Key Points
AMG 330 cytotoxicity against AML cells is proportional to the level of CD33 expression but is not affected by ABC transporter activity.
AMG 330 cytotoxicity is amenable to modulation and augmentation by clinically available drugs such as histone deacetylase or DNA methyltransferase I inhibitors.
Abstract
CD33 is a valid target for acute myeloid leukemia (AML) but has proven challenging for antibody-drug conjugates. Herein, we investigated the cellular determinants for the activity of the novel CD33/CD3-directed bispecific T-cell engager antibody, AMG 330. In the presence of T cells, AMG 330 was highly active against human AML cell lines and primary AML cells in a dose- and effector to target cell ratio–dependent manner. Using cell lines engineered to express wild-type CD33 at increased levels, we found a quantitative relationship between AMG 330 cytotoxicity and CD33 expression; in contrast, AMG 330 cytotoxicity was neither affected by common CD33 single nucleotide polymorphisms nor expression of the adenosine triphosphate–binding cassette (ABC) transporter proteins, P-glycoprotein or breast cancer resistance protein. Unlike bivalent CD33 antibodies, AMG 330 did not reduce surface CD33 expression. The epigenetic modifier drugs, panobinostat and azacitidine, increased CD33 expression in some cell lines and augmented AMG 330-induced cytotoxicity. These findings demonstrate that AMG 330 has potent CD33-dependent cytolytic activity in vitro, which can be further enhanced with other clinically available therapeutics. As it neither modulates CD33 expression nor is affected by ABC transporter activity, AMG 330 is highly promising for clinical exploration as it may overcome some limitations of previous CD33-targeted agents.
Introduction
Acute myeloid leukemia (AML) has served as a paradigm for the therapeutic use of monoclonal antibodies because of well-defined cell-surface antigens and easy tumor accessibility. The most investigated target so far is CD33, a myeloid differentiation antigen found on AML blasts in most patients and, perhaps, leukemic stem cells in some.1,2 Recent randomized phase 3 trials have demonstrated that the CD33 antibody-drug conjugate, gemtuzumab ozogamicin (GO), improves survival for some patients with newly diagnosed AML when added to conventional chemotherapy, with benefit primarily seen for those with favorable-risk disease and, to a smaller extent, intermediate-risk disease.3-5 Although this experience indicates that CD33 is a valid target for this disease,1,2 it is a challenging one for toxin-loaded antibodies due to its relatively low abundance, slow internalization, and drug transporter activity in AML cells. In fact, GO given alone or in combination with other chemotherapeutics is ineffective in many patients and, as a consequence, is currently no longer commercially available in many countries.1,2
Bispecific T-cell engager (BiTE) antibodies are a novel subclass of therapeutic single-chain antibodies.6-8 What distinguishes BiTE antibodies from prior antibody-based therapeutics is that the effector is a cytotoxic T cell rather than a conjugated radioactive isotope, cytotoxic chemotherapy agent, or antibody-dependent cellular cytotoxicity.6-8 Early results from clinical studies with a CD19/CD3 BiTE, blinatumomab, in acute lymphoblastic leukemia suggest that such agents are non–cross-resistant to commonly used chemotherapeutics and can be highly efficacious, even in otherwise chemotherapy-refractory patients.9,10 AMG 330 is a novel CD33/CD3 BiTE antibody developed to recruit T cells to recognize and kill CD33-expressing human AML target cells. AMG 330 has shown activity against AML blasts in initial preclinical studies but the critical cellular characteristics for the cytolytic activity have not been explored in detail.11 Herein, we tested potential variables that may modulate the in vitro cytotoxicity of AMG 330 against human AML, using well-defined AML cell lines and genetically engineered sublines, and then conducted proof-of-principle studies in diagnostic specimens obtained from patients with AML.
Materials and methods
Healthy donor T cells
Mononuclear cells were collected from healthy adult volunteers via leukapheresis under research protocols approved by the Western Institutional Review Board (Olympia, WA). T cells were enriched through magnetic cell sorting (Pan T-Cell Isolation kit II; Miltenyi Biotec) and then frozen in aliquots in liquid nitrogen. Thawed cell aliquots were labeled with 3µM CellVue Burgundy (eBioscience) according to the manufacturer’s instructions.
Parental and engineered human AML cell lines
Human myeloid OCI-AML3, KG-1a, ML-1, NB4, TF-1, and HL-60 cells were maintained as previously described.12-14 Sublines of OCI-AML3 and KG-1a cells overexpressing CD33 to various degrees were generated through transduction with a pRRLsin.cPPT.MSCV lentivirus containing a wild-type human CD33–internal ribosomal entry site–enhanced green fluorescent protein (EGFP) cassette at a multiplicity of infection (MOI) of 0.25 to 100.14 Additional sublines expressing mutant CD33 (A14V, R69G, R304G) were established with lentiviral vectors via standard polymerase chain reaction cloning procedures (supplemental Table 1, available on the Blood Web site) and verified by sequencing. Sublines of HL-60, ML-1, and NB4 cells overexpressing adenosine triphosphate–binding cassette (ABC) transporter proteins were generated through transduction with a pRRLsin.cPPT.MSCV lentivirus containing either wild-type human P-glycoprotein (Pgp [ABCB1]; Pgp complementary DNA kindly provided by Susan E. Kane, Beckman Research Institute of City of Hope, Duarte, CA) or human breast cancer resistance protein (BCRP [ABCG2])15 using an internal ribosomal entry site–EGFP cassette at a MOI of 1 to 100. EGFP-positive cells were isolated by flow cytometry and recultured for further analysis.
Primary human AML cells
Frozen aliquots of Ficoll-isolated mononuclear cells from pretreatment (“diagnostic”) peripheral blood specimens (n = 2; collected via leukapheresis from patients presenting with hyperleukocytosis) or bone marrow specimens (n = 3; with 69%-89% myeloid blasts in thawed aliquots) were obtained from repositories at Fred Hutchinson Cancer Research Center. Patients provided written informed consent in accordance with the Declaration of Helsinki for the collection and use of their biospecimens for research purposes under protocols approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. Clinical data were de-identified in compliance with Health Insurance Portability and Accountability Act regulations.
Quantification of CD33 expression
CD33 expression on AML cell lines and primary AML cells was quantified by flow cytometry using a phycoerythrin (PE)– or PE-Cy7–conjugated CD33 antibody (clone P67.6; BD Biosciences).14 To identify nonviable cells, samples were stained with 4′,6-diamidino-2-phenylindole (DAPI). At least 10 000 events were acquired on a Canto flow cytometer (BD Biosciences), and DAPI− cells were analyzed using FlowJo (TreeStar).
Quantification of drug-induced cytotoxicity
For experiments with AMG 330, AML cells were incubated at 37°C (in 5% CO2 and air) in 96-well round-bottom plates (Falcon; BD Biosciences) at 5 to 10 × 103 cells per well in 225 µL of culture medium containing various concentrations of AMG 330 (kindly provided by Amgen, Amgen Research GmbH, Munich, Germany) as well as T cells at different effector to target (E:T) cell ratios. After 48 hours, cell numbers and drug-induced cytotoxicity, using DAPI to detect nonviable cells, were determined using a LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo Software. AML cells were identified by forward/side scatter properties and negativity for CellVue Burgundy dye. For experiments with other cytotoxic agents, AML cells were incubated in medium containing various concentrations of GO (commercially obtained from Pfizer) or mitoxantrone (Sigma-Aldrich) for 72 hours, after which cell numbers and drug-induced cytotoxicity was quantified by flow cytometry.
Determination of CD33 modulation
To determine CD33 modulation, aliquots of AML cells were left untreated or incubated with either AMG 330 (250 pg/mL) or unlabeled P67.6 (Santa Cruz Biotechnology; 2.5 µg/mL). After 48 hours, cells were washed in ice-cold phosphate-buffered saline (PBS; Invitrogen) to remove unbound antibody and resuspended in PBS containing 2% fetal bovine serum (FBS). As AMG 330 does not compete for binding to CD33 with P67.6, aliquots of untreated and AMG 330-treated cells were incubated with P67.6 or no primary antibody followed by biotin-conjugated rat anti-mouse immunoglobulin G1 (used at 2.5 µg/mL in PBS/2% FBS) and streptavidin-PE (used at 2.5 µg/mL in PBS/2% FBS, both from BD Biosciences). Likewise, aliquots of untreated and P67.6-treated cells were incubated with unconjugated P67.6 or without primary antibody; cells were then washed and incubated with biotin-conjugated rat anti-mouse immunoglobulin G1 monoclonal antibody followed by incubation with streptavidin-PE conjugate. Subsequently, samples were stained with DAPI and analyzed by flow cytometry.
Statistical considerations
Linear median fluorescence intensity values were used to quantify CD33 expression levels. Drug-specific cytotoxicity is presented as: % cytotoxicity = 100 × (1 – live target cellstreated/live target cellscontrol). Results are presented as mean values ± standard error of the mean (SEM). Fifty percent effective concentration (EC50) values were determined using nonlinear regression (4-parameter dose-response curve) using Prism (GraphPad).
Results
To determine the variables that may modulate the in vitro cytotoxicity of AMG 330 against human AML, we selected several human AML cell lines as well-defined model systems. Specifically, we chose OCI-AML3 and KG-1a cells because of their very low levels of endogenous cell-surface expression of CD33 and our ability to generate engineered sublines with various levels of increased CD33.14 We used HL-60, ML-1, NB4, and TF-1 cells as additional models because of their CD33 expression and, in the case of HL-60, ML-1, and NB4 cells, our previous studies showing sensitivity to CD33-targeted therapy with GO.12,13 As prior in vitro investigations of the cytotoxic effect of AMG 330 demonstrated that the T cell killing of targets increased over time,11 we first screened for activity at 24 hours. At this time point, very little specific cytotoxicity could be detected even at higher doses of AMG 330 (data not shown). Therefore, we generally exposed cells to AMG 330 for 48 hours before cytotoxic effects were quantified. Initial studies showed that AMG 330 had no effect on AML cells in the absence of T cells, confirming the absolute requirement for T cells for its cytotoxic effects. In the presence of T cells, the extent of AMG 330-induced specific cytotoxicity was dependent on the concentration of AMG 330 as well as the E:T cell ratio. In addition, these early studies indicated considerable differences in AMG 330-induced cytotoxicity depending on the donor that was chosen as the source for exogenous T cells (supplemental Figure 1, data not shown). Of note, over this 48-hour time period, we did not observe any proliferation of T cells. At a low E:T ratio, we found a higher number of dead AML cells than total T cells, indicating that a T cell, on average, was repeatedly able to effectively lyse AML cells (supplemental Figure 2).
Effect of CD33 expression and CD33 single nucleotide polymorphisms on AMG 330-induced cytotoxicity
To study whether CD33 expression might be a limiting factor for the anti-AML activity of AMG 330, we generated sublines of OCI-AML3 and KG-1a cells engineered to overexpress wild-type CD33 at various levels. As depicted in Figure 1, this set of experiments indicated a quantitative dependence of AMG 330-induced cytotoxicity at the 48-hour time point on the amount of CD33 expressed on the cell surface in both cell lines. Of note, while parental OCI-AML3 cells are sensitive to GO, parental KG-1a cells are resistant to GO, and even overexpression of CD33 does not result in significant sensitization to GO in the absence of a Pgp inhibitor (Walter et al14 and data not shown).
Effect of CD33 expression on AMG 330-induced cytotoxicity. (A) Parental OCI-AML3 and (B) parental KG-1a cells were transduced with wild-type CD33 to generate sublines that expressed CD33 at different levels, as indicated by arbitrary median fluorescence units. Parental cells and corresponding sublines were incubated with increasing concentrations of AMG 330 and various E:T cell ratios using healthy donor T cells. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results (mean ± SEM) are shown as from 4 independent experiments (for quantification of CD33 expression) or from 3 independent experiments performed in duplicate wells using a single healthy donor as source for exogenous T cells (for determination of specific cytotoxicity; qualitatively similar results were obtained with T cells from 2 other healthy donors).
Effect of CD33 expression on AMG 330-induced cytotoxicity. (A) Parental OCI-AML3 and (B) parental KG-1a cells were transduced with wild-type CD33 to generate sublines that expressed CD33 at different levels, as indicated by arbitrary median fluorescence units. Parental cells and corresponding sublines were incubated with increasing concentrations of AMG 330 and various E:T cell ratios using healthy donor T cells. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results (mean ± SEM) are shown as from 4 independent experiments (for quantification of CD33 expression) or from 3 independent experiments performed in duplicate wells using a single healthy donor as source for exogenous T cells (for determination of specific cytotoxicity; qualitatively similar results were obtained with T cells from 2 other healthy donors).
Recent studies in a relatively large cohort of AML patients identified 3 CD33 single nucleotide polymorphisms (SNPs) (A14V, R69G, R304G) in the coding region of CD33 that occurred with a minor allele frequency of >10%.16 To investigate the effect of these SNPs on AMG 330-induced cytotoxicity, we generated sublines of KG-1a cells transduced with mutant forms of CD33 corresponding to the clinically observed CD33 SNPs. When directly comparing sublines expressing equal levels of either wild-type CD33 or these CD33 SNPs, we did not detect any noticeable difference in the activity of AMG 330 (supplemental Figure 3).
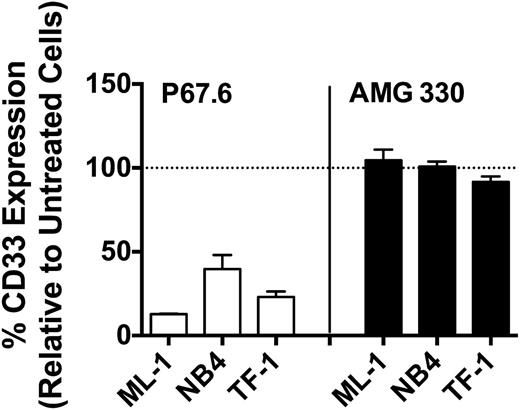
Effect of AMG 330 on CD33 modulation
It has previously been demonstrated that treatment of human AML cells with bivalent CD33 antibodies leads to a decrease (“modulation”) of CD33 cell-surface levels.17,18 Consistent with this, continuous exposure of ML-1, NB4, and TF-1 cells to the bivalent CD33 antibody, P67.6, for 48 hours resulted in significant downregulation of CD33 expression, as depicted in Figure 2. In contrast, continuous exposure of these cell lines to a high concentration of AMG 330 (250 pg/mL) for 48 hours did not result in significant changes in cell-surface display of CD33 (Figure 2). Likewise, when we exposed primary AML cells from 2 patient specimens to AMG 330 (250 pg/mL) for 48 hours, cell-surface levels remained relatively unchanged (103.3% and 92.0%, respectively, compared with untreated cells).
Drug-induced CD33 modulation. After exposure to a bivalent CD33 antibody (P67.6) or AMG 330 for 48 hours, CD33 cell-surface expression was quantified on ML-1, NB4, and TF-1 cells and compared with that of corresponding untreated control cells. Results are shown as a percentage of expression (mean ± SEM) relative to control cells from 3 independent experiments.
Drug-induced CD33 modulation. After exposure to a bivalent CD33 antibody (P67.6) or AMG 330 for 48 hours, CD33 cell-surface expression was quantified on ML-1, NB4, and TF-1 cells and compared with that of corresponding untreated control cells. Results are shown as a percentage of expression (mean ± SEM) relative to control cells from 3 independent experiments.
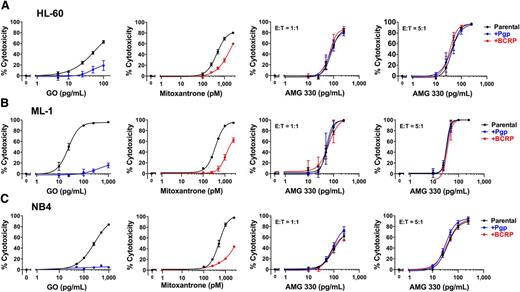
Effect of ABC transporter protein expression on AMG 330-induced cytotoxicity
To study the effect of selected ABC transporter proteins on AMG 330-induced cytotoxicity, we generated sublines of 3 drug-sensitive AML cell lines (HL-60, ML-1, and NB4) that overexpressed wild-type Pgp or BCRP. As expected based on previous studies,15,19 these sublines were more resistant to GO (in the case of Pgp-overexpressing cells) or mitoxantrone (in the case of BCRP-overexpressing cells), respectively, relative to their parental counterparts. In contrast, neither overexpression of Pgp nor BCRP altered target cell killing by AMG 330 (Figure 3).
Effect of Pgp and BCRP expression on AMG 330-induced cytotoxicity. Parental (A) HL-60, (B) ML-1, and (C) NB4 cells and sublines that were transduced with either wild-type Pgp or BCRP at a MOI of 100 were incubated with increasing concentrations of GO or mitoxantrone without healthy donor T cells or AMG 330 and various E:T cell ratios using healthy donor T cells as indicated. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results are shown as mean ± SEM from 3 independent experiments (except 2 independent experiments in the case of AMG 330 cytotoxicity assays in ML-1 cells) performed in duplicate wells using a single healthy donor as source for exogenous T cells.
Effect of Pgp and BCRP expression on AMG 330-induced cytotoxicity. Parental (A) HL-60, (B) ML-1, and (C) NB4 cells and sublines that were transduced with either wild-type Pgp or BCRP at a MOI of 100 were incubated with increasing concentrations of GO or mitoxantrone without healthy donor T cells or AMG 330 and various E:T cell ratios using healthy donor T cells as indicated. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results are shown as mean ± SEM from 3 independent experiments (except 2 independent experiments in the case of AMG 330 cytotoxicity assays in ML-1 cells) performed in duplicate wells using a single healthy donor as source for exogenous T cells.
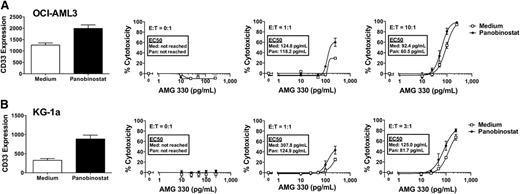
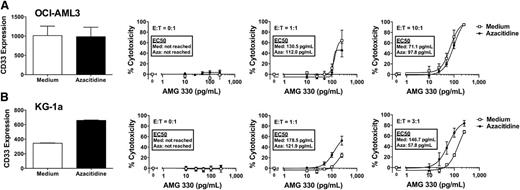
Epigenetic-modifying drugs as sensitizing agents for AMG 330-induced cytotoxicity
Given that we identified the level of CD33 expression as a critical variable for the extent of AMG 330 activity against human AML cells, we were interested in exploring the potential of epigenetic-modifying drugs such as histone deacetylase (HDAC) inhibitors or DNA methyltransferase (DNMT) I inhibitors as sensitizing agents for AMG 330-induced cytotoxicity. This interest was primarily based on the fact that these drugs can lead to cellular maturation and, as noted in previous unpublished studies in our laboratory, upregulation of CD33 expression on AML cell lines. Indeed as shown in Figure 4, 3-day pretreatment with the HDAC inhibitor, panobinostat, resulted in a significant increase in CD33 expression in OCI-AML3 and, markedly, KG-1a cells. More importantly, relative to untreated cells, cells pretreated with panobinostat for 3 days were modestly more sensitive to AMG 330-induced cytotoxicity (KG-1a > OCI-AML3). On the other hand, pretreatment with the DNMT I inhibitor, azacitidine, for 3 days resulted in a significant increase in CD33 expression on KG-1a, but not OCI-AML3, cells. Consistently, after pretreatment with azacitidine, KG-1a, but not OCI-AML3, cells became significantly more sensitive to AMG 330-induced cytotoxicity relative to untreated cells (Figure 5).
Effect of panobinostat pretreatment on CD33 expression and AMG 330-induced cytotoxicity. Parental OCI-AML3 (A) and KG-1a (B) cells were either left untreated or pretreated with panobinostat for 72 hours. Subsequently, CD33 expression was quantified, and cells treated with/without AMG 330 (0-250 pg/mL) and various E:T cell ratios using healthy donor T cells. Forty-eight hours later, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells using a single healthy donor as source for exogenous T cells.
Effect of panobinostat pretreatment on CD33 expression and AMG 330-induced cytotoxicity. Parental OCI-AML3 (A) and KG-1a (B) cells were either left untreated or pretreated with panobinostat for 72 hours. Subsequently, CD33 expression was quantified, and cells treated with/without AMG 330 (0-250 pg/mL) and various E:T cell ratios using healthy donor T cells. Forty-eight hours later, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells using a single healthy donor as source for exogenous T cells.
Effect of azacitidine pretreatment on CD33 expression and AMG 330-induced cytotoxicity. Parental OCI-AML3 (A) and KG-1a (B) cells were either left untreated or pretreated with azacitidine for 72 hours. Subsequently, CD33 expression was quantified, and cells treated with/without AMG 330 (0-250 pg/mL) and various E:T cell ratios using healthy donor T cells. Forty-eight hours later, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells using a single healthy donor as source for exogenous T cells.
Effect of azacitidine pretreatment on CD33 expression and AMG 330-induced cytotoxicity. Parental OCI-AML3 (A) and KG-1a (B) cells were either left untreated or pretreated with azacitidine for 72 hours. Subsequently, CD33 expression was quantified, and cells treated with/without AMG 330 (0-250 pg/mL) and various E:T cell ratios using healthy donor T cells. Forty-eight hours later, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells using a single healthy donor as source for exogenous T cells.
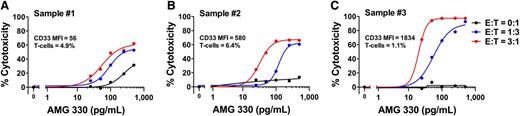
Determinants for AMG 330-induced cytotoxicity against primary human AML cells
Having identified several determinants for the activity of AMG 330 in AML cell lines, we assessed the effects of AMG 330 in a small set of primary specimens from patients with AML. For these proof-of-principle studies, we selected pretreatment specimens from 3 older adult patients (aged 61-79 years) with newly diagnosed normal karyotype AML and varying cell-surface expression levels of CD33 (CD33 median fluorescence intensity of 56, 580, and 1834, respectively). Similar to our cell line experiments, we exposed primary AML cells to various concentrations of AMG 330 in the presence or absence of exogenous T cells from 1 healthy donor for 48 hours before cytotoxic effects were quantified. As shown in Figure 6, the extent of AMG 330-induced specific cytotoxicity was dependent on the concentration of AMG 330 as well as the E:T cell ratio, consistent with our findings in AML cell lines. Furthermore, comparing the BiTE antibody’s cytotoxic effects at similar ratios of added exogenous T cells across these 3 specimens, these limited studies also suggested a dependency of AMG 330 activity on the abundance of cell-surface CD33 expressed on AML blasts.
AMG 330-induced cytotoxicity in primary human AML cells. (A-C) Pretreatment specimens from 3 adult patients with normal karyotype AML were phenotypically assessed to enumerate endogenous T cells and quantify CD33 expression on myeloid blasts. Parallel aliquots of cells were incubated with increasing concentrations of AMG 330 and various E:T cell ratios using healthy donor T cells. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Individual results for samples #1, #2, and #3 are depicted in panels A, B, and C, respectively. Mean values are shown from 1 experiment performed in duplicate wells using a single healthy donor as source for exogenous T cells.
AMG 330-induced cytotoxicity in primary human AML cells. (A-C) Pretreatment specimens from 3 adult patients with normal karyotype AML were phenotypically assessed to enumerate endogenous T cells and quantify CD33 expression on myeloid blasts. Parallel aliquots of cells were incubated with increasing concentrations of AMG 330 and various E:T cell ratios using healthy donor T cells. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Individual results for samples #1, #2, and #3 are depicted in panels A, B, and C, respectively. Mean values are shown from 1 experiment performed in duplicate wells using a single healthy donor as source for exogenous T cells.
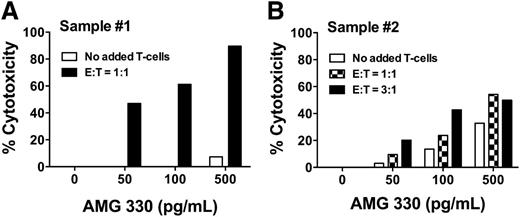
Of note, contrasting our cell line data, we found significant AMG 330-induced cytotoxicity even in the absence of exogenous T cells in 2 of the 3 AML specimens (sample 1 and, to a lesser degree, sample 2; see Figure 6), suggesting that autologous T cells can be redirected by AMG 330 to lyse AML cells, as reported previously.11 Indeed, we observed greater AMG 330-induced cytolysis of primary AML cells if autologous T cells were added (Figure 7).
Effect of autologous T cells on AMG 330-induced cytotoxicity in primary AML cells. (A-B) To test the effect of autologous T cells on AMG 330-induced cytotoxicity of primary human AML cells, frozen aliquots of pretreatment specimens from 2 AML patients were used; both patients presented with hyperleukocytosis, and research specimens were obtained via leukapheresis. One portion of the aliquot was labeled with CellVue Burgundy, and lymphocytes were isolated by fluorescence-activated cell sorting based on CD45 and side scatter properties. A second portion of the aliquot was left untreated and subsequently incubated with increasing concentrations of AMG 330 in the absence or presence of additional isolated autologous T cells in defined E:T cell ratios. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Individual results for Samples #1 and #2 are shown as mean values from 1 experiment performed in duplicate wells, in panels A and B, respectively.
Effect of autologous T cells on AMG 330-induced cytotoxicity in primary AML cells. (A-B) To test the effect of autologous T cells on AMG 330-induced cytotoxicity of primary human AML cells, frozen aliquots of pretreatment specimens from 2 AML patients were used; both patients presented with hyperleukocytosis, and research specimens were obtained via leukapheresis. One portion of the aliquot was labeled with CellVue Burgundy, and lymphocytes were isolated by fluorescence-activated cell sorting based on CD45 and side scatter properties. A second portion of the aliquot was left untreated and subsequently incubated with increasing concentrations of AMG 330 in the absence or presence of additional isolated autologous T cells in defined E:T cell ratios. After 48 hours, cell counts were determined and cytotoxicity was assessed with DAPI staining to quantify drug-specific cytotoxicity. Individual results for Samples #1 and #2 are shown as mean values from 1 experiment performed in duplicate wells, in panels A and B, respectively.
Discussion
The impetus to pursue CD33 as therapeutic target emanated from the notion that some leukemias may predominantly or entirely involve only committed CD33+ myeloid precursors. Specifically, early studies of glucose-6-phosphate dehydrogenase–based X-chromosome inactivation patterns found that clonal dominance was limited to granulocytes and monocytes in some leukemias,20,21 suggesting that mutations leading to leukemic transformation and clonal expansion were acquired only at the level of committed CD33+ myeloid precursors. Indeed, in some of these leukemias, removal of CD33+ cells yielded colony-forming cells with X-chromosome inactivation patterns in a ratio consistent with a nonclonal origin,22,23 indicating that CD33− precursors could be predominantly or completely normal. These data raised the possibility that CD33-targeted therapy could eradicate leukemic stem cells in some AMLs.1,2 In other leukemias, clonal dominance was found in multiple cell lineages, reflecting origination and expansion at the level of pluripotent CD33− hematopoietic stem cells.20,21 However, it remains unclear whether, and to what degree, AML stem cells express CD33, with some data from xenotransplantation studies implying that these cells are CD33+ in most cases.24 Nevertheless, the clinical experience with GO has demonstrated both the value and potential limitations of CD33 antibody-drug conjugates for the treatment of human AML, thus providing the rationale for the development of CD33-directed therapies that use alternative modes of action such as engagement of cytotoxic T cells for elimination of AML cells.
Initial explorations with AMG 330 have indicated potent T-cell–dependent cytolytic activity of this agent against human AML cells ex vivo.11 Our studies significantly extend these findings by providing detailed insight into the cellular characteristics that are critical for the activity of AMG 330. The results presented in this article support 4 major conclusions. First, AMG 330 is highly active against human AML cell lines and primary AML cells at extremely low concentrations (<1 ng/mL). The drug’s activity increases in a dose- and E:T cell ratio–dependent manner and is also proportional to the amount of CD33 expressed on AML cells, identifying CD33 expression as a potential limiting factor for AMG 330 activity. In contrast, the cytolytic activity of AMG 330 is not impacted by the expression of commonly occurring CD33 SNPs. Second, expression of ABC transporter proteins, which is associated with reduced cellular sensitivity to common conventional anti-AML therapeutics as well as GO, does not alter AMG 330 cytotoxicity. Third, unlike bivalent CD33 antibodies, AMG 330 does not lead to decreased cell-surface expression of CD33 over time even after prolonged continuous exposure to AMG 330. And fourth, epigenetic modifier drugs such as panobinostat and azacitidine, which upregulate CD33 expression on some AML cell lines, can augment AMG 330 cytotoxicity. Together, these studies identify important cellular characteristics and principles that may be relevant for the clinical use of AMG 330 as an anti-AML therapeutic.
Approximately 85% to 90% of AML patients have CD33+ disease, as defined by the presence of CD33 on >20% to 25% of the leukemic blasts.25,26 Recent studies have confirmed that the amount of CD33 expressed on leukemic blasts varies significantly (several hundredfold) between individual AML patients.27 Importantly, CD33 expression levels have been associated with prognostic risk factors such as cytogenetic and molecular abnormalities.27 Thus, rather than primarily relying on AML patient specimens to quantify the effect of CD33 on AMG 330-induced cytotoxicity, we took advantage of a well-controlled model system consisting of human AML cell lines expressing low levels of endogenous CD33 and corresponding sublines virally transduced to overexpress wild-type CD33 at various levels. Using this approach, our findings in engineered cell lines unequivocally demonstrate a quantitative relationship between CD33 expression and AMG 330-induced cytotoxicity. Our limited studies in specimens from patients with untreated AML that we selected based on similar (normal) cytogenetics corroborate this dependency of AMG 330 activity on CD33 expression levels, although more comprehensive studies will ultimately be required to conclusively test this relationship in primary human AML cells and determine the impact of potential confounding factors such as cytogenetic/molecular abnormalities, disease stage, etc. Not unexpectedly, the association between AMG 330 activity and CD33 expression levels in our AML cell line experiments was most evident in “suboptimal” conditions, that is, at submaximal doses of AMG 330 administered over a relatively short period of time and in conjunction with lower E:T cell ratios. Nevertheless, this observation suggests the possibility that AMLs, or subpopulations of AML cells within any given leukemia, with lower surface display of CD33 may be less susceptible to AMG 330 if target saturation is low (eg, in the presence of a high CD33+ tumor burden28 ), and may require higher drug concentrations, longer exposure to this drug, or initial debulking of CD33+ cells. Of course, future studies will need to determine whether clinical susceptibility to AMG 330 can be attributed to the debulking of more mature CD33+ AML cells or to the elimination of underlying CD33+ AML stem cells. In contrast to the cell-surface level of CD33, expression of CD33 SNPs did not significantly impact AMG 330-mediated cytolysis. This finding is encouraging given that several CD33 SNPs occur relatively commonly in AML patients and may impact the efficacy of CD33-targeted therapeutics, as recently suggested by analyses of pediatric patients treated with GO-containing multiagent chemotherapy.16
It is well established that ABC transporter activity, in particular mediated by Pgp, is associated with poor prognosis and failure of conventional chemotherapeutics in AML.29,30 Several studies have also demonstrated that expression of Pgp is a major factor contributing to clinical resistance to GO.31,32 Moreover, besides being present on normal hematopoietic stem cells, increasing evidence links ABC drug transporters to protection of malignant stem cells underlying AML and other human cancers from chemotherapeutic agents.33 In that respect, some interest has focused on another ABC transporter, BCRP, which has been found most highly expressed in subpopulations of leukemic cells likely to contain putative AML stem cells.34 Our studies suggest that the anti-AML activity of AMG 330 is neither affected by expression of Pgp nor BCRP or, by extrapolation, perhaps other ABC transporter proteins. This observation implies that AMG 330 may have a much broader activity than GO and may maintain activity even in multidrug-resistant cases where many salvage therapies including GO are of limited benefit. This lack of cross-resistance between AMG 330 and other AML therapeutics that are susceptible to ABC transporter-mediated drug efflux suggests that prior resistance to conventional AML therapy may be a poor predictor of clinical susceptibility to AMG 330, and further evaluation of parameters that correlate with clinical response or resistance will be vital to identify patients that are most suitable for AMG 330-based treatment.
It has been a longstanding observation that, when engaged with bivalent CD33 antibodies, CD33 is internalized and cell-surface levels are modulated.17,18 Although internalization of CD33 is a requirement for the activity of CD33-directed antibody-drug conjugates such as GO,14 removal of CD33 from the cell surface and/or internalization of AMG 330/CD33 complexes could theoretically limit this BiTE antibody’s anti-AML activity by reducing available target binding sites and/or preventing engagement of T cells. It is therefore reassuring that we found no evidence that exposure to relevant doses of AMG 330, that is, doses that effectively lead to complete cytolysis in the presence of T cells, reduces the cell-surface CD33 display on AML cells. This finding indicates that monovalent anti-CD33 constructs such as AMG 330 do not lead to significant target antigen modulation. Similar observations have been made for other cell-surface antigens, for example transferrin receptors35 or Fc receptors (for which rapid recycling between the cell surface and a nonlysosomal endocytic compartment has been described for a monovalent antibody fragment when bound to the receptor),36 and have suggested that ligand valency determines the intracellular fate of antibody/antigen complexes. By extrapolation, our data suggest that prolonged exposure to AMG 330 is feasible from a target expression perspective, and might provide the most efficient way of directing CD33+ AML cells to T-cell–mediated destruction with this BiTE antibody.
Although our studies suggest that AMG 330 is highly active against human AML cells, there is interest in understanding how best to combine this BiTE antibody with other anti-AML chemotherapeutics. To begin developing rational approaches of combinational therapy, we have investigated the possible role of epigenetic-modifying agents, primarily because of the potential to modulate CD33 expression on AML cells. Indeed, our cell-line studies indicate that AML cells can be sensitized to AMG 330 with the use of such agents, perhaps at least partly because of an associated increase in cell-surface display of CD33. Our findings are in line with similar preclinical studies, in which both HDAC inhibitors and DNMT I inhibitors have been shown to sensitize AML cells to CD33-directed therapy with GO.37-39 More importantly, both HDAC inhibitors (especially when used in combination with other agents) and DNMT I inhibitors have shown encouraging results and safety in patients with AML.40-43 Thus, our observation of a chemosensitizing effect of epigenetic-modifying drugs on AMG 330-induced cytotoxicity may be directly translatable to the clinic.
In summary, our data show that AMG 330 causes potent cytolysis in vitro against human AML cells that is proportional to the level of cell-surface CD33 expression. As AMG 330’s activity is not affected by ABC transporter activity nor does the agent lead to decreased CD33 expression after prolonged drug exposure, our findings suggest that AMG 330 may overcome important limitations of previous CD33-targeted therapeutics, including the antibody-drug conjugate, GO. Our observations indicate that AMG 330 may be broadly active against AML cells and may deliver cytotoxic effects even in cells that feature resistance to commonly used AML therapeutics. Together with our observation that AMG 330 cytotoxicity is amenable to modulation and augmentation by clinically available drugs such as HDAC inhibitors or DNMT I inhibitors, these data support our conclusion that AMG 330 should be further explored for the treatment of patients with AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The term BiTE is a registered trademark of Amgen, Inc.
This work was supported by a research contract from Amgen, Inc. (R.B.W.) and by grant P30-CA015704-35S6 (R.B.W.) from the National Institutes of Health, National Cancer Institute (Bethesda, MD).
Authorship
Contribution: G.S.L. and R.B.W. designed research, analyzed and interpreted data, and wrote the manuscript; C.J.G. and K.H.H. performed research, analyzed and interpreted data, and wrote the manuscript; and J.D., K.J.N., G.D.M., A.M.S., R.K., and S.R.F. provided vital reagents, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: J.D., K.J.N., G.D.M., A.M.S., R.K., and S.R.F. are employees of Amgen, Inc. The remaining authors declare no competing financial interests.
Correspondence: Roland B. Walter, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-190, Seattle, WA 98109-1024; e-mail: rwalter@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal