Key Points
A1-A1 inhibits thrombotic properties of anti-β2GPI antibodies in mice.
A1-A1 does not affect thrombus size in the absence of anti-β2GPI antibodies.
Antiphospholipid syndrome (APS) is an autoimmune disorder with increased risk for thrombosis and pregnancy losses. β2-glycoprotein I (β2GPI) is the major antigen for clinically relevant antibodies in APS. We engineered a molecule, A1-A1, which interferes with 2 prothrombotic mechanisms in APS: the binding of β2GPI to negatively charged cellular surfaces and ApoE receptor 2. We studied how A1-A1 affects arterial thrombosis in vivo in lupus-prone (NZW × BXSB)F1 male mice. For the first time, we demonstrated that A1-A1 efficiently reduces thrombus size in vivo in the presence of chronic autoimmune anti-β2GPI antibodies. We have shown that A1-A1 interferes with thrombotic properties of β2GPI/antibody complexes and does not affect normal thrombus formation in the absence of anti-β2GPI antibodies. A1-A1 inhibits prothrombotic properties of β2GPI/antibody complexes in wild-type mice after acute infusion with anti-β2GPI antibodies, as well as in mice expressing persistent autoimmune anti-β2GPI antibodies. A1-A1 reduced thrombus size in a mouse model of APS in the presence of lupus features, suggesting that A1-A1 might effectively interfere with thrombosis not only in primary APS but also in APS secondary to lupus. Our results suggest that A1-A1 could be a prototype for an antithrombotic drug in APS.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disorder with increased risk for thrombosis and pregnancy loss.1,-3 It is diagnosed based on the combination of clinical features and laboratory tests for circulating autoantibodies. Thrombotic events in APS nearly equally occur in veins and arteries, and about 30% of patients with thrombosis are men.4,,-7 Thrombosis in APS correlates with the presence of antibodies to β2-glycoprotein I (β2GPI).8,,-11 The physiological function of β2GPI is not well defined12 and its deficiency in humans or mice does not result in apparent abnormalities.13,,-16 β2GPI acquires prothrombotic properties only after association with antibodies.10,12 Treatment with anti-β2GPI antibodies or recombinant dimers of β2GPI mimicking β2GPI/antibody complexes results in increased thrombus size in animal models of thrombosis and cellular activation in vitro.2,3,17,,-20
It was demonstrated both in vitro and in vivo that exposure of endothelial cells, monocytes, and platelets to β2GPI/anti-β2GPI antibody complexes shifts the cellular phenotype to prothrombotic and proinflammatory (reviewed in Giannakopoulos and Krilis,2 Tripodi et al,3 and Harper et al20 ). Anionic phospholipids, Toll-like receptors 2 and 4 (TLR2 and TLR4), annexin A2, and ApoER2 are cell-surface molecules involved in binding and activation of endothelial cells and monocytes by β2GPI/antibody complexes.21,,,,-26 The binding of β2GPI to anionic phospholipids and the assembly of β2GPI/antibody complexes is considered the first step in the sequence of molecular events leading to cellular activation. In vivo studies in mice support involvement of annexin A2,27 ApoER2,19,24 and TLR428 in the increase of the thrombus size in the presence of anti-β2GPI antibodies.
B2GPI interacts with A1, the first ligand-binding domain of ApoER2.29 We demonstrated that A1 interferes with the binding of β2GPI to anionic phospholipids and constructed a dimer, A1-A1.30,31 A1-A1 inhibits at least 2 prothrombotic interactions of β2GPI/antibody complexes: the binding to ApoER2 and anionic phospholipids on the cellular surfaces. A distinctive feature of A1-A1 compared to A1 is that A1-A1 preferentially interacts with β2GPI bound to anti-β2GPI antibodies.30,31 Because β2GPI is present in the blood at high concentration, 4 μM,32 it is important that a potential drug binds predominantly to pathological β2GPI/antibody complexes.
It is established that injection of APS immunoglobulin G (IgG) positive for anti-β2GPI or purified anti-β2GPI antibodies causes the increase in thrombus size after vessel injury in animal models of thrombosis.17,-19,24,28 It was previously shown that the first ligand-binding domain of ApoER2 can reduce the enhancement of thrombus size in mice when injected along with APS IgG.19 The characteristic feature of APS is that anti-β2GPI antibodies are constitutively present in the patients’ circulation. Persistent autoantibodies affect endothelial function contributing to thrombosis.33,,,-37 In order to evaluate antithrombotic properties of A1-A1 in vivo in the setting resembling APS in humans, we used a mouse model of APS.38,,-41 Male (NZW × BXSB)F1 mice express persistent autoimmune anti-β2GPI antibodies early in life and develop thrombosis of small coronary vessels causing myocardial microinfarcts.
For the first time, we demonstrated that A1-A1 efficiently reduces thrombus size in a laser-induced arterial thrombosis model in vivo in the presence of chronic autoimmune anti-β2GPI antibodies. We have shown that A1-A1 is effective in interfering with thrombotic properties of β2GPI/antibody complexes and does not affect normal thrombus formation in the absence of anti-β2GPI. A1-A1 inhibits prothrombotic properties of β2GPI/antibody complexes in wild-type mice after acute infusion with anti-β2GPI IgG, as well as in mice expressing persistent autoimmune anti-β2GPI antibodies. A1-A1 reduced thrombus size in a mouse model of APS in the presence of lupus features, suggesting that A1-A1 might be effective in interfering with thrombosis not only in primary APS but also in APS secondary to lupus. Our results strongly suggest that the inhibition of the binding of β2GPI/anti-β2GPI antibody complexes to cellular surfaces is a way to interfere with thrombosis in APS, and indicate that A1-A1 can be a prototype for an antithrombotic drug in APS.
Methods
Mice
BALB/c, female NZW, and male BXSB mice were obtained from The Jackson Laboratory. All animal care and experimental procedures were approved by the institutional animal care and use committee of the Beth Israel Deaconess Medical Center.
Proteins
A1 is a fragment of mouse ApoER2 (residues 12-47). A1-A1 was constructed, expressed in Escherichia coli, and purified as previously described.30 A1-A1 was labeled with Atto488 (Sigma-Aldrich) at the N terminus, and separated from A1-A1 and unreacted probe by reverse-phase chromatography. LA6 (residues 212-251 in the low-density lipoprotein receptor [LDLR]) was expressed in E coli and purified following the same procedure used for the purification of A1-A1. Blood samples were collected from APS patients after informed consent was obtained in accordance with the Declaration of Helsinki. Total IgG was purified from the plasma sample of a patient positive for anti-β2GPI antibodies using protein G agarose (Pierce). One milligram of β2GPI (Meridian Life Science) was attached to CarboLink gel (Pierce) and used for isolation of anti-β2GPI IgG from the purified total IgG. Purified total IgG and anti-β2GPI IgG were dialyzed in phosphate-buffered saline (PBS), concentrated, and stored in aliquots at −80°C until use. The patient was a 61-year-old woman with a history of subclinical strokes, aPL-associated livedo reticularis, and recurrent complicated migraines. All 3 laboratory tests for APS remain abnormal on multiple occasions (LA positive, ACA IgG >140 GPL and B2GPI-IgG >100 SGU). Patient plasma used for these studies contained 110 SGU of anti-β2GPI IgG (Inova). Endotoxin, measured with the end point limulus amebocyte lysate assay (Lonza) in preparations of A1-A1, LA6, and IgG, was far below the lowest calibration point of the assay.
Clearance of A1-A1 after intraperitoneal and intravenous administration
Stock solution of Atto488-labeled A1-A1 in PBS supplemented with 250 μM CaCl2 was diluted in saline and injected at a 100-μL volume. Male BALB/c mice had body mass between 27 g and 29 g. Mice were injected intravenously via tail vein with A1-A1 at dose of 0.4 μg/g mouse weight. Blood was withdrawn at 5, 20, 60, 90, 120, and 180 minutes (3 animals per time point). For intraperitoneal injection, we used A1-A1 at a dose of 2 μg/g mouse weight. After intraperitoneal administration, blood was withdrawn at 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, and 24 hours (3 animals per time point). Serum samples were diluted with saline and analyzed using a fluorescence plate reader. Atto488-A1-A1 concentration in the serum was quantified using a calibration curve built with known concentrations of Atto488-A1-A1.
Antibodies to β2GPI
Antibodies to β2GPI were measured by enzyme-linked immunosorbent assay (ELISA) using 96-well plates coated with human β2GPI supplied with the β2GPI-IgG ELISA kit (Inova). Serum samples were diluted 1/50 with the sample diluent from the kit, incubated for 30 minutes on a plate (100 μL per well), and probed with horseradish peroxidase–conjugated donkey anti-mouse IgG (ab7061; Abcam). Bound IgG was detected with TMB (3,3′,5,5′-tetramethylbenzidine) substrate. Each set of measurements contained serial dilutions of mouse monoclonal anti-human β2GPI IgG (Alpha Diagnostic) prepared in the sample diluent and used as standard to generate a standard curve. The range of concentrations of the antibody used to generate standard curves was either from 0 to 7.5 μg/mL or from 0 to 50 μg/mL in a set of 7 or 8 dilutions. The standard data adjusted for a background was fitted either to a linear regression model (dilutions of standard from 0 to 7.5 μg/mL) or to a nonlinear equation f(x) = A/(1 + B/x) (dilutions of standard from 0 to 50 μg/mL). Mean squared error of fit of standard curves in all sets of measurements was better than 0.01.
Reversed-phase chromatography of Atto488-labeled A1-A1
Mouse serum was collected from BALB/c mice and pooled. Three microliters of the stock solution of Atto488-labeled A1-A1 in PBS supplemented with 250 μM CaCl2 was added to 30 μL of mouse serum and incubated at 37°C. At timed intervals, serum samples were analyzed by reversed-phase chromatography by monitoring elution profiles at 500-nm wavelength. Before loading onto a reversed-phase C18 column, 20 μL of 10% acetonitrile with 0.1% trifluoroacetic acid (TFA) in water (buffer A) was added to a 30-μL serum sample. Samples were analyzed using a linear gradient of either 1% per minute or 0.1% per minute of buffer B (acetonitrile with 0.1% TFA).
Intravital microscopy, laser-induced injury, and analysis of platelet accumulation at the site of vascular injury
Mouse surgery and intravital video microscopy of the cremaster muscle microcirculation were performed as previously described.42,-44 Laser injury was created with a Micropoint Laser System (Photonics) focused at the vessel wall. Each new thrombus was generated upstream of the previous one and observed with an Olympus AX microscope with a ×60 water-immersion objective. Platelets were visualized with DyLight649-labeled anti-GPIbβ antibodies (X649; Emfret) infused intravenously at a dose of 0.1 μg/g body weight.
Image analysis was performed using Slidebook. For each collected thrombus, a string of frames was captured every 200 milliseconds starting from a few seconds before the laser injury and collected for the duration of about 5 minutes. In each frame, a background mask was defined in an uninjured portion of vessel upstream of the injury site. The maximum fluorescence intensity of the pixels contained in the mask was determined for all frames in the collected image; their mean value was used as a background fluorescence for each frame in the image. At each time point, the thrombus was described by integrated fluorescence intensity in the corresponding frame corrected for the background fluorescence. The kinetics of thrombus formation was characterized by the plot of the integrated fluorescence signal over time. To account for the variability in thrombus formation, about 30 thrombi were collected for a given set of experimental conditions. A given set of experimental conditions was then described by combined thrombus kinetics calculated as a median value of integrated fluorescence intensity of all thrombi over time. The size of the thrombus was described by the area under the curve of thrombus kinetics. The effect of the inhibitor on thrombus size was evaluated by comparing the areas under the curves of median fluorescence calculated from all thrombi collected in the absence and in the presence of the inhibitor. Statistical significance of the difference between the 2 data sets was calculated using the Wilcoxon rank-sum test by comparing sizes of the individual thrombi.
Results
Serum level of β2GPI antibodies in (NZW × BXSB)F1 mice
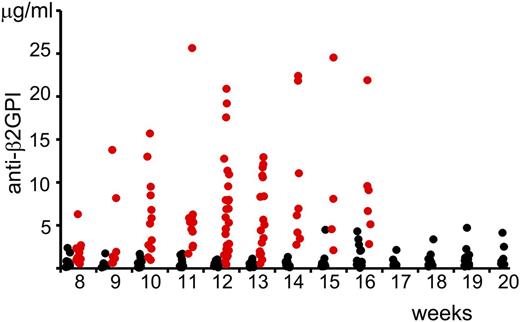
We measured the serum concentration of anti-β2GPI antibodies in (NZW × BXSB)F1 mice depending on age (Figure 1). The statistical significance of the changes in the concentration of anti-β2GPI antibodies in serum samples of mice at different age and gender were compared using the Wilcoxon rank-sum test. Even at the age of 8 weeks, the serum level of anti-β2GPI antibodies was significantly higher in male mice than in female mice (Table 1). Serum concentrations of anti-β2GPI in female mice did not change with age. There was a statistically significant increase (P = .007) in the serum level of anti-β2GP antibodies in 10-week-old male mice compared with 8-week-old male mice. We did not observe a statistically significant difference between serum levels of anti-β2GPI antibodies in male mice with the increase of age from 10 to 16 weeks.
Serum levels of anti-β2GPI IgG in (NZW × BXSB)F1 mice. Serum levels of anti-β2GPI antibodies (males, red circles; females, black circles) were measured by ELISA and the corresponding concentrations were calculated using a standard curve generated with known concentrations of mouse monoclonal anti-human β2GPI IgG.
Serum levels of anti-β2GPI IgG in (NZW × BXSB)F1 mice. Serum levels of anti-β2GPI antibodies (males, red circles; females, black circles) were measured by ELISA and the corresponding concentrations were calculated using a standard curve generated with known concentrations of mouse monoclonal anti-human β2GPI IgG.
Serum levels of anti-β2GPI antibodies
| Mice characteristics . | Values . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, wk | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Females, μg/mL | 0.5 | 0.4 | 0.4 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 | 0.4 | 0.4 | 0.7 | 0.7 | 0.7 |
| (n = 10) | (n = 5) | (n = 10) | (n = 10) | (n = 14) | (n = 16) | (n = 12) | (n = 12) | (n = 22) | (n = 11) | (n = 15) | (n = 17) | (n = 8) | |
| Males, μg/mL | 1.9 | 1.4 | 5.5 | 5.4 | 5.9 | 5.4 | 6.6 | 6.3 | 7.9 | ||||
| (n = 12) | (n = 7) | (n = 12) | (n = 12) | (n = 27) | (n = 17) | (n = 8) | (n = 4) | (n = 6) | |||||
| P* | .007 | .04 | .0002 | .0001 | <.0001 | <.0001 | .0001 | .005 | .0003 | ||||
| Mice characteristics . | Values . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, wk | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Females, μg/mL | 0.5 | 0.4 | 0.4 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 | 0.4 | 0.4 | 0.7 | 0.7 | 0.7 |
| (n = 10) | (n = 5) | (n = 10) | (n = 10) | (n = 14) | (n = 16) | (n = 12) | (n = 12) | (n = 22) | (n = 11) | (n = 15) | (n = 17) | (n = 8) | |
| Males, μg/mL | 1.9 | 1.4 | 5.5 | 5.4 | 5.9 | 5.4 | 6.6 | 6.3 | 7.9 | ||||
| (n = 12) | (n = 7) | (n = 12) | (n = 12) | (n = 27) | (n = 17) | (n = 8) | (n = 4) | (n = 6) | |||||
| P* | .007 | .04 | .0002 | .0001 | <.0001 | <.0001 | .0001 | .005 | .0003 | ||||
Median of concentrations of anti-β2GPI antibodies measured in (NZW × BXSB)F1 mice at different age. The statistical significance of the changes in the concentration of anti-β2GPI antibodies in serum samples of mice at different age and gender is compared using the Wilcoxon rank-sum test.
P values for the differences between serum levels of anti-β2GPI antibodies in the blood samples of female and male (NZW × BXSB)F1 mice at indicated age.
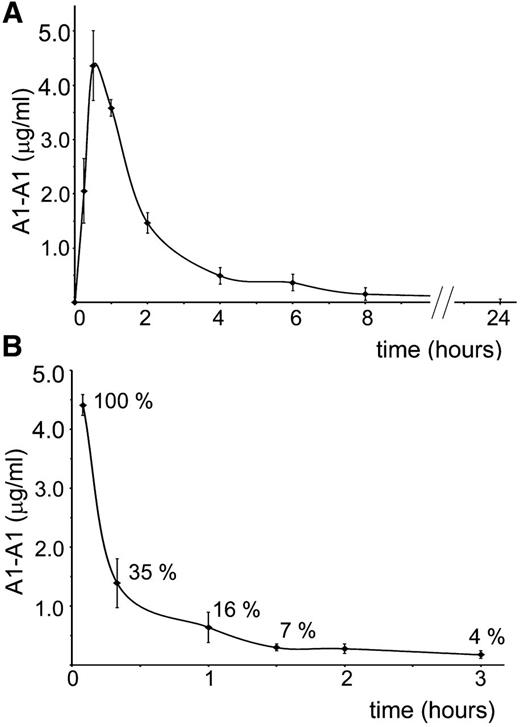
Clearance of A1-A1 from the mouse circulation after intravenous and intraperitoneal administration
For better planning in vivo experiments, we investigated clearance of A1-A1 from the mouse circulation after intraperitoneal and intravenous administration (Figure 2). Fluorescently-labeled A1-A1 was injected intraperitoneally into BALB/c mice at a 2 μg/g mouse dose. The concentration of the inhibitor in the blood reached its maximum in 30 minutes after injection. In 8 hours, A1-A1 was completely cleared from the blood. When 0.4 μg/g A1-A1 was injected intravenously, the amount of the inhibitor in the blood decreased to 16% of the injected dose in 1 hour after A1-A1 administration.
Clearance of A1-A1 from the mouse circulation. Serum concentration of fluorescently-labeled A1-A1 measured in BALB/c mice following (A) intraperitoneal administration with A1-A1 at a dose of 2 μg/g body weight and (B) intravenous injection with A1-A1 at a dose of 0.4 μg/g body weight. Each data point is the mean ± SD (n = 3).
Clearance of A1-A1 from the mouse circulation. Serum concentration of fluorescently-labeled A1-A1 measured in BALB/c mice following (A) intraperitoneal administration with A1-A1 at a dose of 2 μg/g body weight and (B) intravenous injection with A1-A1 at a dose of 0.4 μg/g body weight. Each data point is the mean ± SD (n = 3).
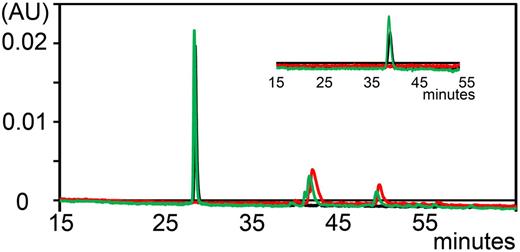
Stability of A1-A1 in mouse serum
Previously, we demonstrated that A1-A1 has a favorable stability in human serum.30 We determined whether A1-A1 is cleaved by proteases in mouse serum in vitro. Degradation of fluorescent A1-A1 was monitored by the reversed-phase high-performance liquid chromatography at 500 nm by comparing chromatograms collected with a linear gradient of 1% per minute up to 60% of acetonitrile/TFA for intact A1-A1 and after 2 hours of incubation with mouse serum at 37°C (Figure 3). To achieve better resolution, we used a linear gradient of 0.1% per minute of acetonitrile/TFA (Figure 3 inset). After incubation with mouse serum, A1-A1 eluted at the same time as intact A1-A1 and no proteolytic fragments of A1-A1 were detected, indicating that A1-A1 remains intact in serum.
Analytical reversed-phase chromatograms of fluorescently-labeled A1-A1 monitored at 500 nm. Chromatogram of A1-A1 eluted with a linear gradient of 1% per minute. A1-A1 is eluted from the column at about 28% of acetonitrile/TFA. (Inset) Chromatogram of A1-A1 eluted with a 0.1% per minute gradient of acetonitrile/TFA starting at 9 minutes from about 26% of acetonitrile/TFA. Elution trace of mouse serum (red), A1-A1 without serum (black), and A1-A1 incubated with serum for 2 hours at 37°C (green).
Analytical reversed-phase chromatograms of fluorescently-labeled A1-A1 monitored at 500 nm. Chromatogram of A1-A1 eluted with a linear gradient of 1% per minute. A1-A1 is eluted from the column at about 28% of acetonitrile/TFA. (Inset) Chromatogram of A1-A1 eluted with a 0.1% per minute gradient of acetonitrile/TFA starting at 9 minutes from about 26% of acetonitrile/TFA. Elution trace of mouse serum (red), A1-A1 without serum (black), and A1-A1 incubated with serum for 2 hours at 37°C (green).
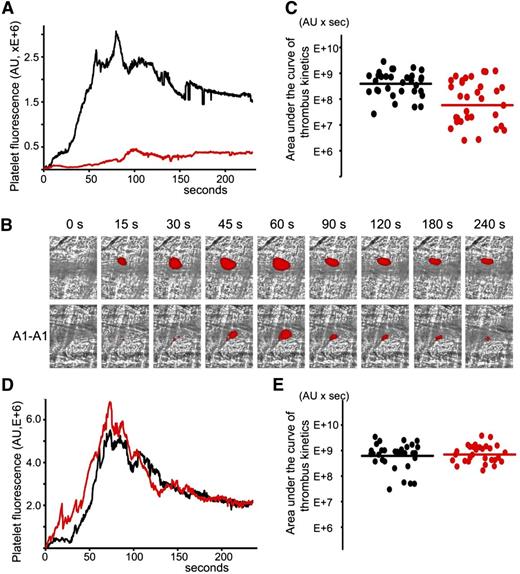
Inhibition of thrombus size by A1-A1 in (NZW × BXSB)F1 mice following the laser-induced vessel injury
A1-A1 was evaluated regarding its ability to inhibit thrombosis in cremaster muscle arterioles of (NZW × BXSB)F1 mice in the presence of persistent autoimmune anti-β2GPI antibodies. The laser-induced injury model in cremaster arterioles was previously used to demonstrate that anti-β2GPI antibodies were directly involved in the pathogenesis of thrombosis.17 We used 13- and 14-week-old (NZW × BXSB)F1 mice. At this age, the median concentration of anti-β2GPI antibodies in male mice was 6 μg/mL. In each mouse, 4 to 9 thrombi were collected in the absence of A1-A1. Then, A1-A1 was infused through the jugular cannula at a dose of 4 μg/g body weight and, after 10 minutes, more thrombi were generated, this time in the presence of A1-A1. The duration of the experiment after the infusion of A1-A1 was no longer than 1 hour. Quantitative data were obtained by analysis of multiple thrombi collected in 5 mice (Figure 4A-C). Both in the absence and in the presence of A1-A1, the thrombus appeared soon after the vessel injury, reached its maximum between 60 and 100 seconds, and remained at the site of injury throughout the observation time of 240 seconds. In the presence of A1-A1, thrombus size, described by the area under the curve of the platelet fluorescence over time, decreased to <15% of the thrombus size observed in the absence of A1-A1. The statistical significance of the thrombus inhibition (P = .001) is confirmed by the Wilcoxon rank-sum test comparing sizes of the individual thrombi collected in the absence and in the presence of the inhibitor (Figure 4C).
Inhibition of thrombus size in male (NZW × BXSB)F1 mice by A1-A1. In vivo imaging of platelet accumulation at the site of injury in male (NZW × BXSB)F1 mice after laser-induced thrombosis in cremaster muscle arterioles in the absence of A1-A1 and after the infusion of A1-A1 at a dose of 4 μg/g mouse. Data were acquired in 13- to 14-week-old mice (A-C) and in 6-week-old mice (D-E). (A) Platelet accumulation during thrombus formation represented by the median-integrated platelet fluorescence studied in 5 mice in the absence of A1-A1 (black curve, 33 thrombi) and in the presence of A1-A1 (red curve, 32 thrombi). Area under the curve of platelet fluorescence over time is a measure of the thrombus size. In the presence of A1-A1, thrombus size is decreased to <15% of the thrombus size in the absence of A1-A1. (B) Snapshots of platelet accumulation at the site of injury over time. Comparison of 2 representative thrombi generated in the same mouse in the absence (top row) and in the presence (bottom row) of A1-A1. (C) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). Inhibition of thrombus size in the presence of A1-A1 is statistically significant (P = .001). (D) Median-integrated platelet fluorescence studied in 5 mice in the absence (black curve, 32 thrombi) and in the presence (red curve, 30 thrombi) of A1-A1. (E) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). The difference between 2 data sets is not statistically significant. Lines on panels C and E correspond to areas under the curves of the median-integrated fluorescence calculated for all thrombi in a data set.
Inhibition of thrombus size in male (NZW × BXSB)F1 mice by A1-A1. In vivo imaging of platelet accumulation at the site of injury in male (NZW × BXSB)F1 mice after laser-induced thrombosis in cremaster muscle arterioles in the absence of A1-A1 and after the infusion of A1-A1 at a dose of 4 μg/g mouse. Data were acquired in 13- to 14-week-old mice (A-C) and in 6-week-old mice (D-E). (A) Platelet accumulation during thrombus formation represented by the median-integrated platelet fluorescence studied in 5 mice in the absence of A1-A1 (black curve, 33 thrombi) and in the presence of A1-A1 (red curve, 32 thrombi). Area under the curve of platelet fluorescence over time is a measure of the thrombus size. In the presence of A1-A1, thrombus size is decreased to <15% of the thrombus size in the absence of A1-A1. (B) Snapshots of platelet accumulation at the site of injury over time. Comparison of 2 representative thrombi generated in the same mouse in the absence (top row) and in the presence (bottom row) of A1-A1. (C) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). Inhibition of thrombus size in the presence of A1-A1 is statistically significant (P = .001). (D) Median-integrated platelet fluorescence studied in 5 mice in the absence (black curve, 32 thrombi) and in the presence (red curve, 30 thrombi) of A1-A1. (E) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). The difference between 2 data sets is not statistically significant. Lines on panels C and E correspond to areas under the curves of the median-integrated fluorescence calculated for all thrombi in a data set.
To confirm the dependence of the observed thrombus inhibition by A1-A1 on the presence of anti-β2GPI antibodies, we repeated experiments in 6-week-old (NZW × BXSB)F1 male mice that did not yet develop anti-β2GPI antibodies. A total of 5 mice were used to collect 32 thrombi prior to infusion of the inhibitor and 30 thrombi after infusion of A1-A1 at a dose of 4 μg/g mouse weight. In the absence of anti-β2GPI antibodies, A1-A1 had no effect on thrombus size (Figure 4D-E).
LA6, a structural homolog of A1 that does not bind β2GPI, does not inhibit thrombus size in (NZW × BXSB)F1 mice after the laser injury
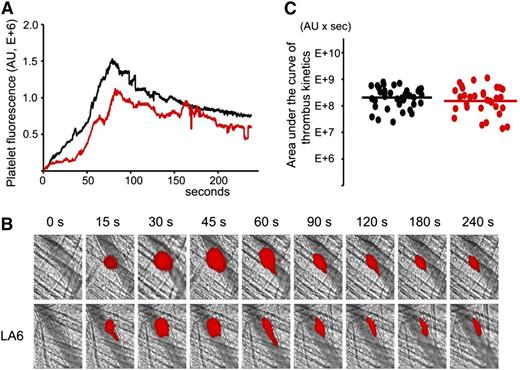
Previously, we demonstrated that LA6 does not bind β2GPI.31 To confirm that the decrease of the thrombus size is specific to the binding of A1-A1 to β2GPI, we used LA6 instead of A1-A1 in intravital experiments in 13- to 14-week-old male (NZW × BXSB)F1 mice. Experiments were performed exactly as described for A1-A1. LA6 was infused into the mouse circulation through the jugular cannula at a dose of 4 μg/g mouse weight. Quantitative data were obtained by analysis of thrombi collected in 6 mice (Figure 5). Although the median thrombus size in the presence of LA6 was 75% of the median thrombus size in the absence of LA6, the difference between the 2 data sets was not statistically significant (P = .42).
LA6, which does not bind β2GPI, does not inhibit thrombus size in (NZW × BXSB)F1mice. In vivo imaging of platelet accumulation at the site of injury in 13- to 14-week-old male (NZW × BXSB)F1 mice after laser-induced thrombosis in cremaster muscle arterioles in the absence of LA6 and after the infusion of LA6 at a dose of 4 μg/g mouse. (A) Median-integrated platelet fluorescence studied in 6 mice in the absence of LA6 (black curve, 37 thrombi) and in the presence of LA6 (red curve, 33 thrombi). (B) Snapshots of platelet accumulation at the site of injury over time. Comparison of 2 representative thrombi generated in the same mouse in the absence (top row) and in the presence (bottom row) of LA6. (C) Sizes of individual thrombi collected in the absence (black circles) and in the presence of LA6 (red circles). Lines correspond to areas under the curves of the median-integrated fluorescence calculated for all thrombi in each data set collected in 6 mice. The difference between 2 data sets is not statistically significant (P = .42).
LA6, which does not bind β2GPI, does not inhibit thrombus size in (NZW × BXSB)F1mice. In vivo imaging of platelet accumulation at the site of injury in 13- to 14-week-old male (NZW × BXSB)F1 mice after laser-induced thrombosis in cremaster muscle arterioles in the absence of LA6 and after the infusion of LA6 at a dose of 4 μg/g mouse. (A) Median-integrated platelet fluorescence studied in 6 mice in the absence of LA6 (black curve, 37 thrombi) and in the presence of LA6 (red curve, 33 thrombi). (B) Snapshots of platelet accumulation at the site of injury over time. Comparison of 2 representative thrombi generated in the same mouse in the absence (top row) and in the presence (bottom row) of LA6. (C) Sizes of individual thrombi collected in the absence (black circles) and in the presence of LA6 (red circles). Lines correspond to areas under the curves of the median-integrated fluorescence calculated for all thrombi in each data set collected in 6 mice. The difference between 2 data sets is not statistically significant (P = .42).
A1-A1 does not affect thrombus size in BALB/c mice in the absence of anti-β2GPI IgG
To demonstrate that the inhibitory effect of A1-A1 on thrombus size depends on the presence of anti-β2GPI antibodies and does not affect normal thrombogenesis, we used wild-type BALB/c mice.
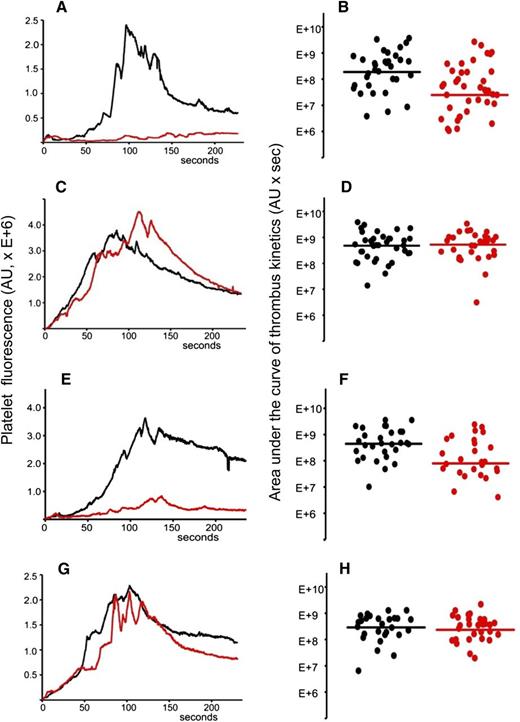
Total IgG was isolated from the plasma sample of APS patient positive for anti-β2GPI antibodies. The IgG preparation contained 20 SGU per μg of purified IgG. It was previously demonstrated that 1 mg of total IgG purified from serum samples of APS patients positive for anti-β2GPI antibodies showed a significant prothrombotic effect in wild-type mice in the laser-induced arterial thrombosis model.17 We infused 2 mg of purified patient IgG via a jugular cannulus. Experiments were performed exactly as described for (NZW × BXSB)F1 mice. After collection of several thrombi in the absence of the inhibitor, A1-A1 (4 μg/g mouse weight) was infused intravenously and more thrombi were collected in the presence of the inhibitor. Data from 4 mice, 33 thrombi in the absence and 39 thrombi in the presence of A1-A1, were combined and analyzed (Figure 6A-B). Thrombus size decreased in the presence of A1-A1 to 13% of the thrombus size observed in the absence of A1-A1 (P = .001). Then, we infused 2 mg of patient IgG into mice and studied the effect of LA6 (4 μg/g mouse) on thrombus size. We did not observe inhibition of thrombus size, indicating that the inhibitory properties of A1-A1 in vivo depend on its binding to β2GPI and confirming results obtained in (NZW × BXSB)F1 mice (Figure 6C-D).
A1-A1 inhibits thrombus size in BALB/c mice in the presence of anti-β2GPI antibodies and does not affect thrombus size in the absence of anti-β2GPI antibodies. (A-B) Anti-β2GPI–positive APS IgG (2 mg per mouse) and A1-A1 (4 μg/g mouse). (A) Median-integrated platelet fluorescence studied in 4 mice in the absence (black curve, 33 thrombi) and in the presence (red curve, 39 thrombi) of A1-A1. In the presence of A1-A1, thrombus size decreased to 13% of the thrombus size in the absence of A1-A1 (P = .001). (B) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). (C-D) Anti-β2GPI–positive APS IgG (2 mg per mouse) and LA6 (4 μg/g mouse). (C) Median-integrated platelet fluorescence studied in 7 mice in the absence (black curve, 37 thrombi) and in the presence (red curve, 30 thrombi) of LA6. (D) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of LA6 (red circles). The difference between 2 data sets is not statistically significant. (E-F) Purified patient anti-β2GPI IgG (16 μg per mouse) and A1-A1 (4 μg/g mouse). (E) Median-integrated platelet fluorescence studied in 4 mice in the absence (black curve, 29 thrombi) and in the presence (red curve, 26 thrombi) of A1-A1. In the presence of A1-A1, thrombus size decreased to 18% of the thrombus size in the absence of A1-A1 (P = .007). (F) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). (G-H) PBS and A1-A1 (4 μg/g mouse). (G) Median-integrated platelet fluorescence studied in 4 mice in the absence (black curve, 28 thrombi) and in the presence (red curve, 29 thrombi) of A1-A1. (H) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). The difference between 2 data sets is not statistically significant. Lines on panels B, D, F, and H correspond to areas under the curves of the median-integrated fluorescence calculated for all thrombi in a data set.
A1-A1 inhibits thrombus size in BALB/c mice in the presence of anti-β2GPI antibodies and does not affect thrombus size in the absence of anti-β2GPI antibodies. (A-B) Anti-β2GPI–positive APS IgG (2 mg per mouse) and A1-A1 (4 μg/g mouse). (A) Median-integrated platelet fluorescence studied in 4 mice in the absence (black curve, 33 thrombi) and in the presence (red curve, 39 thrombi) of A1-A1. In the presence of A1-A1, thrombus size decreased to 13% of the thrombus size in the absence of A1-A1 (P = .001). (B) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). (C-D) Anti-β2GPI–positive APS IgG (2 mg per mouse) and LA6 (4 μg/g mouse). (C) Median-integrated platelet fluorescence studied in 7 mice in the absence (black curve, 37 thrombi) and in the presence (red curve, 30 thrombi) of LA6. (D) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of LA6 (red circles). The difference between 2 data sets is not statistically significant. (E-F) Purified patient anti-β2GPI IgG (16 μg per mouse) and A1-A1 (4 μg/g mouse). (E) Median-integrated platelet fluorescence studied in 4 mice in the absence (black curve, 29 thrombi) and in the presence (red curve, 26 thrombi) of A1-A1. In the presence of A1-A1, thrombus size decreased to 18% of the thrombus size in the absence of A1-A1 (P = .007). (F) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). (G-H) PBS and A1-A1 (4 μg/g mouse). (G) Median-integrated platelet fluorescence studied in 4 mice in the absence (black curve, 28 thrombi) and in the presence (red curve, 29 thrombi) of A1-A1. (H) Area under the curve of individual thrombi collected in the absence (black circles) and in the presence of A1-A1 (red circles). The difference between 2 data sets is not statistically significant. Lines on panels B, D, F, and H correspond to areas under the curves of the median-integrated fluorescence calculated for all thrombi in a data set.
To demonstrate that the inhibition of thrombus size by A1-A1 depends specifically on the presence of anti-β2GPI antibodies, we used purified patient anti-β2GPI IgG autoantibodies. BALB/c mice were infused with 16 μg per mouse of patient anti-β2GPI IgG. Thrombi were collected in 4 mice in the absence of A1-A1 (n = 29) and in the presence of A1-A1 at a dose of 4 μg/g body weight (n = 26) (Figure 6E-F). In the presence of A1-A1, thrombus size decreased to 18% of the thrombus size observed in the absence of A1-A1 (P = .007).
We then studied the effect of A1-A1 on normal thrombus formation in BALB/c mice. Mice were infused with PBS and thrombi generated by the laser injury to the vessel wall in the absence and in the presence of A1-A1 (4 μg/g mouse) (Figure 6G-H). The difference between thrombi collected in 4 mice in the absence of A1-A1 (28 thrombi) and after the infusion of A1-A1 (29 thrombi) was not statistically significant. This result is in line with the absence of any observed effect of A1-A1 on thrombus size in 6-week-old (NZW × BXSB)F1 male mice that do not have anti-β2GPI antibodies.
Taken together, our data strongly suggest that A1-A1 inhibits thrombotic properties of β2GPI/antibody complexes and does not affect normal thrombus formation after laser injury to the vessel wall. The inhibitory effect of A1-A1 depends on the presence of anti-β2GPI antibodies and is caused by the binding of A1-A1 to β2GPI engaged in prothrombotic β2GPI/antibody complex.
Discussion
We engineered a small protein, A1-A1, consisting of 2 identical A1 molecules connected by a flexible linker. A1 is the first ligand-binding module from the ApoER2 that binds domain V from β2GPI.29,31,45 A1-A1 prevents the binding of β2GPI to anionic phospholipids and ApoER2.30,31 Both mechanisms, the binding of β2GPI to negatively charged cellular surfaces and to ApoER2 on platelets in the presence of anti-β2GPI antibodies contribute to thrombosis in APS.19,24,46 Here, we studied the effect of A1-A1 on thrombus size in mice constitutively expressing autoimmune anti-β2GPI antibodies by monitoring thrombus formation in mouse arterioles following laser-induced injury. The presence of persistent anti-β2GPI antibodies is a distinctive feature of APS in humans.1
(NZW × BXSB)F1 male mice spontaneously develop autoimmune anti-β2GPI antibodies, have thrombi in small coronary vessels causing microinfarcts, and display lupus-like characteristics such as anti-DNA antibodies and severe kidney damage.38,,-41 More than 70% of these mice die before 30 weeks of age.38,40,47 For intravital experiments, we used 13- to 14-week-old male mice that had the median concentration of anti-β2GPI antibodies in their blood of 6 μg/mL and 6-week-old male mice that did not yet develop autoimmune anti-β2GPI antibodies.
We demonstrated that when anti-β2GPI are present in the circulation either in autoimmune (NZW × BXSB)F1 mice or in BALB/c mice infused with patient anti-β2GPI IgG, A1-A1 significantly reduced thrombus size in the laser-induced model of arterial thrombosis. The thrombus reduction was due to the binding of A1-A1 to β2GPI because infusion of LA6, a structural homolog of A1 that does not bind β2GPI, did not affect thrombus size. We did not observe thrombus inhibition by A1-A1 in the absence of anti-β2GPI antibodies. These results strongly suggest that A1-A1 is effective in interfering with thrombotic properties of β2GPI/antibody complexes and does not affect normal thrombus formation in the absence of anti-β2GPI antibodies.
We evaluated the effectiveness of A1-A1 in inhibition of thrombotic properties of anti-β2GP antibodies under conditions resembling APS in humans, which is characterized by the presence of persistent autoimmune anti-β2GPI antibodies. Circulating anti-β2GPI antibodies activate endothelium through the binding of β2GPI/antibody complexes to endothelial cells, making vessel walls vulnerable to further damage. Patients with APS often exhibit features of damaged endothelium and the presence of circulating markers of endothelial activation correlates with thrombosis in these patients.33,,,-37 Stimulated endothelial cells and monocytes release proinflammatory cytokines further contributing to thrombosis.48 Chronic systemic inflammation in lupus disease contribute to the high risk for thrombosis in APS patients.49 Male (NZW × BXSB)F1 mice, besides anti-β2GPI antibodies, exhibit typical lupus features, such as anti-DNA antibodies and lupus nephritis.38,41 Our in vivo results suggest that A1-A1 could be effective as an antithrombotic drug both in primary APS and in APS secondary to lupus.
Our data confirm the importance of ApoER2 and anionic phospholipids in conveying thrombotic properties of β2GPI/anti-β2GPI antibody complexes.19,22,24,26 We calculated that the dose of A1-A1 used in vivo is sufficient to completely inhibit the binding of β2GPI/anti-β2GPI antibody complexes to ApoER2 and significantly reduce the binding to anionic phospholipids. Previously, we measured that monomeric A1 binds β2GPI with 1μM affinity and A1-A1 is about 20-fold more efficient than A1 in the inhibition of β2GPI/antibody complexes to anionic phospholipids,30,31 suggesting that the affinity of A1-A1 for β2GPI/anti-β2GPI antibody complexes is about 50nM. Immediately after infusion into mice at a 4 μg/g body weight dose, the concentration of A1-A1 in the blood is about 6μM, providing an excess of the inhibitor to interact with the majority of available β2GPI/antibody complexes.
A1-A1 interferes with the binding of β2GPI/antibody complexes not only to ApoER2 but to all members of the lipoprotein receptor family. This is a beneficial feature of A1-A1 because lipoprotein receptors are expressed on endothelial cells and monocytes and might contribute to APS.50,,-53 It is not clear whether or not other receptors of the LDLR family play a role in APS. Lipoprotein receptors of the LDLR family use multiple small domains structurally similar to A1 to interact with diverse ligands.51,54 The majority of these small ligand-binding modules have similar features allowing them to recognize binding sites centered on basic residues on their ligands.55 Although these same features are used by A1-A1 to bind β2GPI,31 it is unlikely that A1-A1 will bind with a physiologically relevant affinity to other ligands of lipoprotein receptors and significantly affect normal function of these receptors.31,56,-58 Fine tuning the primary sequence of A1-A1 for better binding to β2GPI is a way to avoid possible interference with the native function of ApoER2.
In conclusion, we have shown that A1-A1 is effective in interfering with thrombotic properties of β2GPI/antibody complexes and does not affect normal thrombus formation in the absence of anti-β2GPI. A1-A1 reduced thrombus size in a mouse model of APS in the presence of lupus features, suggesting that A1-A1 might be effective in interfering with thrombosis not only in primary APS but also in APS secondary to lupus. Our data confirm the importance of ApoER2 and anionic phospholipids in conveying thrombotic properties of β2GPI/anti-β2GPI antibody complexes, and suggest that A1-A1 can be a prototype for an effective antithrombotic drug in APS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Drs Bauer, Furie, and Zwicker for referring patients for these studies, Drs Bruce and Barbara Furie for the opportunity to use intravital microscope and valuable advice, and Glenn Merrill-Skoloff for expert technical assistance.
This work was supported by grants from the National Institutes of Health and from the Lupus Research Institute (N.B.).
Authorship
Contribution: A.K., A.P., and N.B. performed experiments and analyzed data; and N.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Natalia Beglova, Division of Hemostasis and Thrombosis, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Ave, CLS 941, Boston, MA 02215; e-mail: nbeglova@bidmc.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal