Key Points
Nonanticoagulant heparin is shown to bind histones and provide cytoprotection in mouse models of sterile inflammation and sepsis.
Extracellular histones are considered to be major mediators of death in sepsis. Although sepsis is a condition that may benefit from low-dose heparin administration, medical doctors need to take into consideration the potential bleeding risk in sepsis patients who are already at increased risk of bleeding due to a consumption coagulopathy. Here, we show that mechanisms that are independent of the anticoagulant properties of heparin may contribute to the observed beneficial effects of heparin in the treatment of sepsis patients. We show that nonanticoagulant heparin, purified from clinical grade heparin, binds histones and prevents histone-mediated cytotoxicity in vitro and reduces mortality from sterile inflammation and sepsis in mouse models without increasing the risk of bleeding. Our results demonstrate that administration of nonanticoagulant heparin is a novel and promising approach that may be further developed to treat patients suffering from sepsis.
Introduction
Sepsis and septic shock are serious clinical problems with high mortality rates for which no adequate treatment currently exists.1 Neutrophils respond to infection with the formation of neutrophil extracellular traps (NETs),2,3 intricate networks containing DNA as the major structural component and proteins like histones and neutrophil elastase, which have antimicrobial properties. Extracellular histones, however, also exhibit cytotoxic activity toward host cells, including the endothelium.4,5 Histone release can thus trigger a feedback cascade, resulting in more cell death and additional release of histones.6 Consequently, extracellular histones are considered interesting therapeutic targets for sepsis treatment.4
Histones are positively charged, and NET-mediated cytotoxicity can be reduced with polysialic acid, a negatively charged polymer.5 We hypothesized that heparin, a negatively charged polysaccharide, blocks histone cytotoxicity and reduces mortality from sterile inflammation and sepsis. Low dose unfractionated heparin (UFH) has been tested in a clinical trial as a complementary treatment of sepsis.7 The study rationale linked infection, inflammation, and coagulation in sepsis and sought to inhibit the coagulation part with low doses of heparin so as not to increase the risk of bleeding in a patient who is already at risk due to sepsis-associated consumption coagulopathy.7,8 Nevertheless, although this study failed to demonstrate a significant benefit on 28-day mortality rate, we hypothesize that the minor beneficial effects of heparin observed might be attributed to a mechanism independent of the anticoagulant properties of heparin. We reasoned that removing the anticoagulant fraction from UFH would yield an antithrombin affinity-depleted heparin (AADH) that neutralizes histone-mediated cytotoxicity and effectively treats sepsis without increasing risk of bleeding. Heparins have the highest negative charge density of any known biological molecule9 and have a strong affinity for histones.10,11 It is, however, not known whether binding of heparin to histones also protects against the cytotoxic effect of histones on endothelial cells and/or in vivo. Here, we investigated the effects of UFH and AADH on histone cytotoxicity. We present data showing that complex formation of histones with heparin reduces their cytotoxicity in an in vitro cell-based cytotoxicity assay. Furthermore, by use of in vivo mouse models of sterile inflammation, a cecal ligation and puncture (CLP) model for sepsis and bacterial lipopolysaccharide (LPS) challenge, we show that AADH increases the survival rate in all 3 animal models.
Study design
AADH was produced by fractionation of UFH via affinity chromatography using an antithrombin-column, and its anticoagulant properties were quantitated via calibrated automated thrombography (CAT). The cytotoxic effects of purified histones, in the presence of UFH and AADH, were tested in vitro via a flow cytometry-based histone cytotoxicity assay employing EA.hy926 cells. Direct binding interaction between histones and AADH was determined using surface plasmon resonance and confocal laser scanning fluorescence microscopy. In vivo, the activities of UFH and AADH were tested in C57Bl6/J mice through a concanavalin A (ConA) challenge model for fatal sterile inflammation, through a CLP model and an LPS challenge model. Survival was used as the primary endpoint in these assays; liver viability assessment was performed to correlate disease severity to organ damage in the animals tested (CLP) and neutrophil lung infiltration, tissue leakage, and bronchoalveolar lavage cytokine levels were determined in the LPS model. Anticoagulant properties of administered UFH and AADH were assessed via CAT and tail bleeding time measurements. Detailed methods are given in the supplemental Data, available on the Blood Web site. The experiments were approved by the animal care committees of the Spanish National Research Council and of the Ludwig-Maximilians-University Munich.
Results and discussion
Antithrombin affinity chromatography yielded a fraction of UFH, AADH, with strongly decreased anticoagulant activity12 (Figure 1A) as determined by CAT, which shows that AADH has retained 0.2% to 0.5% of the anticoagulant activity of UFH. Both UFH and AADH inhibited dose-dependently the cytotoxic activity of purified histones in cell culture (Figure 1B-C). To analyze whether AADH binds to histones, direct binding of AADH to histones was verified by surface plasmon resonance (Figure 1D). Binding affinity between histone H3 and AADH was characterized by an apparent dissociation constant of 86 nM. We further demonstrated in vitro, employing isolated human neutrophils, that addition of AADH to neutrophils that have produced NETs5 (supplemental Figure 1A) results in colocalization of AADH with extracellular DNA fibers and histones (Figure 1E-F; supplemental Figure 1B-C). Thus, both UFH and AADH possess cytoprotective properties, conferred by complexation of histones, because we verified that heparin-histone complexes are devoid of cytotoxic properties (supplemental Figure 1D).
In vitro heparin characterization. (A) In vitro effects of varying amounts of UFH and AADH on thrombin generation in normal pooled mouse plasma as measured by CAT.12 Variable amounts of UFH (B) or AADH (C) and histone H3 (H3) were added to EA.hy926 cells and cell viability was quantitated with flow cytometry. Each bar represents the average ± SD of at least 5 independent experiments. (D) Surface plasmon resonance–analysis of AADH binding to immobilized histone H3 with collected data points in blue and data fit in black. Saturation of binding was verified by analysis of the binding data (insert). (E-F) Fluorimaging analysis of phorbol myristate acetate–stimulated human neutrophils stained with propidium iodide (red), FITC-labeled AADH (green), or anti-histone H2B antibody (blue). (E) A detail showing colocalization (yellow, arrow) of histones, DNA, and AADH on extracellular DNA fibers. (F) Representative presentation of a larger field with NETs, where colocalization (yellow) was observed for histones, DNA, and AADH. Arrows indicate several of the colocalization sites. Bar represents 20 µm for histology. Individual images for the composites (E-F) have been added as supplemental figures. Statistical significance (P < .05) was tested using one-way analysis of variance with Dunnett post hoc test. *Indicates significant difference compared with control samples.
In vitro heparin characterization. (A) In vitro effects of varying amounts of UFH and AADH on thrombin generation in normal pooled mouse plasma as measured by CAT.12 Variable amounts of UFH (B) or AADH (C) and histone H3 (H3) were added to EA.hy926 cells and cell viability was quantitated with flow cytometry. Each bar represents the average ± SD of at least 5 independent experiments. (D) Surface plasmon resonance–analysis of AADH binding to immobilized histone H3 with collected data points in blue and data fit in black. Saturation of binding was verified by analysis of the binding data (insert). (E-F) Fluorimaging analysis of phorbol myristate acetate–stimulated human neutrophils stained with propidium iodide (red), FITC-labeled AADH (green), or anti-histone H2B antibody (blue). (E) A detail showing colocalization (yellow, arrow) of histones, DNA, and AADH on extracellular DNA fibers. (F) Representative presentation of a larger field with NETs, where colocalization (yellow) was observed for histones, DNA, and AADH. Arrows indicate several of the colocalization sites. Bar represents 20 µm for histology. Individual images for the composites (E-F) have been added as supplemental figures. Statistical significance (P < .05) was tested using one-way analysis of variance with Dunnett post hoc test. *Indicates significant difference compared with control samples.
To further test our hypothesis on the cytoprotective activities of AADH and translate our in vitro findings into an in vivo system, we next evaluated the effects of AADH on the overall mortality rate in a mouse model of fatal liver injury using ConA-triggered activation of T cells to mimic sterile inflammation (Figure 2A). It has been described that extracellular histones are the major mediators of death in this model.13 We measured plasma levels of histone H3 by semiquantitative western blotting13 and showed that ConA injection increased circulating histone H3 levels in all animals (Figure 2B). In treated animals, appearance of histones was delayed and from our in vitro studies and reported in vivo cytotoxicity of extracellular histones, we conclude that histones detected in the treated group are at least partly complexed to AADH. Injection of ConA caused significant differences in 24-hour survival between the AADH-treated and control groups (P < .0005) (Figure 2A), with only a moderate effect of AADH on thrombin formation (supplemental Figure 1E). We verified that the moderate anticoagulant effect seen is independent of any antithrombin-mediated activities of AADH and can be attributed to heparin cofactor II stimulation, the plasma concentration of which does not allow complete inhibition of thrombin formation.14 Together, our in vitro and in vivo results strongly indicate that AADH treatment results in complexation and inactivation of circulating histones with a concomitant improvement of survival, in agreement with the observation that AADH abolished cytotoxic effects of histones on endothelial cells by complexation (supplemental Figure 1D). In contrast to UFH, AADH treatment of unchallenged mice with a dose 5 times that of UFH caused only moderate anticoagulation of blood plasma (supplemental Figure 1F) and no significant prolongation of tail bleeding time (supplemental Figure 1G). Furthermore, AADH treatment had no significant effects on plasma levels of the cytokines interleukin-6, interleukin-10, and tumor necrosis factor at 3 and 6 hours after ConA challenge (data not shown).
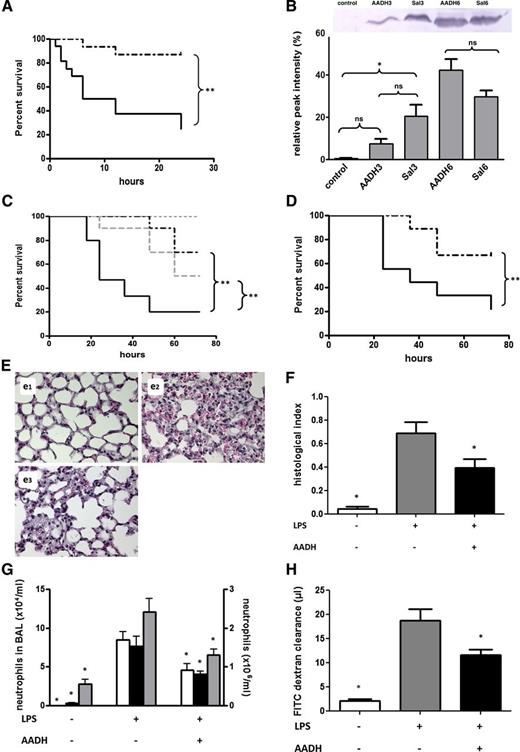
In vivo effects of AADH. (A) Twenty-four–hour survival in mice challenged through intravenous injection of 30 mg/kg ConA following intraperitoneal injection of 570 µg AADH (n = 15, dashed line) or saline (n = 16, solid line). Found differences are significant (P = .0005, with hazard ratio = 8.384 and 95% confidence interval (CI) of ratio 2.371-21.79). (B) Plasma of saline- or AADH-treated mice was collected 3 or 6 hours after ConA challenge and pooled (n = 6). Histone H3 content was characterized by western blotting. For comparison, a pooled mouse plasma sample from mice not challenged with ConA is shown. Relative densities as determined by densitometry using ImageJ are indicated. (C) Seventy-two–hour survival in CLP-challenged C57Bl/6 mice treated by intraperitoneal injection with 570 μg AADH 12 hours before, directly after, and 12 hours after CLP (prophylaxis, n = 10, dashed black line) or only 4 hours after CLP (n = 10, dashed gray line). Saline-treated, CLP-challenged mice are shown as a solid black line (n = 15), whereas survival of sham-treated animals is shown as a dotted gray line (n = 15). Differences between AADH-treated and nontreated groups are significant (for both the prophylactic and treatment regimes, with P = .0031 and P = .0275, respectively, and hazard ratios = 5.471 and 3.345, 95% CI of ratio 1.774-16.87 and 1.143-9.787, respectively. (D-H) Effect of AADH in a mouse model of LPS-induced sepsis. C57Bl/6 mice were treated with LPS 20 mg/kg i.p. and 570 µg AADH after 1 hour of the LPS challenge. (D) Survival in mice receiving LPS and vehicle (solid line) and mice receiving LPS plus AADH (dashed line) (n = 9/group). The difference between groups is significant, with P = .0375 and hazard ratio = 5.4328 and 95% CI of ratio 1.088-17.22. (E-H) Mice were euthanized 8 hours after LPS challenge. (E) Representative histological hematoxylin and eosin-stained sections of lungs from mice receiving saline (e1), LPS + vehicle control (e2), or LPS + AADH (e3). (F) Quantification of histological analysis, n = 4 per bar. (G) Quantification of neutrophils in the bronchoalveolar lavage (BAL) (white bars, left y-axis) of adherent, intravascular (black bars, right y-axis), and interstitial (gray bars, right y-axis) neutrophils. Discrimination between neutrophils in either location was made based on an antibody to Ly6G injected 5 minutes prior to euthanasia (n = 6-8 for each bar). (H) Assessment of lung plasma leakage based on the exudation of the plasma tracer FITC dextran (n = 6-8). Statistical significance was tested using one-way analysis of variance with Dunnett post hoc test. *Indicates significant difference (P < .05) compared with the LPS-challenged group.
In vivo effects of AADH. (A) Twenty-four–hour survival in mice challenged through intravenous injection of 30 mg/kg ConA following intraperitoneal injection of 570 µg AADH (n = 15, dashed line) or saline (n = 16, solid line). Found differences are significant (P = .0005, with hazard ratio = 8.384 and 95% confidence interval (CI) of ratio 2.371-21.79). (B) Plasma of saline- or AADH-treated mice was collected 3 or 6 hours after ConA challenge and pooled (n = 6). Histone H3 content was characterized by western blotting. For comparison, a pooled mouse plasma sample from mice not challenged with ConA is shown. Relative densities as determined by densitometry using ImageJ are indicated. (C) Seventy-two–hour survival in CLP-challenged C57Bl/6 mice treated by intraperitoneal injection with 570 μg AADH 12 hours before, directly after, and 12 hours after CLP (prophylaxis, n = 10, dashed black line) or only 4 hours after CLP (n = 10, dashed gray line). Saline-treated, CLP-challenged mice are shown as a solid black line (n = 15), whereas survival of sham-treated animals is shown as a dotted gray line (n = 15). Differences between AADH-treated and nontreated groups are significant (for both the prophylactic and treatment regimes, with P = .0031 and P = .0275, respectively, and hazard ratios = 5.471 and 3.345, 95% CI of ratio 1.774-16.87 and 1.143-9.787, respectively. (D-H) Effect of AADH in a mouse model of LPS-induced sepsis. C57Bl/6 mice were treated with LPS 20 mg/kg i.p. and 570 µg AADH after 1 hour of the LPS challenge. (D) Survival in mice receiving LPS and vehicle (solid line) and mice receiving LPS plus AADH (dashed line) (n = 9/group). The difference between groups is significant, with P = .0375 and hazard ratio = 5.4328 and 95% CI of ratio 1.088-17.22. (E-H) Mice were euthanized 8 hours after LPS challenge. (E) Representative histological hematoxylin and eosin-stained sections of lungs from mice receiving saline (e1), LPS + vehicle control (e2), or LPS + AADH (e3). (F) Quantification of histological analysis, n = 4 per bar. (G) Quantification of neutrophils in the bronchoalveolar lavage (BAL) (white bars, left y-axis) of adherent, intravascular (black bars, right y-axis), and interstitial (gray bars, right y-axis) neutrophils. Discrimination between neutrophils in either location was made based on an antibody to Ly6G injected 5 minutes prior to euthanasia (n = 6-8 for each bar). (H) Assessment of lung plasma leakage based on the exudation of the plasma tracer FITC dextran (n = 6-8). Statistical significance was tested using one-way analysis of variance with Dunnett post hoc test. *Indicates significant difference (P < .05) compared with the LPS-challenged group.
To further investigate the potential therapeutic value of AADH, we injected AADH in a mouse CLP model known to mimic human sepsis15 and an LPS injection model that mimics TLR-mediated septic shock.15 As observed in the ConA model, AADH is able to significantly increase survival both when given prior to cecal puncture as well as when given 4 hours after puncture (Figure 2C) and when given 1 hour after LPS challenge (Figure 2D). Protection of organs is likely to contribute to overall survival as was concluded from a liver viability assessment after CLP (supplemental Figure 1H) and lung tissue histological analysis (Figure 2E). Administration of AADH appears to reduce neutrophil influx, protects against intrapulmonary protein leakage and capillary-alveolar leakage, and is accompanied by reduction of cytokine release (Figure 2F-G; supplemental Figure 1I-J).
We conclude that AADH, a low anticoagulant fraction of UFH, has therapeutic potential to treat sepsis and other hyperinflammatory conditions in which excessive neutrophil activation with release of histones is part of the host response. Given the huge clinical experience with UFH and the anticipated safe profile of AADH, we expect low thresholds for commencing clinical studies in the near future.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Netherlands Organization for Scientific Research (medium investment grant to G.A.F.N.) and the Cardiovascular Research Institute Maastricht (to G.A.F.N.). O.S. is supported by the Netherlands Organization for Scientific Research (VIDI project 91712303) and the Deutsche Forschungsgemeinschaft (SO876/3-1, SO876/6-1, FOR809, SFB914-B08).
Authorship
Contribution: K.C.A.A.W., P.G.d.F., H.C.H., and O.S. conducted most of the experimental work and wrote the manuscript; H.C.H., C.A., C.P.R., A.O.-G., N.M.D., and R.S. made essential experimental contributions; P.G.d.F., C.P.R., and G.A.F.N. designed experimental plans and interpreted data; G.A.F.N. and C.P.R. drafted the manuscript; G.A.F.N. designed and directed the project; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: H.C.H., C.P.R., and G.A.F.N. are inventors of a patent application owned by the Maastricht University and claiming nonanticoagulant heparin use in sepsis. The remaining authors declare no competing financial interests.
Correspondence: G. A. F. Nicolaes, Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, PO Box 616, 6200 MD Maastricht, The Netherlands; e-mail: g.nicolaes@maastrichtuniversity.nl.
References
Author notes
K.C.A.A.W. and P.G.d.F. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal