Key Points
The small molecule Me6TREN is a new potent and efficacious mobilizing agent of HSPCs and works more effectively than G-CSF or AMD3100.
Me6 mobilizes murine HSPCs and functions by upregulating MMP-9 expression and disrupting the SDF-1α/CXCR4 axis.
Abstract
Mobilization of hematopoietic stem and progenitor cells (HSPCs) from bone marrow into the blood circulation has been widely used for hematopoietic transplantation. However, the current methods of cytokine- or small-molecule–stimulated HSPC mobilization are far from satisfactory. New mobilizing agents are needed to increase the number of stem cells in peripheral blood for effective reconstitution of hematopoiesis. Here, we report that the molecule Me6TREN (Me6) can induce rapid mobilization of hematopoietic progenitor cells and that Me6 exhibits more significant effects than granulocyte colony-stimulating factor (G-CSF) or AMD3100. Me6 also mobilizes long-term repopulating cells, which successfully engraft and expand in a multilineage fashion in primary and secondary transplant recipients. Mechanistically, Me6 inhibits both the SDF-1α–induced migration and VLA-4–mediated adhesion of mouse and human hematopoietic cells. Me6 appears to mobilize HSPCs by activating MMP-9 expression and disrupting the SDF-1α/CXCR4 axis. Therefore, Me6 may become a new potent and efficacious mobilizing agent of HSPCs.
Introduction
The transplantation of hematopoietic stem and progenitor cells (HSPCs) has been used to treat various diseases, including malignant and nonmalignant hematologic conditions, immunodeficiencies, and metabolic disorders. The infused HSPCs can replace and reconstitute the hematopoietic and immune systems, rescuing patients from the effects of chemotherapy or radiotherapy. Thus, harvesting sufficient HSPCs is important for autologous or allogeneic stem cell transplantation.1,2
Adult HSPCs reside within a unique bone marrow (BM) microenvironment. BM is the primary source of HSPCs. Recently, peripheral blood (PB) has replaced BM as the preferred source of HSPCs in the stem cell transplants. However, under steady-state conditions, the number of HSPCs circulating in the PB is insufficient for efficient hematopoietic and immune system reconstitution. Approaches for mobilizing HSPCs from BM into the blood circulation are clinically important because more cells are needed for transplantation. The SDF-1α/CXCR4 chemotactic axis and the VCAM-1/VLA-4 adhesive axis play pivotal roles in HSPC mobilization.3,4 HSPC egress from the BM microenvironment can be stimulated by the CXCR4 antagonists and agonists, small-peptide analogs of SDF-1α, small-molecule inhibitors of VLA-4, or antibodies against VLA-4.5-9 In addition, granulocyte colony-stimulating factor (G-CSF) and other hematopoietic cytokines also mobilize HSPCs, mainly through the suppression of SDF-1α production.10 Some proteases, such as neutrophil elastase, matrix metalloproteinase-9 (MMP-9), and CD26, are involved in HSPC mobilization by cleaving and inactivating the adhesion molecule VCAM-1 or the chemokine SDF-1α.11-15 Disruption of the SDF-1α/CXCR4 or the VCAM-1/VLA-4 axis contributes to the HSPC mobilization.
Currently, G-CSF is the most commonly used mobilization agent in clinical transplantation. However, for optimal effect, G-CSF requires repeated dosing, which is often associated with side effects. Moreover, some patients or normal donors respond poorly to G-CSF.16 Among other agents capable of mobilizing HSPCs, plerixafor (AMD3100) is the only molecule used for patients with non-Hodgkin lymphoma and multiple myeloma that has been approved by the US Food and Drug Administration (FDA).17,18 Clinical studies have shown that plerixafor may be a more rapid and less toxic alternative to traditional G-CSF–based mobilization.19 However, the mobilization effect of plerixafor alone is modest,20 and nearly one third of healthy donors mobilized with plerixafor require more than one apheresis to obtain the maximal number of CD34+ cells. More robust mobilizing agents are required to optimize the autologous and allogeneic HSPC transplants.
Here we report that, in mouse models, the small molecule Tris[2-(dimethylamino)ethyl]amine) (Me6TREN; hereafter Me6) can mobilize HSPCs effectively and rapidly. Me6 mobilizes long-term repopulating cells in primary and secondary transplant recipients, suggesting that Me6 can be used as a novel HSPC mobilizing agent. The HSPC mobilization mechanism of Me6 involves the activation of MMP-9 expression and the perturbation of the SDF-1α/CXCR4 axis.
Methods
Treatment with mobilizers
For time-effect analysis, mice were subcutaneously injected with 2.5 mg/kg Me6 and were euthanized at 0, 2, 4, 12, 24, 48, 72, and 96 hours after injection. For dose-effect analysis, various doses of Me6 (0, 2.5, 5, and 10 mg/kg) were administered in 100 μL phosphate-buffered saline (PBS) and PB was collected 12 hours after injection. AMD3100 was injected at a dose of 5 mg/kg, and PB was collected 1 hour later. G-CSF was injected at a dose of 2.5 µg per mouse twice a day for 4 days, and PB was harvested 20 hours after the last injection. Control mice were injected with PBS. In the G-CSF and Me6 combination experiments, the mice were injected with 5 mg/kg Me6 at 8 hours after the last injection of G-CSF, and PB was collected 12 hours after injection. For the G-CSF and AMD3100 combination, the mice were given 5 mg/kg AMD3100 at 19 hours after the last injection of G-CSF, and PB was harvested 1 hour later. The study was approved by the Animal Care and Use Committee of the Animal Center at the Academy of Military Medical Science (Beijing, China).
Flow cytometric analysis
For blood cell lineage detection, the cells were stained with antibodies against CD3, CD11b, Ter-119, B220, Ly-6G, or matched isotype controls (eBioscience). Multicolor flow cytometry panels were used to determine the following cell subpopulations: T cells, CD3+/CD19− (CD3+/CD8+; CD3+/CD4+)21 ; B cells,22 CD3−/CD19+; myeloid dendritic cells,23,24 CD11chighCD11b+B220−; plasmacytoid dendritic cells,24-26 CD11cdimCD11b−B220+PDCA-1+. For HSPC analysis, these cells were stained with allophycocyanin (APC)–Cy7- or APC-labeled lineage antibodies (including CD4, CD11b, Ter-119, B220, Ly-6G, CD127, CD8a), APC- or phycoerythrin (PE) -conjugated anti–c-Kit antibody and PerCP-Cy5.5- or PE-Cy7–conjugated anti–Sca-1 antibodies (eBioscience). Apoptosis was measured by using propidium iodide and Annexin-V staining. For detection of engraftment and chimerism, blood cells were stained with PE or fluorescein isothiocyanate–labeled antibodies against CD45.1 or CD45.2 (eBioscience). To examine the CXCR4 expression on mobilized cells (Lin−Sca-1+c-Kit+ [LSK]) following Me6, G-CSF, or AMD3100 administration, blood cells were suspended in PBS and incubated with APC-Cy7–labeled lineage antibodies, PerCP-Cy5.5–conjugated anti–Sca-1, APC-conjugated anti–c-Kit, and AF488-conjugated anti-CXCR4 antibodies for 30 minutes. Immunostained cells were analyzed by flow cytometry.
Transplantation experiments
For noncompetitive transplantation experiments, C57BL/6 donor mice were injected with 5 mg/kg Me6 or 5 mg/kg AMD3100. PB was collected after 12 hours from Me6-treated mice and after 1 hour from AMD3100-treated mice. Mononuclear cells (MNCs) were isolated from 1.5 mL of blood and transplanted into individual recipient C57BL/6 mice that were lethally irradiated (800 cGy) before intravenous injection of the cells. Recipient mice that were inoculated with the cells obtained from the blood of PBS-treated mice or with normal BM MNCs (5 × 106 cells per mouse) were used as controls. The survival times of the mice were monitored for 4 months. For competitive transplantation experiments, lethally irradiated C57BL/6 mice (CD45.1) received MNCs isolated from 2.0 mL PB from mice (CD45.2) that had received different treatments. Blood was collected from CD45.2 mice treated with Me6 for 12 hours or AMD3100 for 1 hour. Recipient mice were also given 5 × 105 congenic BM competitor cells (CD45.1). At 2 and 6 months after transplantation, the numbers of CD45.1+ and CD45.2+ cells were detected by flow cytometry. At 6 months after transplantation, the multilineage reconstitution capacity of donor cells was analyzed by flow cytometry. For secondary repopulation, 5 × 106 BM MNCs from primary repopulation mice were transplanted into lethally irradiated (950 cGy) CD45.1 mice. PB was harvested 6 months later, and the CD45.2:CD45.1 ratio was measured.
Statistical analysis
The results are expressed as the mean ± standard deviation. The paired t test was used for determination of statistical significance between two groups; one-way or two-way analysis of variance was used to compare means among three or more independent groups. P values less than .05 were considered to be statistically significant.
Results
Screening for small molecules that can mobilize Lin−Sca-1+ cells
To obtain new chemical compounds with the capacity to mobilize HSPCs, we developed an assay that used collected PB from mice that were dosed with different chemicals and evaluated cell markers by flow cytometry. Using this assay, we found that mice injected with the Me6 chemical compound (Figure 1A) had an increased number of Lin−Sca-1+ cells in the PB (supplemental Figure 1 as found on the Blood Web site). These results suggest that Me6 may be a novel HSPC mobilizing agent. By performing in vitro cytotoxicity experiments, we found that Me6 showed no significant cytotoxic effect on primary human fibroblasts and Jurkat T cells (supplemental Figure 2A-B), and there was no obvious difference in the apoptosis rate of PB MNCs between PBS- and Me6-treated mice (supplemental Figure 2C). Acute toxicity test results showed that Me6 had a median lethal dose (LD50) value of 1.11 g/kg in mice, suggesting a very low toxicity in vivo.
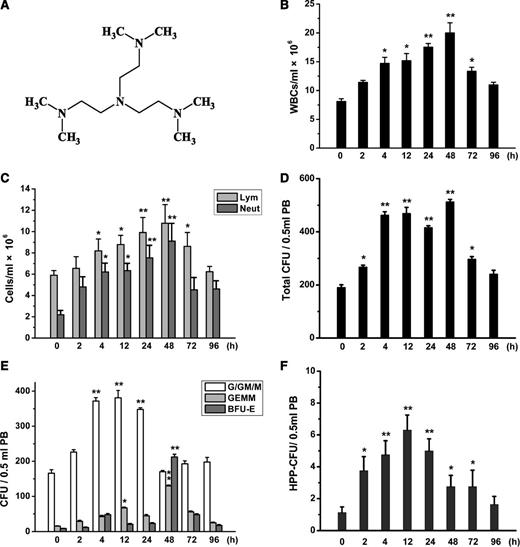
Time-dependent mobilization of WBCs and HPCs by Me6. (A) Chemical structure of Me6. (B) Time-dependent mobilization of WBCs, especially (C) neutrophils (Neut) and lymphocytes (Lym) in PB of mice injected with 2.5 mg/kg Me6. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-hours group. (D) Time-response analysis of total CFUs, including (E) CFU-G/GM/M, CFU-GEMM, BFU-E, and (F) HPP-CFU in PB of C57BL/6 mice injected with 2.5 mg/kg Me6. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-hours group.
Time-dependent mobilization of WBCs and HPCs by Me6. (A) Chemical structure of Me6. (B) Time-dependent mobilization of WBCs, especially (C) neutrophils (Neut) and lymphocytes (Lym) in PB of mice injected with 2.5 mg/kg Me6. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-hours group. (D) Time-response analysis of total CFUs, including (E) CFU-G/GM/M, CFU-GEMM, BFU-E, and (F) HPP-CFU in PB of C57BL/6 mice injected with 2.5 mg/kg Me6. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-hours group.
Time-response analysis of the effects of Me6 on WBC and hematopoietic progenitor cell mobilization
To determine the onset time of the mobilization of BM into PB by Me6, C57BL/6 mice were treated with 2.5 mg/kg of Me6 for different times. Hemogram analysis showed an increase in peripheral white blood cells (WBCs), neutrophils, and lymphocytes starting at 2 hours after Me6 injection, which remained elevated for 2 hours to 4 days (Figure 1B-C). Therefore, a single administration of Me6 was sufficient for rapid and long-lasting increase in WBCs, neutrophils, and lymphocytes.
Because HSPC mobilization appears to correlate with leukocytosis, we then used colony-forming unit (CFU) assays to evaluate the ability of Me6 to mobilize hematopoietic progenitor cells (HPCs) and analyzed the time-effect relationship between Me6 administration and HPC mobilization. MNCs from the PB of the Me6-injected mice (2.5 mg/kg) were subjected to CFU assay. The total CFUs increased at 2 hours and persisted at high levels from 4 to 48 hours after injection (Figure 1D). The number of CFU-granulocyte/granulocyte-macrophage/megakaryocyte (CFU-G/GM/M) also increased at 2 hours and peaked at 12 hours (Figure 1E). The CFU-G/GM/M number remained high until 24 hours and then gradually decreased. The mobilization effects on CFU-granulocyte, erythrocyte, megakaryocyte, macrophage (CFU-GEMM) by Me6 reached a high level at 12 hours (4.41-fold increase) and peaked at an 8.5-fold increased mobilization at 48 hours (Figure 1E). The pattern of burst-forming unit-erythroid (BFU-E) differed from that of the other cell types, increasing 24.64-fold at 48 hours compared with the controls (Figure 1E). We also measured the kinetics of the high proliferative potential (HPP)-CFUs of PB from Me6-treated mice. The peak mobilization of HPP-CFUs occurred at 12 hours after injection, with an approximately 5.6-fold increase compared with the controls (Figure 1F).
Dose-response analysis of Me6 on WBC and HPC mobilization
To detect the dose-response of Me6 on mobilization, C57BL/6 mice were injected with PBS and 2.5, 5, and 10 mg/kg Me6. Increases in total WBC, neutrophil, and lymphocyte counts in PB were observed in response to all of these doses (Figure 2A-B). The maximal mobilization of WBCs occurred at a dose of 5 mg/kg of Me6. In addition, Me6 led to notable mobilization of B cells and T cells (CD4+ and CD8+ T cells) (supplemental Figure 3A-C). Dendritic cell (myeloid dendritic cell and plasmacytoid dendritic cell) precursors were not significantly mobilized into the blood by Me6 (supplemental Figure 3D).
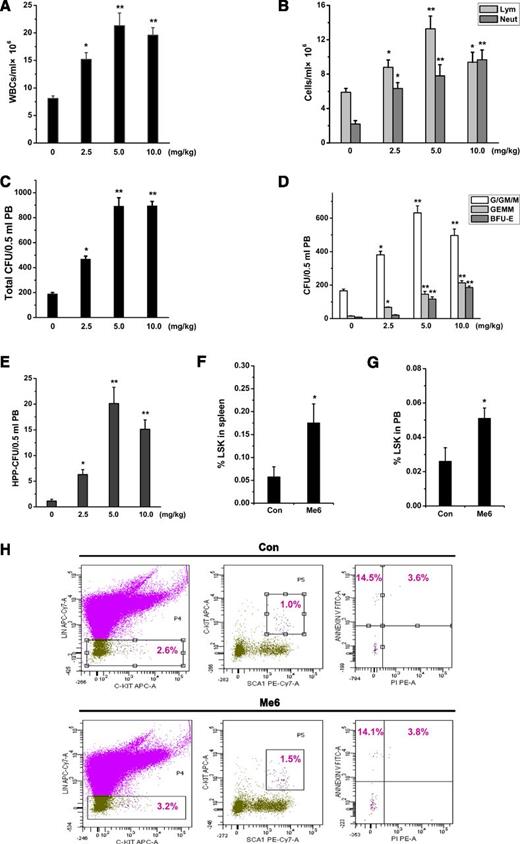
Dose-response effect of Me6 on the mobilization of HPCs into the blood. (A) Mobilization of WBCs, especially (B) neutrophils and lymphocytes induced by the administration of Me6 at different doses. C57BL/6 mice were injected subcutaneously with 0, 2.5, 5, or 10 mg/kg of Me6. Twelve hours after injection, the mice were euthanized and PB cells were obtained. The blood cells were counted by using a hemocytometer. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-mg/kg group. (C) Dose-response analysis of total CFUs, including (D) CFU-G/GM/M, CFU-GEMM, BFU-E, and (E) HPP-CFU in PB of C57BL/6 mice 12 hours after subcutaneous injection using 0, 2.5, 5, or 10 mg/kg Me6. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-mg/kg group. PB cells were obtained and evaluated by CFU assay. (F-G) The percentage analysis of LSK cells in PB and spleen of mice at 12 hours after administration of 5 mg/kg Me6 or PBS. *P < .05 vs the control group. (H) Apoptosis phenotype of PB LSK cells was assessed by using propidium iodide and Annexin-V staining. Mice were subcutaneously injected with 5 mg/kg Me6 and were euthanized 12 hours after injection. *P < .05 vs the control group. Con: control.
Dose-response effect of Me6 on the mobilization of HPCs into the blood. (A) Mobilization of WBCs, especially (B) neutrophils and lymphocytes induced by the administration of Me6 at different doses. C57BL/6 mice were injected subcutaneously with 0, 2.5, 5, or 10 mg/kg of Me6. Twelve hours after injection, the mice were euthanized and PB cells were obtained. The blood cells were counted by using a hemocytometer. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-mg/kg group. (C) Dose-response analysis of total CFUs, including (D) CFU-G/GM/M, CFU-GEMM, BFU-E, and (E) HPP-CFU in PB of C57BL/6 mice 12 hours after subcutaneous injection using 0, 2.5, 5, or 10 mg/kg Me6. n = 6 mice per group. *P < .05 and **P < .01 vs the 0-mg/kg group. PB cells were obtained and evaluated by CFU assay. (F-G) The percentage analysis of LSK cells in PB and spleen of mice at 12 hours after administration of 5 mg/kg Me6 or PBS. *P < .05 vs the control group. (H) Apoptosis phenotype of PB LSK cells was assessed by using propidium iodide and Annexin-V staining. Mice were subcutaneously injected with 5 mg/kg Me6 and were euthanized 12 hours after injection. *P < .05 vs the control group. Con: control.
We next assayed the number of CFUs in mice treated with different doses of Me6. Me6 at 5 mg/kg caused the maximal mobilization of total CFUs and CFU-G/GM/M (Figure 2C-D). At a dose of 10 mg/kg, we observed an increase in CFU-GEMM and BFU-E (Figure 2D) of approximately 9.52- and 13.54-fold, respectively. Me6 at 15 mg/kg did not exhibit a greater effect on HPC mobilization than the doses of 5 and 10 mg/kg (supplemental Figure 4A-D). The HPP-CFU assay showed that the maximal mobilization of primitive HPCs occurred at a dose of 5 mg/kg (17.89-fold; Figure 2E).
We then determined the HSPC number in PB and spleens of mice treated with 5 mg/kg Me6. HSPC subpopulations were defined by using cell surface markers to identify LSK cells. Me6 treatment resulted in a significant increase in LSK cells in PB and spleens 12 hours after injection (Figure 2F-G). However, LSK cells from Me6-injected and control mice showed no significant difference in apoptosis (Figure 2H).
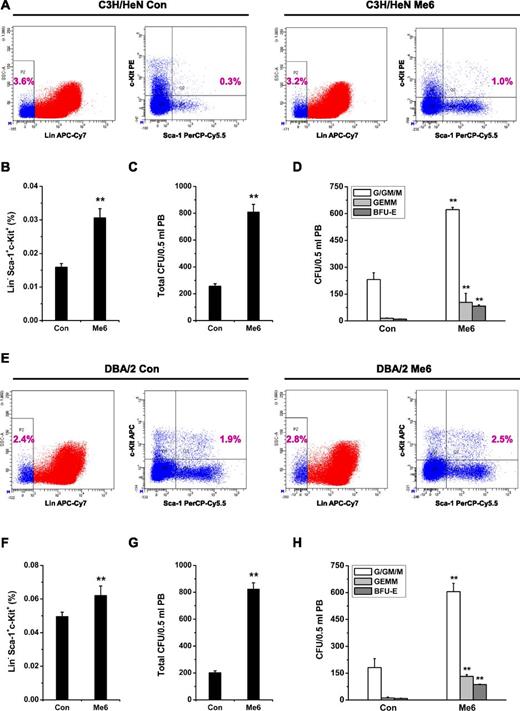
To evaluate whether the mobilization of HSPCs induced by Me6 is a generalizable effect, we performed the similar experiments in 2 more murine strains besides C57BL/6 (ie, C3H/HeN and DBA/2). The results showed that Me6 caused significant mobilization effects on LSK cells and CFU numbers in these two strains (Figure 3A-F).
Me6-induced mobilization of HSPCs in C3H/HeN and DBA/2 mouse strains. (A-B) Representative flow cytometry plots and percentage of LSK in PB of C3H/HeN mice subcutaneously injected with 5 mg/kg Me6. (C-D) Analysis of (C) total CFUs, and (D) CFU-G/GM/M, CFU-GEMM, and BFU-E in PB of C3H/HeN mice 12 hours after subcutaneous injection using 5 mg/kg Me6. (E-F) DBA/2 mice were injected with 5 mg/kg Me6 and PB was harvested after 12 hours for LSK analysis. (G-H) Total CFUs, CFU-G/GM/M, CFU-GEMM, and BFU-E in PB of DBA/2 mice 12 hours after Me6 treatment. n = 8 mice per group. **P < .01 vs the control group.
Me6-induced mobilization of HSPCs in C3H/HeN and DBA/2 mouse strains. (A-B) Representative flow cytometry plots and percentage of LSK in PB of C3H/HeN mice subcutaneously injected with 5 mg/kg Me6. (C-D) Analysis of (C) total CFUs, and (D) CFU-G/GM/M, CFU-GEMM, and BFU-E in PB of C3H/HeN mice 12 hours after subcutaneous injection using 5 mg/kg Me6. (E-F) DBA/2 mice were injected with 5 mg/kg Me6 and PB was harvested after 12 hours for LSK analysis. (G-H) Total CFUs, CFU-G/GM/M, CFU-GEMM, and BFU-E in PB of DBA/2 mice 12 hours after Me6 treatment. n = 8 mice per group. **P < .01 vs the control group.
Me6 synergizes with G-CSF to mobilize HPCs
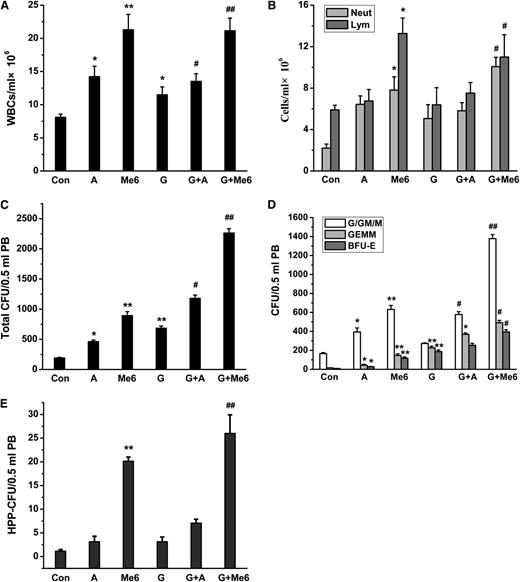
To compare the effect of Me6 on WBC and HPC mobilization when used together with AMD3100 and G-CSF, each agent was injected into the mice separately. PB was collected at 1 hour after administering AMD3100 (5 mg/kg), 12 hours after administering Me6 (5 mg/kg), or 20 hours after the last injection of G-CSF (2.5 µg per mouse twice a day for 4 days). We observed a greater mobilization of WBCs, neutrophils, and lymphocytes after Me6 administration alone compared with G-CSF or AMD3100 alone (Figure 4A-B). The mobilization of neutrophils was maximized with a combination of G-CSF and Me6 (Figure 4B). Moreover, Me6 alone produced more CFUs (especially CFU-G/GM/M) and HPP-CFU than G-CSF or AMD3100 alone (Figure 4C-E). The combination of Me6 and G-CSF produced a highly synergistic effect resulting in the mobilization of HPP-CFUs and total CFUs, including CFU-GEMM, CFU-G/GM/M, and BFU-E (32.20-, 8.30-, and 45.68-fold, respectively).
WBC and HPC mobilization effect in PB of mice injected with G-CSF, plerixafor, or Me6, alone or in combination. (A) WBCs, including (B) neutrophils and lymphocytes in the blood were measured by using a hemocytometer. (C) Total CFUs, including (D) CFU-G/GM/M, CFU-GEMM, BFU-E, and (E) HPP-CFU in PB were analyzed. Plerixafor, Me6, or G-CSF was injected into C57BL/6 mice alone or in combination. For the combination treatment, C57BL/6 mice were first injected subcutaneously with G-CSF (2.5 µg per mouse) twice a day for 4 days. Eight or 19 hours after the last injection of G-CSF, the mice were injected with 5 mg/kg of either Me6 or plerixafor; PB was harvested after 20 hours for G-CSF–treated mice, 1 hour after injection for plerixafor-treated mice, and 12 hours after injection for Me6-treated mice. n = 6 mice per group. *P < .05 and **P < .01 vs the control group; #P < .05 and ##P < .01 vs the G-CSF group. A, plerixafor; G, G-CSF; M, Me6.
WBC and HPC mobilization effect in PB of mice injected with G-CSF, plerixafor, or Me6, alone or in combination. (A) WBCs, including (B) neutrophils and lymphocytes in the blood were measured by using a hemocytometer. (C) Total CFUs, including (D) CFU-G/GM/M, CFU-GEMM, BFU-E, and (E) HPP-CFU in PB were analyzed. Plerixafor, Me6, or G-CSF was injected into C57BL/6 mice alone or in combination. For the combination treatment, C57BL/6 mice were first injected subcutaneously with G-CSF (2.5 µg per mouse) twice a day for 4 days. Eight or 19 hours after the last injection of G-CSF, the mice were injected with 5 mg/kg of either Me6 or plerixafor; PB was harvested after 20 hours for G-CSF–treated mice, 1 hour after injection for plerixafor-treated mice, and 12 hours after injection for Me6-treated mice. n = 6 mice per group. *P < .05 and **P < .01 vs the control group; #P < .05 and ##P < .01 vs the G-CSF group. A, plerixafor; G, G-CSF; M, Me6.
Me6 mobilizes HSCs with long-term repopulating capacity
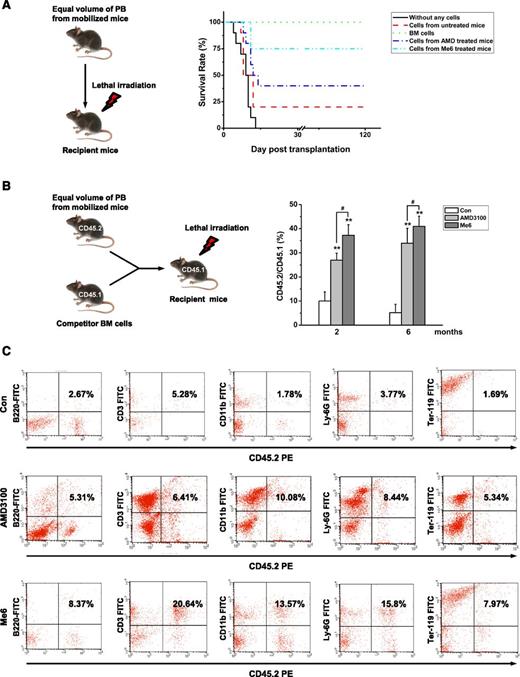
To further determine whether Me6 mobilizes long-term repopulating cells, lethally irradiated C57BL/6 recipients were transplanted with PB from C57BL/6 donors administered Me6, AMD3100, or PBS. The cell-transplanted mice were observed for at least 4 months. All irradiated mice that did not receive cell transplantation died within 14 days, and 100% of mice that were transplanted with BM cells survived. The survival rate of mice transplanted with Me6-mobilized blood was significantly higher than that of mice receiving AMD3100 (75% vs 40%; Figure 5A). These results suggest that Me6-mobilized HSPCs were much more effective in the long-term rescue of irradiated donors.
Me6 mobilizes HSCs with long-term repopulating capacity. (A) Survival rate analysis of the recipient C57BL/6 mice that were transplanted with PB MNCs of donor C57BL/6 mice subjected to different treatments. The C57BL/6 donor mice were injected with PBS, 5 mg/kg Me6, or 5 mg/kg plerixafor (AMD; AMD3100). PB was collected after 12 hours for Me6-treated mice and after 1 hour for plerixafor-treated mice and transplanted into recipient mice that were lethally irradiated (800 cGy) before intravenous injection of the cells. The cells that were obtained from 1.5 mL of blood were transplanted into individual recipient mice. As a control, one group of recipient mice received cells that were obtained from normal BM, and another group of mice did not receive any cells. n = 10 mice per group. (B-C) Competitive repopulation assay. Lethally irradiated C57BL/6 mice (CD45.1) received MNCs from 2.0 mL PB from mice (CD45.2) that had received different treatments. Donor PB was collected at 12 hours for Me6-treated CD45.2 mice and at 1 hour for plerixafor-treated CD45.2 mice. Mice also received 5 × 105 congenic BM competitor cells (CD45.1+). The results are shown as (B) the percentage of donor CD45.1+ cells vs recipient CD45.2+ cells and (C) the percent lineage of the CD45.1+ cells in PB of primary CD45.2 recipients 6 months after transplantation. n = 6 mice per group. **P < .01 vs the control group; #P < .05 vs the plerixafor group. FITC, fluorescein isothiocyanate.
Me6 mobilizes HSCs with long-term repopulating capacity. (A) Survival rate analysis of the recipient C57BL/6 mice that were transplanted with PB MNCs of donor C57BL/6 mice subjected to different treatments. The C57BL/6 donor mice were injected with PBS, 5 mg/kg Me6, or 5 mg/kg plerixafor (AMD; AMD3100). PB was collected after 12 hours for Me6-treated mice and after 1 hour for plerixafor-treated mice and transplanted into recipient mice that were lethally irradiated (800 cGy) before intravenous injection of the cells. The cells that were obtained from 1.5 mL of blood were transplanted into individual recipient mice. As a control, one group of recipient mice received cells that were obtained from normal BM, and another group of mice did not receive any cells. n = 10 mice per group. (B-C) Competitive repopulation assay. Lethally irradiated C57BL/6 mice (CD45.1) received MNCs from 2.0 mL PB from mice (CD45.2) that had received different treatments. Donor PB was collected at 12 hours for Me6-treated CD45.2 mice and at 1 hour for plerixafor-treated CD45.2 mice. Mice also received 5 × 105 congenic BM competitor cells (CD45.1+). The results are shown as (B) the percentage of donor CD45.1+ cells vs recipient CD45.2+ cells and (C) the percent lineage of the CD45.1+ cells in PB of primary CD45.2 recipients 6 months after transplantation. n = 6 mice per group. **P < .01 vs the control group; #P < .05 vs the plerixafor group. FITC, fluorescein isothiocyanate.
We performed a competitive repopulation experiment to further assess the repopulating ability of Me6-mobilized HSPCs. Lethally irradiated CD45.1 recipients were transplanted with 2.0 mL of C57BL/6 CD45.2 donor blood cells in combination with 5 × 105 CD45.1 BM cells. Six months after transplantation, the recipient mice were euthanized, and the PB was analyzed for the presence of donor (CD45.2+) vs recipient (CD45.1+) repopulation. Long-term engraftment rates of the Me6-mobilized cells were significantly higher than those of the AMD3100-mobilized cells (Figure 5B). To investigate whether the engrafted stem cells repopulated all leukocyte lineages in lethally irradiated recipient mice, we analyzed the lineage percentage of donor blood cells in recipient PB. Mice transplanted with Me6-mobilized cells had more CD45.2-derived B220+, CD3+, CD11b+, Ly-6G+, and Ter-119+ cells compared with mice that received AMD3100-mobilized cells (Figure 5C). Secondary transplantation experiments showed more donor cell engraftment in the Me6 group than in the AMD3100 group (supplemental Figure 5). These results strongly indicate that Me6 is a powerful mobilizing agent of long-term, multilineage, self-renewing HSCs.
Inhibition of migration and adhesion by Me6
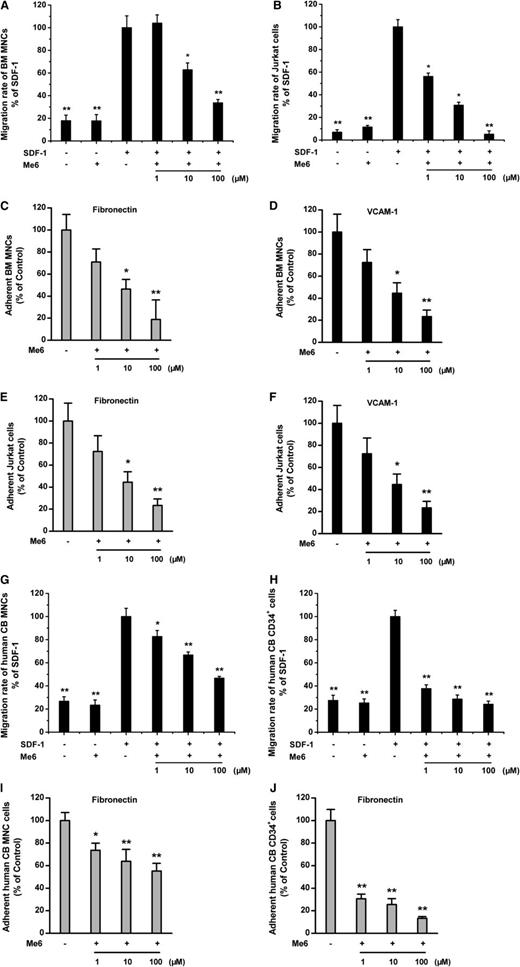
To determine the mechanisms of Me6-induced HSPC mobilization, we investigated the involvement of the SDF-1α/CXCR4 chemotactic axis and the VCAM-1/VLA-4 adhesive axis. We first tested the effects of Me6 on SDF-1α–induced migration of mouse BM MNCs and human Jurkat cells. Me6 significantly inhibited the migration of mouse MNCs toward SDF-1α. At concentrations of 10 μM and 100 μM, Me6 induced approximately 30% and 65% inhibition of SDF-1α–induced chemotaxis, respectively (Figure 6A). Similarly, Me6 caused a dose-dependent inhibition of the SDF-1α–induced chemotaxis of Jurkat cells (Figure 6B). Furthermore, Me6 significantly inhibited the adhesion of MNCs and Jurkat cells to fibronectin and VCAM-1 in a dose-dependent manner (Figure 6C-F). Importantly, Me6 also showed the capacity to block the SDF-1α–induced migration of human cord blood MNCs and CD34+ HSCs (Figure 6G-H) and inhibit adhesion of these cells to fibronectin (Figure 6I-J). Therefore, Me6 inhibits both the SDF-1α–induced migration and VLA-4–mediated adhesion of mouse and human hematopoietic cells in vitro.
Inhibition of migration and adhesion by Me6. (A-B) Me6 inhibited the migration of mouse BM MNCs and human Jurkat cells toward SDF-1α in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the SDF-1α group. (C-F) Me6 significantly inhibited the adhesion of BM MNCs and human Jurkat cells to fibronectin and VCAM-1 in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the control group. (G-H) Me6 inhibited the migration of human cord blood (CB) MNCs and CD34+ HSCs toward SDF-1α in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the SDF-1α group. (I-J) Me6 significantly inhibited the adhesion of human cord blood MNCs and CD34+ HSCs to fibronectin in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the control group.
Inhibition of migration and adhesion by Me6. (A-B) Me6 inhibited the migration of mouse BM MNCs and human Jurkat cells toward SDF-1α in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the SDF-1α group. (C-F) Me6 significantly inhibited the adhesion of BM MNCs and human Jurkat cells to fibronectin and VCAM-1 in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the control group. (G-H) Me6 inhibited the migration of human cord blood (CB) MNCs and CD34+ HSCs toward SDF-1α in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the SDF-1α group. (I-J) Me6 significantly inhibited the adhesion of human cord blood MNCs and CD34+ HSCs to fibronectin in a dose-dependent manner. n = 6. *P < .05 and **P < .01 vs the control group.
Me6 activates the MAPK and AKT pathways and induces MMP-9 expression
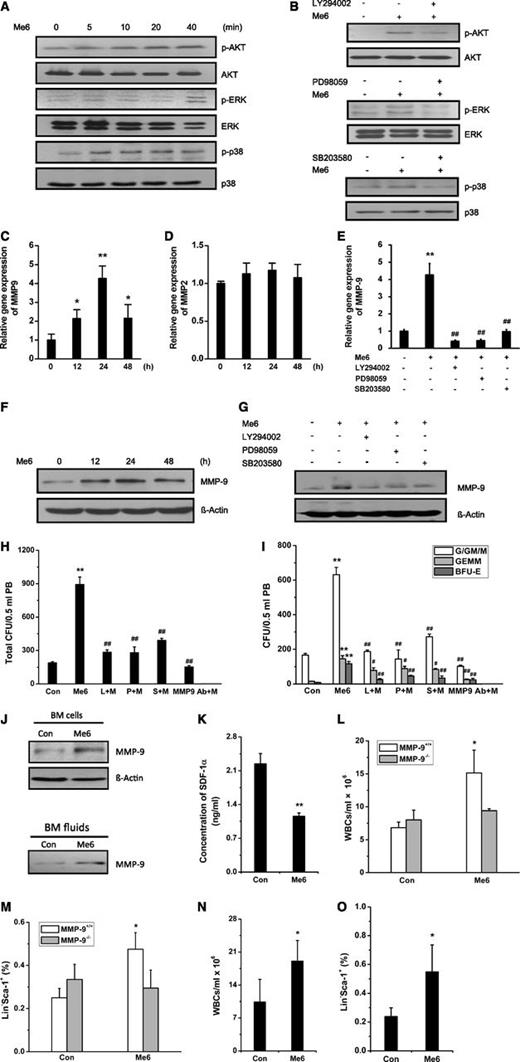
To further explore the effect of Me6 on the SDF-1α/CXCR4 pathway, we performed phospho-MAPK array detection. The exposure of Jurkat cells to SDF-1α resulted in different degrees of activation of ERK, p38, and AKT. Surprisingly, Me6 did not inhibit the SDF-1α–activated signaling but increased SDF-1α–induced phospho-AKT, phospho-ERK, and phospho-p38 levels (supplemental Figure 6), suggesting that Me6 might function as an agonist. Western blotting analysis showed that Me6 gradually increased the phosphorylation of AKT, ERK, and p38 (Figure 7A), although the cAMP pathway was not significantly activated (data not shown). The activation of AKT, ERK, and p38 by Me6 was significantly inhibited when cells were pretreated with the PI3K/AKT inhibitor LY294002, the ERK inhibitor PD98059, and the p38 inhibitor SB203580, respectively (Figure 7B).
HSPC mobilization by Me6 is MMP9-dependent through activating the AKT, ERK, and p38 signal pathways. (A) Human Jurkat cells were stimulated with Me6 for 0, 5, 10, 20, and 40 minutes. The cell lysates were prepared and subjected to western blot analysis to detect the total protein level and phosphorylation level of AKT, ERK, and p38. (B) Human Jurkat cells were stimulated with Me6 for 40 minutes without pretreatment or following a 40-minute pretreatment with the selective PI3K/AKT inhibitor LY294002, ERK inhibitor PD98059, or p38 inhibitor SB203580. Total and phosphorylation level of AKT, ERK, and p38 were analyzed. (C) Jurkat cells (1 × 106) were cultured with Me6 for 0, 12, 24, and 48 hours. Real-time quantitative polymerase chain reaction (qPCR) was performed to show that Me6 treatment significantly upregulated the expression of MMP-9 mRNA. (D) The expression level of MMP-2 mRNA was not affected by Me6. (E) Inhibition of PI3K/AKT, ERK, or p38 pathways significantly attenuated the expression of MMP-9 mRNA in the Me6-treated Jurkat cells. A total of 1 × 106 Jurkat cells were cultured for 24 hours in medium alone, with Me6, or with Me6 and a selective signaling pathway inhibitor (LY294002, PD98059, or SB203580). The cells were collected for analysis of the MMP-9 mRNA levels by qPCR. (F) Me6 treatment upregulated the expression level of MMP-9 protein in Jurkat cells. (G) The upregulation of MMP-9 protein levels was significantly inhibited by LY294002, PD98059, or SB203580. (H-I) Analysis of (H) total CFU, and (I) CFU-G/GM/M, CFU-GEMM, and BFU-E in the PB of C57BL/6 mice single injected with PBS or 5 mg/kg Me6; or injected with both Me6 and LY294002, PD98059, SB203580, or neutralizing anti–MMP-9 antibody (clone 6-6B). LY294002, PD98059, SB203580, or anti–MMP-9 antibody was injected 1 hour prior to Me6 administration. n = 6 mice per group. **P < .01 vs the PBS group. #P < .05 and ##P < .01 vs the Me6 group. (J) The expression of MMP-9 protein was increased in BM cells from mice treated with Me6. The secretion of MMP-9 protein was increased in BM fluids from Me6-treated mice compared with the control mice. The concentration of protein in the BM fluids was determined by using the bicinchoninic acid (BCA) protein assay system, and the samples with equivalent protein concentrations were loaded onto an sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. (K) The concentration of SDF-1α in BM fluids was detected by enzyme-linked immunosorbent assay. BM fluids were collected from mice treated with PBS or Me6 12 hours after injection. n = 3 mice per group. **P < .01 vs the control group. (L) Mobilization of WBCs by Me6 was significantly inhibited in MMP-9−/− mice. (M) The percentages of Lin−Sca-1+ cells showed little change in Me6-treated MMP-9−/− mice compared with PBS-treated MMP-9−/− control mice. MMP-9−/− and MMP-9+/+ mice were treated with 5 mg/kg Me6 or PBS. After 12 hours, blood WBCs and HPCs (Lin−Sca-1+) were counted. n = 3 mice per group. *P < .05 vs the control MMP-9+/+ group. (N-O) Me6 significantly induced mobilization of WBCs and HPCs (Lin−Sca-1+) in wild-type BM-MMP-9−/− mice. BM cells from MMP-9+/+ WT mice were transplanted into lethally irradiated MMP-9−/− mice. Six weeks after the BM transplantation, these BM-reconstructed mice were subcutaneously injected with PBS or Me6; 12 hours later, blood WBCs and HPCs (Lin−Sca-1+) were counted. n = 4 mice per group. * P < .05 vs the PBS control group. L, LY294002; M, Me6; P, PD98059; S, SB203580.
HSPC mobilization by Me6 is MMP9-dependent through activating the AKT, ERK, and p38 signal pathways. (A) Human Jurkat cells were stimulated with Me6 for 0, 5, 10, 20, and 40 minutes. The cell lysates were prepared and subjected to western blot analysis to detect the total protein level and phosphorylation level of AKT, ERK, and p38. (B) Human Jurkat cells were stimulated with Me6 for 40 minutes without pretreatment or following a 40-minute pretreatment with the selective PI3K/AKT inhibitor LY294002, ERK inhibitor PD98059, or p38 inhibitor SB203580. Total and phosphorylation level of AKT, ERK, and p38 were analyzed. (C) Jurkat cells (1 × 106) were cultured with Me6 for 0, 12, 24, and 48 hours. Real-time quantitative polymerase chain reaction (qPCR) was performed to show that Me6 treatment significantly upregulated the expression of MMP-9 mRNA. (D) The expression level of MMP-2 mRNA was not affected by Me6. (E) Inhibition of PI3K/AKT, ERK, or p38 pathways significantly attenuated the expression of MMP-9 mRNA in the Me6-treated Jurkat cells. A total of 1 × 106 Jurkat cells were cultured for 24 hours in medium alone, with Me6, or with Me6 and a selective signaling pathway inhibitor (LY294002, PD98059, or SB203580). The cells were collected for analysis of the MMP-9 mRNA levels by qPCR. (F) Me6 treatment upregulated the expression level of MMP-9 protein in Jurkat cells. (G) The upregulation of MMP-9 protein levels was significantly inhibited by LY294002, PD98059, or SB203580. (H-I) Analysis of (H) total CFU, and (I) CFU-G/GM/M, CFU-GEMM, and BFU-E in the PB of C57BL/6 mice single injected with PBS or 5 mg/kg Me6; or injected with both Me6 and LY294002, PD98059, SB203580, or neutralizing anti–MMP-9 antibody (clone 6-6B). LY294002, PD98059, SB203580, or anti–MMP-9 antibody was injected 1 hour prior to Me6 administration. n = 6 mice per group. **P < .01 vs the PBS group. #P < .05 and ##P < .01 vs the Me6 group. (J) The expression of MMP-9 protein was increased in BM cells from mice treated with Me6. The secretion of MMP-9 protein was increased in BM fluids from Me6-treated mice compared with the control mice. The concentration of protein in the BM fluids was determined by using the bicinchoninic acid (BCA) protein assay system, and the samples with equivalent protein concentrations were loaded onto an sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. (K) The concentration of SDF-1α in BM fluids was detected by enzyme-linked immunosorbent assay. BM fluids were collected from mice treated with PBS or Me6 12 hours after injection. n = 3 mice per group. **P < .01 vs the control group. (L) Mobilization of WBCs by Me6 was significantly inhibited in MMP-9−/− mice. (M) The percentages of Lin−Sca-1+ cells showed little change in Me6-treated MMP-9−/− mice compared with PBS-treated MMP-9−/− control mice. MMP-9−/− and MMP-9+/+ mice were treated with 5 mg/kg Me6 or PBS. After 12 hours, blood WBCs and HPCs (Lin−Sca-1+) were counted. n = 3 mice per group. *P < .05 vs the control MMP-9+/+ group. (N-O) Me6 significantly induced mobilization of WBCs and HPCs (Lin−Sca-1+) in wild-type BM-MMP-9−/− mice. BM cells from MMP-9+/+ WT mice were transplanted into lethally irradiated MMP-9−/− mice. Six weeks after the BM transplantation, these BM-reconstructed mice were subcutaneously injected with PBS or Me6; 12 hours later, blood WBCs and HPCs (Lin−Sca-1+) were counted. n = 4 mice per group. * P < .05 vs the PBS control group. L, LY294002; M, Me6; P, PD98059; S, SB203580.
MMP-9 is involved in the migration and egress of stem cells from BM.11,27 The MMP-9 expression can be activated by the MAPK and AKT pathways.28,29 In Jurkat cells, Me6 significantly increased MMP-9 but not MMP-2 messenger RNA (mRNA) levels, especially at 24 hours posttreatment (Figure 7C-D). Me6 also upregulated the expression of MMP-9 mRNA in human cord blood MNCs (supplemental Figure 7). Remarkably, this upregulation of MMP-9 by Me6 was significantly inhibited by LY294002, PD98059, and SB203580 (Figure 7E). Me6 treatment also resulted in increased MMP-9 protein expression (Figure 7F), which was attenuated by the addition of the PI3K/AKT, p38, or ERK inhibitors (Figure 7G). Importantly, the capacity of Me6 to mobilize HPCs into PB was remarkably inhibited by LY294002, PD98059, SB203580, and MMP-9–neutralizing antibody. Notably, the inhibitory effect of anti–MMP-9 antibody was stronger than that of the kinase inhibitors (Figure 7H-I).
We then analyzed MMP-9 levels in Me6-treated mice. The mice were injected with 5 mg/kg Me6. After 12 hours, BM MNCs and BM fluids were collected for the detection of MMP-9 protein. Me6 administration increased MMP-9 protein expression in BM MNCs and the secretion of MMP-9 into BM fluids (Figure 7J). Quantitative polymerase chain reaction results demonstrated a significant increase in MMP-9 mRNA levels in BM MNCs from Me6-treated mice (data not shown). It has been reported previously that MMP-9 degrades the SDF-1α proteins in BM.15 Enzyme-linked immunosorbent assay results showed that the level of SDF-1α in the BM fluids of Me6-treated mice was decreased by approximately 0.5-fold compared with the controls (Figure 7K).
To further evaluate the requirement of MMP-9 in Me6-induced HSPC mobilization, MMP-9 knockout (FVB MMP-9−/−) mice were injected with Me6 or PBS. The Me6-induced mobilization of WBCs was significantly inhibited in MMP-9−/− mice (Figure 7L). The percentages of Lin−Sca-1+ cells showed little change in Me6-treated MMP-9−/− mice compared with PBS-treated MMP-9−/− mice (Figure 7M). However, Me6 induced significant WBC and HPC mobilization in wild-type mice. To further confirm the important role of MMP-9 expression in BM cells, we reconstructed the BM cells of MMP-9−/− mice with that from wild-type mice (wild-type BM-MMP-9−/− mice). Notably, Me6 administration produced a significant WBC and HPC mobilization effect in these mice (Figure 7N-O). Taken together, the in vitro and in vivo results indicate that Me6 upregulates MMP-9 expression via the activated ERK, p38, and AKT pathways, which subsequently decreases the concentration of SDF-1α in BM, thereby promoting the egress of HSPCs into the blood circulation.
Discussion
Several types of HSPC mobilizing agents have been reported. Hematopoietic growth factors, such as G-CSF, thrombopoietin, stem cell factor, and FMS-like tyrosine kinase 3 ligand, have the capability to mobilize HSPCs.30-32 Among these agents, G-CSF is the only FDA-approved agent for clinical HSCT. Hematopoietic cytokines have relatively moderate effects and require multiple doses and several days to reach their maximum effects. In contrast, chemokines and small-molecule compounds can induce rapid mobilization of HSPCs, with peak mobilization achieved within minutes to hours of administration. Until now, only one small molecule, plerixafor, has been FDA-approved for use in mobilizing CD34+ cells in patients with non-Hodgkin lymphoma and multiple myeloma. The use of more efficient mobilization agents would increase the comfort and safety of donors and reconstitute the hematopoietic system of patients in a shorter time than G-CSF. Therefore, there is substantial interest in the development of small molecules that can mobilize HSPCs. Small molecules have several advantages over native cytokines, such as lower immunogenicity, lower synthesis and manufacturing costs, and improved bioavailability. These advantages may make small molecules more accessible for clinical use.
Here, we report that the small molecule Me6 may be a potent HSPC mobilizing agent. Me6 is an alkaloid analog that contains multiple hydrogen-bonding acceptor sites. It is frequently used as a ligand for introducing transition metals into hydrophobic surroundings.33,34 So far, the biologic activity of Me6 remains unclear. We show that a single subcutaneous injection of Me6 resulted in significant leukocytosis, indicating that Me6 may elicit the mobilization of HSPCs into PB. Importantly, Me6 exhibited no significant cytotoxic effect in vitro or in vivo and was safe for use in mice at its effective dose. By using CFU assays, we found that injection of Me6 resulted in rapid mobilization of HPCs. The mobilization effect of progenitor cells by a single administration of Me6 was maintained for 3 days. Compared with AMD3100, which causes HPC mobilization in 1 to 8 hours after administration,18,35 the relatively longer-lasting effect of Me6 on HPC mobilization suggests that it may have a different mobilization mechanism. We found that the combination of Me6 and AMD3100 induced a significant synergistic effect on the mobilization of total CFUs and different types of CFUs (supplemental Figure 8A-B), which further indicated that different mobilization mechanisms were used by Me6 and AMD3100. Me6 presented a generalized effect on HPC mobilization in different strains of mice. Repeat dosing of Me6 (≤3 times, 24 hours apart) also caused a significant HPC mobilization effect (supplemental Figure 9A-B), which suggested that egress of HPCs into the blood was not desensitized by Me6 repeated treatment. By analyzing CFU numbers in PB, we found that Me6 was more effective at mobilization than AMD3100 or G-CSF administration alone, and that Me6 synergized with G-CSF or AMD3100 to mobilize HSPCs. In homing experiments, no significant difference in the percentage of CD45.1+ cells homing to BM of CD45.2 mice was observed among the Me6, AMD3100, and G-CSF groups. However, a significantly higher percentage of CD45.1+Lin−Sca-1+ cells was found in the BM of CD45.2 mice transplanted with Me6-mobilized PB MNCs than in that of CD45.2 mice transplanted with cells from PBS-, AMD3100- or G-CSF–treated mice (supplemental Figure 10A). Given that Me6-mobilized PB MNCs showed no significant changes in CXCR4 expression in the LSK cell population (supplemental Figure 10B), more intricate experiments are needed to understand the regulatory mechanisms of Me6 in enhancing homing of HPCs. Using competitive transplantation experiments, we found that HSPCs mobilized by Me6 demonstrated better engraftment and repopulation capacities than HSPCs mobilized by AMD3100, which might be due to the enhanced mobilization and homing capacity of HSPCs induced by Me6.
Numerous studies have suggested that the SDF-1α/CXCR4 axis plays a critical role in HSPC mobilization.36-38 Disruption of the SDF-1α/CXCR4 axis by either CXCR4 antagonists/agonists18 or by proteases or cell surface peptidases results in the mobilization of HSPCs into the circulation. The mechanisms of Me6-mediated HSPC mobilization might involve the direct or indirect disruption of the SDF-1α/CXCR4 interaction. We found that Me6 blocked the SDF-1α–induced migration of mouse BM MNCs and human Jurkat cells. Interestingly, Me6 presented stronger capacity for inhibiting the migration or adhesion of human CD34+ HSPCs than murine or human MNCs, which indicated the higher potential of Me6 in human HSPC mobilization.
We also found that Me6 did not inhibit the SDF-1α–activated signaling in Jurkat cells. Surprisingly, Me6 activated the PI3K/AKT, p38, and ERK pathways, indicating that Me6 might function as an agonist that indirectly blocked the SDF-1α/CXCR4 interaction. Pretreatment with certain agonists or the overexpression of S1P inhibits SDF-1α–induced migration and indirectly blocks the SDF-1α/CXCR4 axis but activates downstream pathways and promotes the egress of HSPCs.39,40 We postulated that Me6 upregulated the expression of certain target genes via the activation of signaling pathways. Gene expression microarray data (GEO accession number: GSE51974) and KEGG pathway analysis revealed that two genes, Jun and Fos, were significantly upregulated by Me6 (supplemental Figure 11A) and that two transcriptional complexes, activator protein-1 and nuclear factor κB, were activated (supplemental Figure 11B). The MAPK and AKT signaling pathways are involved in the activation of these transcription factors and promote the expression of MMP-9. We found that Me6 upregulated the expression of MMP-9 via AKT, p38, and ERK pathways in Jurkat cells and the BM cells. MMP-9 can cleave SDF-1α proteins and disrupts the SDF-1α/CXCR4 interaction in BM forcing the release of HSPCs into the PB.15 The in vivo experiments demonstrated that the SDF-1α level in the BM fluids of Me-6–treated mice was lower than that of the controls by approximately 0.5-fold. Importantly, Me6-induced HSPC mobilization was markedly impaired in MMP-9−/− mice, confirming that MMP-9 is the important downstream target of Me6.
We show, for the first time, that the compound Me6 can rapidly mobilize WBCs and HSPCs. HSPCs mobilized by Me6 demonstrate more efficient engraftment and appear to have a greater competitive repopulation capacity than those mobilized by AMD3100. These effects may be partially due to the activation of MMP-9 expression and the disruption of the SDF-1α/CXCR4 axis. Me6 may become a new potent and efficacious mobilizing agent of HSPCs in BM transplants, either alone or in combination with G-CSF.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (2013AA020107), the National Basic Research Program of China (2011CB964804, 2010CB945500), and the National Natural Science Foundation of China (31101040).
Authorship
Contribution: Y. Li, J. Zhang, and X.P. designed the research, analyzed the data, and wrote the manuscript; J. Zhang, X.R., and W.S. performed most of the experiments and analyzed the data; S.W., H.C., Z.W., Y.Z., and R.Z. assisted in the transplantation experiments; Y. Lv, B.Z., L.C., J. Zhou, X.N., and L.H. assisted in the in vitro experiment; and W.Y. analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yanhua Li, Beijing Institute of Transfusion Medicine, 27 Taiping Rd, Haidian District, Beijing 100850, China; e-mail: shirlylyh@126.com; and Xuetao Pei, Beijing Institute of Transfusion Medicine, 27 Taiping Rd, Haidian District, Beijing 100850, China; e-mail: peixt@nic.bmi.ac.cn.
References
Author notes
J. Zhang, X.R., and W.S. contributed equally to this study.