to correlate with shorter survival (Table 3). Hence, low platelet counts predict for shorter PFS and OS as well, while the other factors independently predicting for shorter OS were aggressive disease as indicated by high LDH and intensive pretreatment.
Grade 3 and 4 anemia and leukopenia were seen in 18.7% of patients, while grade 3 and 4 thrombocytopenia were noted in 32% and 6%, respectively (Table 4). Grade 3 and 4 infections were observed in 20% of patients, and 2 patients (3%) died as a result of infection, 1 as a result of severe pneumonia and 1 as a result of sepsis. Herpes zoster reactivation occurred in 6 patients, in 3 during and in 3 without ongoing antiviral prophylaxis. Mild grade 1/2 polyneuropathy was documented in 39 patients (49%), grade 3 in 4 patients (5%), and grade 4 in 1 patient (1%). The frequency of neuropathy increased with increasing duration of therapy from 19% grade 2 polyneuropathy at baseline to 52% at cycle 8. Grade 3 and 4 polyneuropathy were not observed at baseline but were noted in 7% of patients at the last treatment cycle.
Hematologic and nonhematologic toxicity
| Toxicity . | Grade . | |||||
|---|---|---|---|---|---|---|
| 1-2 . | 3 . | 4 . | ||||
| No. . | % . | No. . | % . | No. . | % . | |
| Hematologic | ||||||
| Anemia | 12 | 15 | 2 | 3 | ||
| Leucopenia | 13 | 16 | 1 | 1 | ||
| Thrombocytopenia | 25 | 32 | 5 | 6 | ||
| Nonhematologic | ||||||
| Infection/sepsis | 34 | 43 | 13 | 16 | 3/2* | 4/3* |
| Herpes zoster | 3 | 4 | 3 | 4 | — | |
| Diarrhea | 17 | 22 | 6 | 8 | — | |
| Constipation | 21 | 27 | 3 | 4 | — | |
| Nausea, emesis | 26 | 33 | 1 | 1 | — | |
| Polyneuropathy | 39 | 49 | 4 | 5 | 1 | 1 |
| Exanthema | 6 | 8 | — | 1 | 1 | |
| Insomnia/fatigue | 32 | 40 | 2 | 3 | — | |
| Toxicity . | Grade . | |||||
|---|---|---|---|---|---|---|
| 1-2 . | 3 . | 4 . | ||||
| No. . | % . | No. . | % . | No. . | % . | |
| Hematologic | ||||||
| Anemia | 12 | 15 | 2 | 3 | ||
| Leucopenia | 13 | 16 | 1 | 1 | ||
| Thrombocytopenia | 25 | 32 | 5 | 6 | ||
| Nonhematologic | ||||||
| Infection/sepsis | 34 | 43 | 13 | 16 | 3/2* | 4/3* |
| Herpes zoster | 3 | 4 | 3 | 4 | — | |
| Diarrhea | 17 | 22 | 6 | 8 | — | |
| Constipation | 21 | 27 | 3 | 4 | — | |
| Nausea, emesis | 26 | 33 | 1 | 1 | — | |
| Polyneuropathy | 39 | 49 | 4 | 5 | 1 | 1 |
| Exanthema | 6 | 8 | — | 1 | 1 | |
| Insomnia/fatigue | 32 | 40 | 2 | 3 | — | |
Values for Grade 5.
Discussion
This prospective phase 2 trial with BBD showed a remarkable RR of 60.8% and, when MRs were included, in 75.9% of patients with relapsing/refractory disease. In the per-protocol analysis of the 71 patients who had a least 2 cycles of therapy, the respective values were 67.6%, and 84.5%. In patients pre-exposed to bortezomib, lenalidomide, or both drugs, a tendency for lower RRs was observed. RRs were also lower in patients exposed to ≥2 prior lines of lenalidomide-based therapy, and in those with refractory or relapsing disease during the last treatment line before start of the BBD regimen, while a PR or better was noted in 5 of the 8 patients who were pre-exposed to 2 or 3 lines of bortezomib. This indicates that the BBD regimen can result in meaningful responses in a remarkable proportion of patients, even after multiple retreatments and in those relapsing after or refractory to the previous treatment line.
The short time to response (31 days) and to best response (111 days) is clinically relevant, because rapid tumor control usually corresponds with fast improvement of clinical symptoms. Similar findings have also been reported in studies using bendamustine with lenalidomide and corticosteroids.15,16
Our results compare well with data from other phase 2 studies using bendamustine-bortezomib-corticosteroid combinations. Hrusovsky and Heidtmann17 reported in an abstract a RR of 72.5% and, when MRs were included, of 85% obtained in 40 patients with a median of 4 prior treatment lines. Pönisch et al18 studied a heterogeneous group of patients with good or restricted bone marrow function and observed an RR of 69%.
Recently, the Intergroupe Francophone du Myelome presented data obtained with BBD in elderly patients (median age, 75.8 years) progressing on or after 1 line of therapy without previous exposure to bortezomib.19 Responses were observed in 57.5% of patients after 4 cycles and after longer follow-up in 67.1% of patients. Apart from grade 3 and 4 hematologic toxicities, severe infections were noted in 7 of 73 patients. The RRs reported are comparable to those of our study, although we included younger but more heavily pretreated patients. Interim data from an Italian study20 using a similar regimen showed an RR of 77% in 30 patients who completed at least 1 cycle.
Bendamustine has been tested as single-agent treatment and in combination regimens with 1 or 2 additional drugs other than bortezomib. In a dose-escalation study starting at 60 mg/m2 of bendamustine on days 1 and 2, the maximum-tolerated dose (MTD) was 100 mg/m2 on days 1 and 2 per 28-day cycle. The ORR in the 31 patients treated with second-line therapy after relapsing from single or double autologous transplantation was 55%.21 Berenson et al22 conducted a phase 1/2 trial aiming to establish the MTD of bendamustine in combination with a fixed low dose of bortezomib (1 mg/m2) without corticosteroids in heavily pretreated patients. A dose of 90 mg/m2 bendamustine given on days 1 and 4 was defined as MTD, but the RR was only 33% and, when MRs were included, 48%. This indicates that bortezomib should be used in the conventional dose of 1.3 mg/m2 when combined with bendamustine and that corticosteroids should not be excluded from the regimen.22
Combinations of bendamustine with immunomodulatory drugs have also been evaluated. Thalidomide was combined with bendamustine and prednisone, and reported RRs ranged from 26% in a compassionate use program enrolling highly pretreated patients with a median of 5 prior lines of therapy23 to 85% in a trial with thalidomide dose escalation from 50 to 200 mg in patients substantially less exposed to previous therapy.24 In both reports, bendamustine was administered at a dose of 60 mg on days 1, 8, and 15, with the latter administration often withheld because of hematotoxicity. Because pharmacologic data show no accumulation of bendamustine in patients with renal failure,25 and bortezomib, likewise, has only little renal excretion,26 both drugs can be administered in patients with reduced glomerular filtration rate without dose reductions. Actually, a report on a small group of patients with stage 4 or 5 renal impairment showed no untoward toxicity and an RR of 55% when a single dose of 120 mg/m2 bendamustine was given in combination with daily 100 mg thalidomide and weekly dexamethasone for the first 3 weeks of a 4-week cycle.27
Bendamustine has also successfully been combined with lenalidomide and dexamethasone. Lentzsch et al15 established bendamustine 75 mg/m2 days 1 and 2, lenalidomide 10 mg days 1 to 21, and dexamethasone 40 mg once per week in patients with a median of 3 prior treatment lines as MTD, while in another trial, the MTD was not reached with bendamustine 75 mg/m2 on days 1 and 2 and lenalidomide 25 mg on days 1 to 21 in patients with a median of 2 prior treatment lines.16 Reported response rates without MR were 50% in the former trial and 75% in the latter trial. In both studies, several patients had prior exposure to lenalidomide.
In our study, PFS was 9.7 months. In multivariate analysis, prior lenalidomide exposure, prior stem cell transplantation, and low baseline platelet counts were established as factors correlating with PFS independently from each other, which accords with clinical experience. Low platelet count reflects poor bone marrow reserve and/or aggressive myeloma, and lenalidomide was used in patients in later treatment lines than other drugs. For OS, multivariate analysis revealed that higher baseline LDH, more than 2 prior treatment lines, and low platelet counts were associated with inferior outcome.
Prospective comparisons confirming the supposed additional value of adding bendamustine to bortezomib-dexamethasone are not currently available, but comparisons with 2-drug bortezomib-based treatments in the relapsed/refractory setting indicate superior activity of the 3-drug BBD regimen. RRs obtained with bortezomib with or without dexamethasone were 38% in the APEX trial28 and amounted to 44% in pretreated patients receiving bortezomib plus pegylated liposomal doxorubicin.29 A recent study showed synergistic activity between bendamustine and bortezomib,30 which may partly explain the higher RRs obtained in our patients with prior bortezomib exposure compared with those obtained in the Retrieve study, which showed a response rate of 40% with bortezomib retreatment after prior response to this drug.1 The activity of bortezomib in patients with high-risk cytogenetics and the finding that bendamustine overcomes the adverse effect of p53 deletions3 make both drugs ideal candidates for combined use in patients with FISH-defined high-risk cytogenetics. This notion is supported by our data showing almost similar RRs and comparable PFS and OS in patients with or without cytogenetically defined high-risk features, a finding which accords with results obtained with another bendamustine-based combination incorporating lenalidomide.15 The BBD regimen also resulted in similar PFS in patients with low (stage I/II) or advanced ISS stage. OS, however, was inferior in ISS stage III patients, indicating divergent efficacy of post relapse treatments in the differently characterized risk populations.
The BBD regimen was relatively well tolerated although grade 3/4 hematologic toxicities were seen in up to one third of patients. Severe infections, partly in the absence of neutropenia, have previously been observed by other groups.19 Grade 3/4/5 infections were noted in 23% of our patients, with 2 patients having a fatal outcome. Hence, granulocyte colony-stimulating factor prophylaxis should be considered, particularly in frail elderly patients. Herpes zoster prophylaxis is mandatory, but clinical virus reactivation may occur even during adequate prophylaxis.
Although few individual patients reported even better neurologic tolerance of bortezomib when it was given together with bendamustine than during their previous exposure to the proteasome inhibitor, the frequency of neuropathy increased with increasing duration of therapy from 19% grade 2 polyneuropathy at baseline to 52% at cycle 8. Grade 3/4 polyneuropathy was not recorded at baseline but was noted at a frequency of 7% in the last cycle. Two patients discontinued treatment because of this adverse event.
In summary, this study depicts the combination of BBD as effective treatment for patients with relapsed/refractory myeloma. The regimen induced responses in two thirds of patients and produced a PFS of 9.7 months and OS of 25.6 months in patients with a median of 2 prior treatment lines, frequently containing bortezomib and/or lenalidomide, and is active in patients with FISH-defined high-risk cytogenetics. The treatment- and disease-associated risk for infections mandates adequate supportive care management but, in general, the regimen was well tolerated.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Axel Hinke for statistical support and Raphaela Oswald for secretarial assistance in the preparation of the manuscript.
This work was supported by the Austrian Forum Against Cancer.
Authorship
Contribution: H.L., E.R., and N.Z. analyzed the data; and H.L., H.K., C.L., E.R., W.L., N.Z., R.G., A.S., L.P., A.W., and Z.A. had access to the primary clinical data, contributed to writing and/or commenting on the manuscript, and approved the manuscript.
Conflict-of-interest disclosure: H.L. received honoraria and research funding from Janssen-Cilag, Celgene, and Mundipharma; N.Z. received honoraria from Janssen-Cilag and Mundipharma; and R.G. received honoraria from Celgene, Mundipharma, and Merck. The remaining authors declare no competing financial interests.
Corresponding author: Heinz Ludwig, Department of Medicine I, Center for Oncology, Hematology and Palliative Care, Wilhelminenspital, Montleartstrasse 37, 1160 Vienna, Austria; e-mail: heinz.ludwig@wienkav.at.