Key Points
The clinically based NCCN-IPI is a robust prognostic tool for the rituximab era that better discriminates low- and high-risk DLBCL patients compared with the IPI.
The NCCN-IPI outperforms the IPI by refined categorization of age and LDH, and the identification of disease involvement at specific extranodal sites.
Abstract
The International Prognostic Index (IPI) has been the basis for determining prognosis in patients with aggressive non-Hodgkin lymphoma (NHL) for the past 20 years. Using raw clinical data from the National Comprehensive Cancer Network (NCCN) database collected during the rituximab era, we built an enhanced IPI with the goal of improving risk stratification. Clinical features from 1650 adults with de novo diffuse large B-cell lymphoma (DLBCL) diagnosed from 2000-2010 at 7 NCCN cancer centers were assessed for their prognostic significance, with statistical efforts to further refine the categorization of age and normalized LDH. Five predictors (age, lactate dehydrogenase (LDH), sites of involvement, Ann Arbor stage, ECOG performance status) were identified and a maximum of 8 points assigned. Four risk groups were formed: low (0-1), low-intermediate (2-3), high-intermediate (4-5), and high (6-8). Compared with the IPI, the NCCN-IPI better discriminated low- and high-risk subgroups (5-year overall survival [OS]: 96% vs 33%) than the IPI (5 year OS: 90% vs 54%), respectively. When validated using an independent cohort from the British Columbia Cancer Agency (n = 1138), it also demonstrated enhanced discrimination for both low- and high-risk patients. The NCCN-IPI is easy to apply and more powerful than the IPI for predicting survival in the rituximab era.
Introduction
The International Prognostic Index (IPI) is a powerful prognostic tool developed more than 20 years ago based on the clinical characteristics of more than 1000 patients with diffuse, aggressive lymphomas treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-like chemotherapy.1 It has since served as the basis for determining prognosis among patients with diffuse large B-cell lymphoma (DLBCL). Five clinical characteristics including age, lactate dehydrogenase (LDH), number of extranodal sites, Ann Arbor stage, and Eastern Cooperative Oncology Group (ECOG) performance status were used to risk stratify and identify 4 discrete risk categories. Assignment to the high-risk subgroup (4 or 5 risk factors) was associated with a 26% 5-year overall survival (OS). Since the late 1990s, the addition of rituximab (R) to conventional CHOP or CHOP-like regimens for DLBCL has resulted in a major improvement in survival across all risk groups.2,3 As a result, the capacity of the IPI to discriminate between risk groups has declined, especially among the higher-risk patients.4,5 Analysis of pooled data from 3 European trials (MInT, MegaCHOEP, and RICOVER-60)4 that enrolled adult DLBCL patients treated with R-containing regimens, demonstrated a 5-year OS in the IPI-defined high-risk group of approximately 50%. The Kaplan-Meier curves for OS showed a convergence of high-intermediate (H-I) and high (H) risk categories. Efforts to improve the model’s discrimination have focused on adding new clinical prognostic factor(s) to the initial index,6 regrouping the original IPI score,5 or specifically focusing on elderly patients (E-IPI).7 These approaches, however, have resulted in only incremental improvements. Using the revised IPI (R-IPI), Sehn et al reported a 5-year OS that remained no lower than 50% in the poor-risk group from the British Columbia lymphoid cancer registry.5 New tools for the rituximab era are needed.
In parallel, efforts to characterize the biological basis for prognosis in DLBCL using immunohistochemical or molecular techniques have identified a variety of biomarkers and gene signatures with prognostic significance.8-13 For the most part, these novel prognostic markers are independent of the clinically based IPI but add little to its prognostic power.14 The results of immunohistochemical (IHC) staining are often inconsistent,15 and molecular markers/signatures are expensive, technically challenging, and currently not standardized for clinical care. Other than the IPI, which predates the introduction of rituximab, there is still a lack of robust prognostic tools for initial risk stratification for routine clinical use. Using the raw data collected for the NCCN NHL database in the rituximab era, we built a new prognostic model based solely on clinical features. We subsequently validated this model using a population-based registry cohort from the British Columbia Cancer Agency (BCCA).
Patients, materials, and methods
The NCCN NHL database project was reviewed and approved by the institutional review board at each participating institution.
Patient cohort
The NCCN NHL database for prognostic index development.
The formation and data structure of this multicenter NCCN NHL database have been previously reported.16 There are 7 participating NCCN cancer centers, including (1) City of Hope Comprehensive Cancer Center, (2) Dana Farber Cancer Institute/Brigham and Women’s Cancer Center, (3) Fox Chase Cancer Center, (4) The University of Texas MD Anderson Cancer Center, (5) Roswell Park Cancer Institute, (6) University of Michigan Comprehensive Cancer Center, and (7) Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Sequential patients seen at the aforementioned centers who were older than 18 years with newly diagnosed de novo DLBCL between June 1, 2000, and December 31, 2010 were included, with follow-up through December 31, 2011. Patients were required to be cancer free for 5 years before lymphoma diagnosis. De novo DLBCL cases were diagnosed at each center based on the NCCN protocol that included only cases with no evidence of more than one histologic subtype and no prior documented history of lymphoma. Institutional review board approval was obtained for the NCCN dataset used for clinical research at all participating institutions, as well as for BCCA dataset used for validation, and informed consent was obtained in accordance with the Declaration of Helsinki when required by the participating institutions.
Baseline clinical and disease characteristics included age, gender, Ann Arbor stage (I-IV), number and specific sites of extranodal involvement including the spleen, normalized LDH (ratio to the institutional upper limit of normal), ECOG performance status (PS, 0-4), presence of bulky disease (≥10 cm), and B symptoms (defined as recurrent fever, night sweats, or >10% weight loss). Sites of involvement were determined by a hierarchical order based on verified findings available—first by histology, then by image, and then by physical examination if demonstrated. Involvement of the bone marrow and central nervous system (CNS) were established histologically. Neither β2-microglobulin nor IHC markers, such as Ki-67 or cell of origin (GCB vs non-GCB), were mandated for reporting and thus were not evaluated. Patients with HIV, hepatitis C virus, or hepatitis B virus were not excluded from the cohort. Initial treatment, outcomes, and corresponding dates were queried related to disease progression and death, or if neither of those, then the date of the last follow-up visit. We included all eligible patients who were diagnosed and treated in the rituximab era, receiving first-line therapy, during the study period. OS at 5 years was the primary outcome, defined as death from any cause. Progression-free survival (PFS) at 5 years was determined as the earliest of death, disease recurrence, or indication of disease progression on therapy.17
The BCCA Centre for Lymphoid Cancer database for external validation.
The BCCA lymphoid cancer database is population-based and includes patients with lymphoma treated throughout the province of British Columbia (BC), encompassing both tertiary center and community hospital settings. In the current study, all consecutive adult patients with biopsy-proven and centrally reviewed new diagnoses of DLBCL treated with R-CHOP with curative intent since March 1, 2001 (date when rituximab became available in BC), and with complete clinical information, were included. Patients were excluded if they had evidence of secondary malignancy or an underlying indolent lymphoproliferative disorder, or the presence of a major illness that precluded an attempt at curative therapy.5 Clinical variables and outcome information comparable to those studied in the NCCN DLBCL cohort were available. Follow-up information was available through October 2012.
Statistical methods
Development of NCCN-IPI.
Descriptive statistics of baseline clinical features were generated as proportions (categorical factors) or means, standard deviations, and interquartile ranges (continuous factors). Extreme outlier values, such as those in LDH, were verified by chart review of the original data. Only cases with complete required clinical information were used in the analysis. This approach was unlikely to introduce bias because data were missing at random without knowledge of outcomes.1
The training set of the NCCN cohort consisted of randomly selected 85% of the sample, and the remaining 15% was initially reserved for internal validation. Using the training set, the univariate association between individual clinical prognostic factors and OS was analyzed using the Cox proportional hazard model, with P < .1 considered statistically significant. An indicator for each individual extranodal site (CNS, bone marrow, liver/gastrointestinal [GI] tract, lung, spleen, and other sites) was included. Special efforts were made to examine the linearity assumption of age and LDH ratio as continuous variables with respect to effects on survival using restricted cubic splines,18 followed by refined categorization of both variables in the Cox model to minimize the Martingale residuals.19 For age, this was done by 15- to 20-year increments instead of dichotomizing the age at 60. This was performed similarly for the LDH ratio based on the linearity check and how it minimized the Martingale residuals in the model.
Significant factors from univariate selection were kept in the multivariate analysis by using backward selection and the SCORE method for the best predictor set. Global model fitting was assessed by Akaike Information Criteria (AIC), in which a lower value indicated a better fit. Index scores were assigned proportionally to the estimates of the relative contribution of the significant factors in the final prognostic model. Risk strata were formed according to the proximity of Kaplan-Meier (K-M) curves for each score value (0-8). Patients were also risk stratified according to the original IPI and were compared with the NCCN-IPI for model fitting and 5-year OS rates across different risk groups.
Validation of NCCN-IPI and comparison with IPI.
Our findings were initially examined using the internal validation sample consisting of 15% of the NCCN dataset. Subsequently, validation of the NCCN-IPI was carried out using the independent BCCA cohort. Datasets from NCCN and BCCA were compared for patient demographic and clinical characteristics. Discrimination of the NCCN-IPI and IPI was measured by the concordance probability estimate (CPE) along with its 95% confidence interval (CI) in both training and validation data for OS.20 A higher CPE indicated better discrimination. Capacity of prognostication by the 2 indices was visually compared side by side based on KM curves of each risk stratum for 5-year OS. Finally, interrater-weighted κ statistics (95% CI) were calculated to compare the degree of agreement (0 ≤ κ ≤ 1, 0 = no agreement, 1 = perfect agreement) in risk stratification by the NCCN-IPI and IPI in both the NCCN and BCCA cohorts.21 All analyses were conducted using SAS software (SAS Institute Inc., Cary, NC). P values < .05 were considered statistically significant unless otherwise specified.
Results
Cohort characteristics
The training set of the NCCN cohort consisted of 1935 patients, of whom 1650 had complete clinical information to be included in the analysis. The BCCA external validation cohort consisted of 1138 patients with complete information. The presenting characteristics of patients in both cohorts were compared in Table 1. Of note, patients in the NCCN cohort were younger compared with the BCCA cohort (median age 57 vs 63 years), had better ECOG PS (PS 2-4: 11% vs 37%) but a higher percentage of extranodal involvement in major organs (36% vs 25%) including in the CNS, bone marrow, liver/GI tract, or lung. Median follow-up was 3.1 years in the NCCN cohort and 3.2 years in the BCCA cohort.
Characteristics of de novo DLBCL patients in the NCCN and BCCA cohorts
| Cohort characteristics . | NCCN (n = 1650) . | BCCA (n = 1138) . |
|---|---|---|
| Male gender | 54% | 60% |
| Age (y), mean (SD) | 57 (16) | 63 (15) |
| Age >60 y, % | 46% | 60% |
| LDH ratio >1, % | 50% | 49% |
| Ann Arbor stage (III-IV), % | 59% | 55% |
| ECOG PS (≥2), % | 11% | 37% |
| Extranodal disease,* % | 36% | 25% |
| Cohort characteristics . | NCCN (n = 1650) . | BCCA (n = 1138) . |
|---|---|---|
| Male gender | 54% | 60% |
| Age (y), mean (SD) | 57 (16) | 63 (15) |
| Age >60 y, % | 46% | 60% |
| LDH ratio >1, % | 50% | 49% |
| Ann Arbor stage (III-IV), % | 59% | 55% |
| ECOG PS (≥2), % | 11% | 37% |
| Extranodal disease,* % | 36% | 25% |
Lymphomatous involvement in the bone marrow, CNS, liver/GI tract, or lung.
Development of NCCN-IPI
The univariate analysis of baseline characteristics revealed that age, LDH ratio, advanced stage (Ann Arbor stage III-IV), ECOG PS (≥2), and presence of bulky disease significantly affected OS, whereas B symptoms did not. Gender had only borderline significance (P = .16). Lymphomatous involvement in major organs (bone marrow, CNS, liver/GI tract, or lung) appeared to be a stronger predictor (P < .001) than the number of extranodal sites (>1), which was not significant. Each of the aforementioned extranodal sites was chosen as being individually significant and remained significant as a group in the selection. Splenic, genitourinary, or bone involvement was not significant. The effect of continuous age on survival was linear and a 15- to 20-year increment of age (ie, >40-60, >60-75, and >75 years), with age ≤40 as the reference category, provided the optimal model fit (supplemental Figure 1). However, the effect of normalized LDH was not linear and reached a plateau at a ratio of 3 (supplemental Figure 2). In turn, the LDH ratio was categorized into >1-3 and >3 using normal LDH (ratio ≤1) as a reference. This better accommodated the model variance and minimized the Martingale residual (supplemental Figures 3.1 and 3.2).
Following the model iterations in multivariate analysis, the final prognostic index consisted of 5 factors, as shown in Table 2. Numbers of extranodal sites (>1) in the current multivariate model again were observed to be not predictive (HR 1.0, 95% CI 0.8-1.3, P = .91). Based on the model estimates, the current NCCN-IPI used a maximum of 8 scoring points for categorized age (>40-60, 1 point (pt); >60-75, 2 pts; >75, 3 pts) and LDH ratio (>1-3, 1 pt; ≥3, 2 pts) upper limit of normal in addition to extranodal disease in major organs (bone marrow, CNS, liver/GI tract, or lung), Ann Arbor stage III-IV, and ECOG PS (≥2), each having a score of 1 (Table 3). This model showed better model fitting (a smaller AIC) compared with the same data stratified by the original IPI score.
Clinical factors prognostic of overall survival from multivariate selection in the NCCN cohort
| NCCN (n = 1650) . | HR . | 95% CI . | P value . | Score . |
|---|---|---|---|---|
| Age | ||||
| ≤40 y | 1.0 | 0 | ||
| 41-60 y | 2.4 | (1.4-4.2) | .0002 | 1 |
| 61-75 y) | 3.2 | (2.0-5.3) | <.0001 | 2 |
| >75 y | 6.1 | (3.5−10.6) | <.0001 | 3 |
| LDH-R ≤1 | 1.0 | 0 | ||
| LDH-R (>1-3) | 2.1 | (1.6-2.7) | <.0001 | 1 |
| LDH-R >3 | 3.3 | (2.3-4.8) | <.0001 | 2 |
| ECOG PS ≥2 | 1.9 | (1.5-2.4) | <.0001 | 1 |
| Ann Arbor stage, III-IV | 1.5 | (1.1-2.0) | .0062 | 1 |
| Extranodal disease* | 1.5 | (1.2-1.9) | .0008 | 1 |
| NCCN (n = 1650) . | HR . | 95% CI . | P value . | Score . |
|---|---|---|---|---|
| Age | ||||
| ≤40 y | 1.0 | 0 | ||
| 41-60 y | 2.4 | (1.4-4.2) | .0002 | 1 |
| 61-75 y) | 3.2 | (2.0-5.3) | <.0001 | 2 |
| >75 y | 6.1 | (3.5−10.6) | <.0001 | 3 |
| LDH-R ≤1 | 1.0 | 0 | ||
| LDH-R (>1-3) | 2.1 | (1.6-2.7) | <.0001 | 1 |
| LDH-R >3 | 3.3 | (2.3-4.8) | <.0001 | 2 |
| ECOG PS ≥2 | 1.9 | (1.5-2.4) | <.0001 | 1 |
| Ann Arbor stage, III-IV | 1.5 | (1.1-2.0) | .0062 | 1 |
| Extranodal disease* | 1.5 | (1.2-1.9) | .0008 | 1 |
HR, hazard ratio; LDH-R, LDH ratio.
Lymphomatous involvement in bone marrow, CNS, liver/GI tract, or lung.
The NCCN-IPI
| NCCN-IPI . | Score . |
|---|---|
| Age, y | |
| >40 to ≤60 | 1 |
| >60 to ≤75 | 2 |
| >75 | 3 |
| LDH, normalized | |
| >1 to ≤3 | 1 |
| >3 | 2 |
| Ann Arbor stage III-IV | 1 |
| Extranodal disease* | 1 |
| Performance status ≥2 | 1 |
| NCCN-IPI . | Score . |
|---|---|
| Age, y | |
| >40 to ≤60 | 1 |
| >60 to ≤75 | 2 |
| >75 | 3 |
| LDH, normalized | |
| >1 to ≤3 | 1 |
| >3 | 2 |
| Ann Arbor stage III-IV | 1 |
| Extranodal disease* | 1 |
| Performance status ≥2 | 1 |
Disease in bone marrow, CNS, liver/GI tract, or lung.
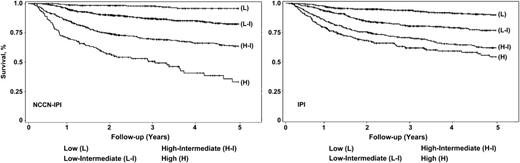
Four distinct risk groups were formed based on K-M curves for 5-year OS: low (L, 0-1 pt), low-intermediate (L-I, 2-3 pts), high-intermediate (H-I, 4-5 pts), and high (H, ≥6 pts). This model showed better discrimination of outcomes (both OS and PFS) compared with the original IPI and identified a high-risk group (8% of the cohort) with a 5-year OS of 33% (95% CI, 22%-45%) (Table 4 and Figure 1).
Comparison of NCCN-IPI to IPI for risk stratification and outcomes of 5-y OS and PFS in the NCCN and BCCA cohorts
| . | Score . | 5-y OS . | 5-y PFS . | |||
|---|---|---|---|---|---|---|
| . | NCCN-IPI . | IPI . | NCCN-IPI . | IPI . | NCCN-IPI . | IPI . |
| NCCN cohort (n = 1650) | ||||||
| Low | 0-1 (19%)* | 0-1 (38%) | 96% | 90% | 91% | 85% |
| L-I | 2-3 (42%) | 2 (26%) | 82% | 77% | 74% | 66% |
| H-I | 4-5 (31%) | 3 (22%) | 64% | 62% | 51% | 52% |
| High | ≥6 (8%) | 4-5 (14%) | 33% | 54% | 30% | 39% |
| BCCA cohort (n = 1138) | ||||||
| Low | 0-1 (12%) | 0-1 (33%) | 96% | 84% | 94% | 81% |
| L-I | 2-3 (37%) | 2 (24%) | 77% | 72% | 72% | 66% |
| H-I | 4-5 (37%) | 3 (22%) | 56% | 54% | 54% | 54% |
| High | ≥6 (14%) | 4-5 (21%) | 38% | 43% | 35% | 41% |
| . | Score . | 5-y OS . | 5-y PFS . | |||
|---|---|---|---|---|---|---|
| . | NCCN-IPI . | IPI . | NCCN-IPI . | IPI . | NCCN-IPI . | IPI . |
| NCCN cohort (n = 1650) | ||||||
| Low | 0-1 (19%)* | 0-1 (38%) | 96% | 90% | 91% | 85% |
| L-I | 2-3 (42%) | 2 (26%) | 82% | 77% | 74% | 66% |
| H-I | 4-5 (31%) | 3 (22%) | 64% | 62% | 51% | 52% |
| High | ≥6 (8%) | 4-5 (14%) | 33% | 54% | 30% | 39% |
| BCCA cohort (n = 1138) | ||||||
| Low | 0-1 (12%) | 0-1 (33%) | 96% | 84% | 94% | 81% |
| L-I | 2-3 (37%) | 2 (24%) | 77% | 72% | 72% | 66% |
| H-I | 4-5 (37%) | 3 (22%) | 56% | 54% | 54% | 54% |
| High | ≥6 (14%) | 4-5 (21%) | 38% | 43% | 35% | 41% |
Percent of cohort.
NCCN IPI vs IPI in risk stratification in the NCCN DLBCL training cohort.
Validation of NCCN-IPI
Whereas the internal validation sample (n = 301) showed better risk discrimination with the NCCN-IPI than with the IPI (5-y OS 42% vs 63%, respectively, in the high-risk group and 96% vs 90% in the low-risk group; supplemental Figure 4), we proceeded to validate with the substantially larger external dataset from the BCCA.
The NCCN-IPI was found to be prognostic in the BCCA cohort and, notably, 14% of patients were classified as high risk. The 5-year K-M estimates of OS between NCCN-IPI and IPI differed substantially in the high-risk group—38% (95% CI, 29%-46%) vs 43% (95% CI, 36%-49%), as well as in the low-risk category, 96% (95% CI, 90%-99%) vs 84% (95% CI, 80%-88%). The high mortality rate (43%) observed in the high-risk category from the BCCA cohort could be related to senior age and overall poorer performance status compared with the NCCN cohort. The absolute difference in survival between the low- and high-risk groups was 58% with NCCN-IPI stratification compared with 41% with IPI stratification in the BCCA cohort (Table 4 and Figure 2).
NCCN IPI vs IPI in risk stratification in the BCCA DLBCL validation cohort.
The NCCN-IPI outperformed the IPI in both cohorts, with a higher CPE in discrimination (NCCN cohort: 0.80 vs 0.74; BCCA cohort: 0.77 vs 0.74) and better global model fit measure with lower AIC (supplemental Table 1). The weighted κ statistics suggested that the risk classification by the 2 indices agreed to a moderate degree and were sufficiently different in both cohorts (weighted κ: 0.60) (supplemental Table 1). The greater capacity of the NCCN-IPI to risk stratify was maintained in the external validation.
Discussion
For the past 20 years, the IPI has been the basis for initial risk stratification for patients with DLBCL, facilitating treatment selection, balance within clinical trials, comparison among studies, and discussion with patients regarding prognosis. However, its capacity to discriminate among risk groups has declined with the addition of rituximab to anthracycline-containing therapy. The enhanced NCCN-IPI, built on unselected patients with newly diagnosed DLBCL from NCCN centers with contemporary data, has shown an improved capacity to discriminate clinically important risk groups.
The search for novel indices using IHC markers or gene expression signatures has not yet led to a robust index for routine clinical use.8-13,22,23 This is largely the result of intrinsic limitations in the application of these markers related to technical limitations or poor reproducibility. The extent to which biologic markers improve the prognostic value of the IPI has been the subject of recent debate.14 Furthermore, controversy remains as to which gene set and/or collection of IHC markers is best for prognostication.24 In addition, there is substantial statistical complexity to building such a composite model. Whether the addition of biological markers, such as translocations involving MYC, BCL2, and BCL6 to the NCCN-IPI, will enhance its capacity to prognosticate will require investigation.
The current NCCN-IPI was developed using traditional clinical factors but provides increased capacity to discriminate both high-risk (with 5-y OS <50%) and low-risk patients with previously untreated DLBCL. In contrast to the original IPI, which was based on patients with “diffuse aggressive lymphomas” enrolled in clinical trials, the NCCN-IPI derives from unselected patients with a confirmed diagnosis of DLBCL enrolled at participating NCCN institutions in the rituximab era. Although patients seen at academic medical centers likely represent some degree of self-selection, the validation of the NCCN-IPI in the independent population-based BCCA cohort, which includes patients treated in the community setting, supports its generalizability. Differences in patient characteristics between the 2 cohorts represent a study strength rather than a limitation. Compared with the E-IPI, a modification of the IPI specifically for elderly patients, the NCCN-IPI similarly identifies advanced age (60-75 and >75) to be associated with incremental risk and encompasses such risk in a single index. Moreover, there have been reports associating gender, especially in combination with older age and low body mass index, with worse outcomes, possibly related to rituximab metabolism.25 The addition of gender to the model did not substantially enhance its capacity to predict outcome, thus interactions of gender with age and body mass index were not explored further for this analysis. It was our intention to keep the index simple to use and applicable to all patients.
Both the NCCN-IPI and original IPI include a similar set of clinical factors for prognostication and recognize 4 risk groups, with the former applying a refined categorization of age and normalized LDH to better capture the associated increased risk of mortality. This was achieved by observing the best evidence during statistical modeling. Indeed, these 2 characteristics may be surrogates for disease biology. Klapper et al26 reported that age at diagnosis of DLBCL correlated with the molecular profile with genetic alterations previously shown to confer a poor prognosis in DLBCL accumulating with age. These included, for example, ABC subtype, MYC or BCL2 expression, gains and translocations affecting the BCL6 locus, and other complex cytogenetic alterations. When age was incorporated into their multivariate analyses, much of the genetic complexity lost its prognostic significance, further supporting the notion of age as an important surrogate in this context. Similarly, in an earlier study, if LDH was merely dichotomized into “normal” vs “abnormal,” the model did not fit as well. Refined categorization to capture increments in LDH reduces the Martingale residuals,27 providing better risk prognostication and supporting LDH as a proxy for disease aggressiveness. In the current study, LDH replaced the presence of bulky disease as a risk factor once included in the model. A third change in the NCCN-IPI was related to the presence of extranodal sites. Lymphomatous involvement of major organs including bone marrow, CNS, liver/GI tract, or lung appeared to be a stronger predictor than merely the number of extranodal sites used in the IPI, likely also reflecting more aggressive disease. For example, CNS involvement carries a poor prognosis and has been associated with MYC translocations, itself a marker of high-risk disease.28
Limitations of the current study are principally those typically associated with observational studies using large health care databases. We attempted to safeguard against data entry errors by verification of original records for outlier values. Close to 15% of subjects in the original cohort had missing values in one or more clinical features and were deleted from the data analysis. Because information was collected at presentation irrespective of outcomes, it is likely that these were missing at random and therefore should not bias the results. Although, as anticipated, the difference in discrimination in the high-risk group was less dramatic in the BCCA validation cohort (5-year OS: NCCN-IPI 38% vs IPI 43%) compared with that in the NCCN cohort (NCCN-IPI 33% vs IPI 54%), the difference in discrimination of the low-risk groups was more so (NCCN-IPI 96% vs IPI 84% compared with NCCN-IPI 96% vs IPI 90%, respectively). The BCCA cohort had higher proportions of elderly males and patients with documented poor performance status. These differences may explain a lower 5-year OS in the BCCA high-risk group. It is worth noting that both cohorts are unselected and from real-world settings. It will be important to validate the NCCN-IPI using pooled clinical trial data for its performance.
In conclusion, the NCCN-IPI is a robust and useful tool to stratify prognostically relevant subgroups of DLBCL patients in the current era of rituximab-based therapy. Compared with the IPI and other modifications of the IPI, it better incorporates 2 known continuous prognostic variables, age and LDH, in a rational way that is both simple to apply and valid in the rituximab era. With its enhanced capacity to discriminate risk groups, it has value in treatment planning and in discussions of prognosis. Its utility can also be found in stratification of future randomized clinical trials. Because there is continued enthusiasm for defining a high-risk group in the R-CHOP era, the NCCN-IPI will be useful in identifying candidates for novel approaches including in postremission therapies such as intensification with autologous stem cell transplant or consolidation/maintenance with new targeted agents. Patients in the lowest risk group may be treated with traditional R-CHOP with excellent outcomes. Whether the NCCN-IPI will retain its robust capacity for risk stratification in the context of targeted therapies and novel biomarkers will need to be investigated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Authorship
Contribution: Z.Z., A.W.R., and J.N.W. designed and performed research study, analyzed data, and wrote the manuscript; A.C.-T., A.V., and J.N. performed research and studied and analyzed data; and L.I.G., L.H.S., A.S.L., A.D.Z., G.A.A., M.A.R., A.N., M.S.K., M.S.C., M.M., R.D.G., J.M.C., and J.W.F. designed research study, collected data, and contributed significantly to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jane N. Winter, Division of Hematology/Oncology, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St Clair Street, Suite 850, Chicago, IL 60611; e-mail: j-winter@northwestern.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal