Key Points
Heme, released from hemoglobin, elicits vaso-occlusion in transgenic sickle mice via endothelial TLR4 signaling.
Heme/TLR4 signaling activates NF-κB and triggers vaso-occlusion through Weibel-Palade body degranulation and adhesion molecule expression.
Abstract
Treatment of sickle cell disease (SCD) is hampered by incomplete understanding of pathways linking hemolysis to vaso-occlusion. We investigated these pathways in transgenic sickle mice. Infusion of hemoglobin or heme triggered vaso-occlusion in sickle, but not normal, mice. Methemoglobin, but not heme-stabilized cyanomethemoglobin, induced vaso-occlusion, indicating heme liberation is necessary. In corroboration, hemoglobin-induced vaso-occlusion was blocked by the methemoglobin reducing agent methylene blue, haptoglobin, or the heme-binding protein hemopexin. Untreated HbSS mice, but not HbAA mice, exhibited ∼10% vaso-occlusion in steady state that was inhibited by haptoglobin or hemopexin infusion. Antibody blockade of adhesion molecules P-selectin, von Willebrand factor (VWF), E-selectin, vascular cell adhesion molecule 1, intercellular adhesion molecule 1, platelet endothelial cell (EC) adhesion molecule 1, α4β1, or αVβ3 integrin prevented vaso-occlusion. Heme rapidly (5 minutes) mobilized Weibel-Palade body (WPB) P-selectin and VWF onto EC and vessel wall surfaces and activated EC nuclear factor κB (NF-κB). This was mediated by TLR4 as TAK-242 blocked WPB degranulation, NF-κB activation, vaso-occlusion, leukocyte rolling/adhesion, and heme lethality. TLR4−/− mice transplanted with TLR4+/+ sickle bone marrow exhibited no heme-induced vaso-occlusion. The TLR4 agonist lipopolysaccharide (LPS) activated ECs and triggered vaso-occlusion that was inhibited by TAK-242, linking hemolysis- and infection-induced vaso-occlusive crises to TLR4 signaling. Heme and LPS failed to activate VWF and NF-κB in TLR4−/− ECs. Anti-LPS immunoglobulin G blocked LPS-induced, but not heme-induced, vaso-occlusion, illustrating LPS-independent TLR4 signaling by heme. Inhibition of protein kinase C, NADPH oxidase, or antioxidant treatment blocked heme-mediated stasis, WPB degranulation, and oxidant production. We conclude that intravascular hemolysis in SCD releases heme that activates endothelial TLR4 signaling leading to WPB degranulation, NF-κB activation, and vaso-occlusion.
Introduction
During hemolysis, heme may accumulate in plasma derived from hemoglobin (Hb) released by lysed erythrocytes. Heme exerts multiple adverse effects, including leukocyte activation and migration, adhesion molecule and cytokine upregulation, and oxidant production.1-4
Sickle cell disease (SCD) is characterized by chronic unrelenting hemolysis, inflammation and coagulation, adhesion of blood cells, vaso-occlusion, and ischemia-reperfusion injury leading to strokes and organ infarctions. Endothelial cells (ECs), leukocytes, and platelets are chronically exposed to Hb and heme that may promote activated proinflammatory and prothrombotic phenotypes.5-8 To explain the basis for such phenotypes, substantial attention has been directed to hemolysis in SCD; indeed, in some patients, hemolysis may be especially brisk (hyperhemolysis) during acute painful episodes.9 Endothelial dysfunction with diminished nitric oxide (NO) bioavailability and increased adhesion molecule expression is a known consequence of hemolysis and systemic inflammatory states.10 Interest also resides in the oxidative cytotoxic effects of heme in SCD.11
The present study examined a potential mechanism that links these 3 salient phenotypic features of SCD: hemolysis, vaso-occlusion, and inflammation. Specifically, this study examined the hypothesis that heme, released from plasma Hb during hemolysis, is an extracellular cell-signaling molecule and a causal factor in the development of vascular inflammation and vaso-occlusion in SCD.
Methods
Heme and Hb reagents
Stroma-free Hb was a gift from Sangart. Methemoglobin (metHb) and cyanomethemoglobin (cyanometHb) were prepared as described previously.12 Initial studies employed clinical grade Panhematin (Ovation Pharmaceuticals). Subsequent studies employed hematin prepared immediately before use by mixing 10 mg hemin chloride (Frontier Scientific), 10 mg D-sorbitol (Sigma-Aldrich), and 6.9 mg sodium carbonate (Sigma-Aldrich) in 5.7 mL sterile saline (Baxter) for 30 minutes in the dark. All heme preparations, appropriately diluted in saline, were filtered at 0.22 µm. Protoporphyrin IX (PPIX; Frontier Scientific) preparations were prepared identically to heme. The structures of heme (ferrous PPIX), hemin (ferric PPIX), and PPIX (no iron) are shown in supplemental Figure 1 (available on the Blood Web site). The term “heme” is used generically to refer to both heme and “hemin.” Endotoxin levels were monitored using a Limulus amebocyte lysate test (GenScript). All Hb/heme preparations contained <0.1 endotoxin units (EU)/mL. Human haptoglobin was a gift from Bio Products Laboratory. Size-exclusion high-performance liquid chromatography profiles of haptoglobin showed the following molecular weight distribution: 60% with 2 αβ (dimer, haptoglobin 1-1), 21% with 3 αβ (trimer, mostly haptoglobin 1-2), and 19% larger forms (polymer, mostly haptoglobin 2-2). Hemopexin was purified from rabbit plasma or human recombinant hemopexin was purchased (Athens Research & Technology).13
Mice
All animal experiments were approved by the University of Minnesota’s Institutional Animal Care and Use Committee. We used male and female NY1DD14 and HbSS-Townes15 transgenic sickle mice. The NY1DD and HbSS-Townes mice are on C57BL/6 and mixed genetic backgrounds, respectively. The NY1DD mice are homozygous for deletion of the mouse βmajor globin and express a human α and βS globin transgene. NY1DD mice have no anemia and a red blood cell (RBC) half-life of 7 days (J.D.B. and G.M.V., unpublished data); C57BL/6 mice (RBC half-life, 24 days)16 served as controls. The HbSS-Townes mice were created by knocking in human α and βS globins into the sites where murine α and β globins were knocked out. HbSS-Townes mice have severe anemia and an RBC half-life of 2.5 days.16 HbAA-Townes control mice (RBC half-life, 16 days)16 were created by replacing the βS with βA. HbAS-Townes mice (RBC half-life, 11 days)16 were created by breeding HbSS with HbAA mice. TLR4−/− mice (The Jackson Laboratory; C57BL/6 background) were transplant recipients and donors of murine pulmonary vein ECs (mPVECs).
Measurement of stasis
Dorsal skinfold chambers (DSFCs) were implanted on NY1DD, HbSS-, HbAS-, and HbAA-Townes and C57BL/6 mice at age 8 to 10 weeks and NY1DD/TLR4−/− and NY1DD/TLR4+/+ chimeric bone marrow (BM) transplant mice at age 20 to 22 weeks as described elsewhere.17 At 3 days postimplantation, flowing subcutaneous venules (n = 20-30) in the DSFCs were randomly selected at baseline in each mouse. Mice were then given a bolus infusion (0.012 mL/g body weight) via tail vein with the indicated doses of saline, water, Hb, metHb, cyanometHb, heme, or lipopolysaccharide (LPS; Escherichia coli, serotype O111:B4; Sigma-Aldrich) or exposed to 1-hour hypoxia (7%O2/93%N2) followed by 1-hour H/R. Venules selected at baseline were reexamined 1 hour after infusion or H/R. Venules without visible blood flow were counted as static and the percent static venules calculated. In some studies, HbSS and HbAA mice were not given a stimulus and venules were reexamined on days 1 and 2 after baseline to determine steady-state stasis. For inhibitor studies, we infused haptoglobin, hemopexin, methylene blue (Sigma-Aldrich), PPIX, apocynin (Santa Cruz Biotechnology), diphenyleneiodonium (DPI) (Sigma-Aldrich), calphostin C (Tochris Bioscience), starch-conjugated deferoxamine (S-DFO; Bo Hedlund), N-acetylcysteine (NAC; Sigma-Aldrich), TAK-242 (InvivoGen), or blocking immunoglobulin G (IgG) to P-selectin (clone RB40.34), E-selectin (clone 10E9.6), vascular cell adhesion molecule 1 (VCAM-1) (clone 429), intercellular adhesion molecule 1 (ICAM-1) (clone 3E2), platelet EC adhesion molecule 1 (PECAM-1) (clone 35-95; BD Biosciences), αVβ3 and α4β1 integrins (clones LM609 and PS/2; Millipore), von Willebrand factor (VWF) (Cedarlane; CL20176A-R), thrombomodulin (clone MAB3894; R&D Systems) or LPS (clone WN1 222-5; Hycult Biotech). For negative controls, we infused the appropriate control IgG (BD Biosciences, BioLegend, Jackson ImmunoResearch), vehicle, or 6% hetastarch in saline (B. Braun Medical). Total infusion volumes (inhibitors + Hb/heme/LPS) were limited to 0.012 mL/g body weight (∼20% blood volume). Hb concentrations were normalized to heme (4 hemes per Hb).
Plasma heme
Plasma heme was measured by pseudoperoxidase activity.18
Cell culture
Immunofluorescence staining for P-selectin and VWF in HUVECs and murine tissue
HUVECs in 0.1% fetal bovine serum (FBS) were incubated with heme (20 µM) for 15 minutes, hemoglobin A (HbA) or hemoglobin S (HbS) (20 µM heme) for 24 hours, or Hb for 24 hours followed by heme for 15 minutes or H/R (3 hours in 95% N2/5% CO2 followed by 1 hour in 95% air/5% CO2). Haptoglobin, hemopexin, apocynin, calphostin C, or TAK-242 was used as an inhibitor. Cells were fixed in 4% paraformaldehyde, stained with anti–P-selectin (Santa Cruz Biotechnology) and anti-VWF IgG (Cedarlane), and visualized with appropriate secondary IgG labeled with fluorescein isothiocyanate (FITC) (P-selectin) and tetramethylrhodamine-5(and 6)-isothiocyanate (TRITC) (VWF). Nuclei were counterstained with 4′,6-Diamidino-2-phenylindole (DAPI; Santa Cruz Biotechnology). Cells were mounted using Fluoromount-G (Southern Biotech) and visualized, and images were acquired using a FluoView FV1000 BX2 upright confocal microscope (Olympus, Center Valley, PA) with a ×60 objective at room temperature and processed with FluoView (Olympus) and Adobe Photoshop software (San Jose, CA).
For tissue staining, C57BL/6 and NY1DD mice were administered saline, heme, or histamine, a known Weibel-Palade body (WPB) agonist. After 15 minutes, mice were sacrificed; organs were removed and placed into optimal cutting temperature compound (Sakura Finetek), snap-frozen, and stored at −85°C. Six-micrometer tissue sections were mounted on glass slides and fixed in 4% paraformaldehyde. Slides were blocked with 3% donkey serum, stained, mounted, and visualized as described for HUVECs. Nonspecific staining was assessed using nonimmune IgG. Dorsal skin samples from C57BL/6 and NY1DD mice were fixed in Zamboni’s and processed and analyzed as described elsewhere20 using anti-VWF IgG.
Oxidative Stress in HUVEC
Intracellular reactive oxygen species (ROS) levels were measured using a cell-permeable fluorescent probe, dichlorodihydrofluorescein diacetate (DCFH-DA), as described previously.21
Enzyme immunoassay for VWF
mPVECs from TLR4+/+ and TLR4−/− mice were incubated with heme, Hb, or LPS for 15 minutes (n = 6 wells/treatment). Cells were fixed and cell surface VWF was measured by enzyme immunoassay using sheep anti-VWF IgG (Abcam) and donkey anti-sheep IgG-alkaline phosphatase conjugate (Jackson ImmunoResearch).
BM transplants
Chimeric mice were generated by harvesting BM from NY1DD mice (βS+/+) and transplanting the BM into lethally irradiated TLR4−/− or TLR4+/+ (C57BL/6) mice. Recipients (8-10 weeks of age) were irradiated with 2 doses of 5 Gy (X-RAD 320 Biological Irradiator) 3 hours apart. During the 3-hour interval, BM donors were killed and BM was collected from both femurs. Ten million BM cells were injected via tail vein into each irradiated recipient. Drinking water containing 0.2% neomycin sulfate (Sigma-Aldrich) was given to transplanted mice for 3 weeks immediately after transplantation. Twelve weeks posttransplant, globin phenotype was determined by Hb isoelectric focusing. Chimeric mice were employed 12 to 14 weeks after transplant.
Leukocyte rolling and adhesion
NF-κB activation in HUVEC and mPVEC
HUVEC or mPVEC isolated from TLR+/+ and TLR−/− mice, grown to confluence, were incubated with serum-free media containing LPS, TNF-α, or heme or pretreated with TAK-242 followed by heme. Cells were washed and nuclear extracts were prepared using a nuclear extraction kit (Affymetrix). Western blots were performed using a rabbit IgG to nuclear factor κB (NF-κB) phospho-p65 (Ser536)23 (Cell Signaling Technology).
Heme-induced lethality in HbSS mice
HbSS and HbAA mice (n = 6 mice per treatment) were given a bolus infusion of heme at time zero. One treatment group received TAK-242 30 minutes before heme infusion. Time of death after heme infusion was recorded.
Statistics
Analyses were performed with SigmaStat 3.5 for Windows (Systat Software). Comparisons of multiple treatment groups were made using 1-way analysis of variance (Holm-Sidak method). An unpaired t test was used for 2 groups with equal variances.
Results
Heme induces vaso-occlusion in sickle, but not normal, mice
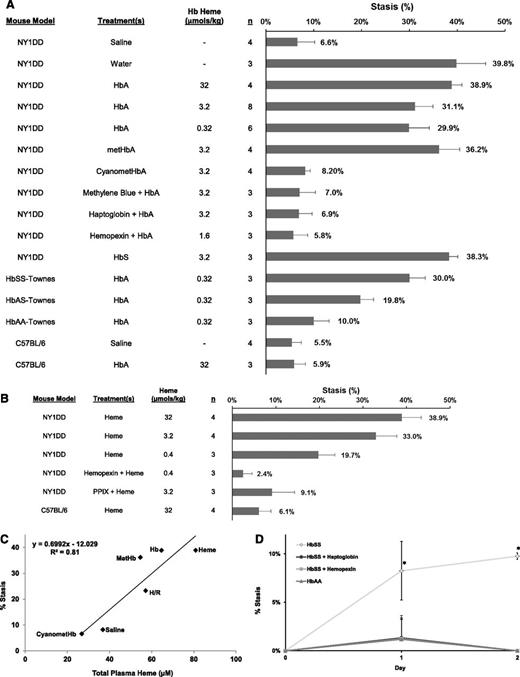
Because hemolysis is fundamental to the pathobiology of SCD, we tested whether hemolysis and plasma Hb induce vaso-occlusion (percent stasis) in 2 SCD models (NY1DD and HbSS-Townes) and 2 nonsickling control groups (C57BL/6 and HbAA-Townes). NY1DD sickle mice administered saline had 6.6% stasis after 1 hour (Figure 1A). NY1DD mice administered water to induce hemolysis24 had 39.8% stasis (P < .05, water vs saline). Likewise, HbA at 32, 3.2, or 0.32 µmol/kg induced 38.9%, 31.1%, and 29.9% stasis 1 hour after infusion (P < .05, HbA vs saline).
Hemolysis and plasma heme liberated from Hb induce stasis in transgenic sickle mice. (A) Percent stasis was measured in the subcutaneous venules of NY1DD, HbSS, HbAS, HbAA, and C57BL/6 mice with DSFCs. Flowing venules were selected and mapped at baseline (20-35 venules per mouse). Mice were given a bolus infusion (0.012 mL/g) of the following treatments at the indicated Hb doses: saline (control), water (to induce hemolysis in vivo), HbA, metHbA, cyanometHbA, methylene blue (2 mg/kg, IV) + HbA, haptoglobin (3.2 µmol/kg, IV) + HbA, hemopexin (1.6 µmol/kg, IV) + HbA, or HbS. Percent stasis was measured using intravital microscopy 1 hour after infusion. The numbers of mice (n) in each treatment group are indicated. Bars are mean % stasis + standard deviation (SD) with mean stasis values written above the bars. (B) Percent stasis was measured in the subcutaneous venules of NY1DD and C57BL/6 mice with DSFCs as described in panel A. Mice were given a bolus infusion (0.012 ml/g) of the following treatments at the indicated heme dosages: heme, hemopexin (0.4 µmol/kg, IV) + heme, and PPIX (40 µmol/kg, IP 60 minutes preheme) + heme. (C) Correlation between percent stasis and total plasma heme concentrations. NY1DD sickle mice (n = 3/treatment) were infused with saline, heme, Hb, metHb or cyanometHb, or exposed to 1 hour of hypoxia (7%O2) followed by 1 hour of hypoxia-reoxygenation (H/R). Percent stasis and total plasma heme were measured 1 hour after treatments. Values are mean % stasis and mean total plasma heme. (D) Percent stasis was measured in HbSS and HbAA mice in steady state. DSFCs were implanted on day −3. Flowing venules were selected at baseline on day 0. The same venules were reexamined for stasis on days 1 and 2. HbAA mice were untreated. HbSS mice were untreated or infused with human haptoglobin (900 µg/g) or hemopexin (34 µg/g) on days 0, 1, and 2. n = 3 mice per group; *P < .05 for HbSS vs HbSS + haptoglobin, HbSS + hemopexin or HbAA mice.
Hemolysis and plasma heme liberated from Hb induce stasis in transgenic sickle mice. (A) Percent stasis was measured in the subcutaneous venules of NY1DD, HbSS, HbAS, HbAA, and C57BL/6 mice with DSFCs. Flowing venules were selected and mapped at baseline (20-35 venules per mouse). Mice were given a bolus infusion (0.012 mL/g) of the following treatments at the indicated Hb doses: saline (control), water (to induce hemolysis in vivo), HbA, metHbA, cyanometHbA, methylene blue (2 mg/kg, IV) + HbA, haptoglobin (3.2 µmol/kg, IV) + HbA, hemopexin (1.6 µmol/kg, IV) + HbA, or HbS. Percent stasis was measured using intravital microscopy 1 hour after infusion. The numbers of mice (n) in each treatment group are indicated. Bars are mean % stasis + standard deviation (SD) with mean stasis values written above the bars. (B) Percent stasis was measured in the subcutaneous venules of NY1DD and C57BL/6 mice with DSFCs as described in panel A. Mice were given a bolus infusion (0.012 ml/g) of the following treatments at the indicated heme dosages: heme, hemopexin (0.4 µmol/kg, IV) + heme, and PPIX (40 µmol/kg, IP 60 minutes preheme) + heme. (C) Correlation between percent stasis and total plasma heme concentrations. NY1DD sickle mice (n = 3/treatment) were infused with saline, heme, Hb, metHb or cyanometHb, or exposed to 1 hour of hypoxia (7%O2) followed by 1 hour of hypoxia-reoxygenation (H/R). Percent stasis and total plasma heme were measured 1 hour after treatments. Values are mean % stasis and mean total plasma heme. (D) Percent stasis was measured in HbSS and HbAA mice in steady state. DSFCs were implanted on day −3. Flowing venules were selected at baseline on day 0. The same venules were reexamined for stasis on days 1 and 2. HbAA mice were untreated. HbSS mice were untreated or infused with human haptoglobin (900 µg/g) or hemopexin (34 µg/g) on days 0, 1, and 2. n = 3 mice per group; *P < .05 for HbSS vs HbSS + haptoglobin, HbSS + hemopexin or HbAA mice.
Methemoglobin A (metHbA), which can release heme,25 induced 36.2% stasis, whereas equimolar heme-stabilized cyanomethemoglobin A (cyanometHbA) induced 8.2% stasis (Figure 1A; P < .05, metHbA vs cyanometHbA), suggesting that metHb plays a role in stasis. Infusion of HbA and methylene blue, a metHb reducing agent,26 inhibited stasis (7.0%) compared with HbA alone (31.1%) (P < .05, methylene blue + HbA vs HbA). Binding HbA with equimolar haptoglobin also inhibited stasis (6.9%) (P < .05, haptoglobin + HbA vs HbA). Similarly, the high affinity heme-binding protein hemopexin inhibited HbA-induced stasis (5.8%) (P < .05, hemopexin + HbA vs HbA). These data indicate that Hb induces stasis in NY1DD mice through the release of heme from metHb.
HbS infused into NY1DD mice induced 38.3% stasis, which was 7.2% more than the stasis induced by equimolar amounts of HbA (Figure 1A; P < .05, HbS vs HbA). This suggests that HbS may lose heme more readily than HbA in NY1DD mice.
We also infused HbA into HbSS-, HbAS-, and HbAA-Townes mice (Figure 1A). HbA (0.32 µmol/kg) induced 30.0%, 19.8%, and 10.0% stasis, respectively (P < .05, all pairwise comparisons). At this physiological dose, percent stasis in HbSS mice was not significantly different than NY1DD mice. Finally, HbA (32 µmol/kg) infused into C57BL/6 mice induced 5.9% stasis that was significantly lower than stasis in NY1DD mice (38.9%, P < .05) but similar to stasis in C57BL/6 mice infused with saline (5.5%), indicating that Hb induces stasis in sickle, but not normal, mice.
Because liberated heme appeared to be the active, stasis-inducing molecule, we examined stasis in mice after infusion of heme. Heme at 32, 3.2, or 0.4 µmol/kg induced 38.9%, 33.0%, and 19.7% stasis 1 hour after infusion (P < .05, heme vs saline; Figure 1B), which is comparable to stasis induced by HbA. Equimolar amounts of hemopexin inhibited heme-induced stasis (2.4%, P < .05, hemopexin + heme vs heme). Similar to Hb, heme (32 µmol/kg) infused into C57BL/6 mice induced only 6.1% stasis (P < .05, C57BL/6 vs NY1DD; Figure 1B), indicating that, like Hb, heme induces stasis in sickle, but not wild-type, mice.
To evaluate the specificity of heme-induced stasis, we examined whether PPIX, a tetrapyrrole ring identical to heme but lacking iron, antagonizes heme-mediated stasis. PPIX (40 µmol/kg) was administered by intraperitoneal (IP) injection to NY1DD mice followed 1 hour later by an IV infusion of heme (3.2 µmol/kg). PPIX inhibited heme-mediated stasis at 1 hour after heme infusion (9.1% stasis with PPIX + heme vs 33.0% stasis with heme alone, P < .05; Figure 1B). Thus, redox-active iron in heme is required for heme-mediated stasis.
Mean percent stasis was plotted against mean total plasma heme (Figure 1C) in NY1DD mice 1 hour after infusion of heme, Hb, metHb, or cyanometHb or exposure to H/R. Stasis was correlated to total plasma heme (r2 = 0.81). Since the half-life of RBCs in HbSS mice (2.5 days) is short, we hypothesize that sickle mice are chronically hemolyzing and releasing Hb and heme into plasma. We examined steady-state stasis in HbSS- and HbAA-Townes mice, which have total plasma heme levels of 52.6 ± 18.3 µM and 28.8 ± 3.1µM, respectively (P < .05). DSFCs were implanted and 3 days later flowing venules were selected at baseline (day 0). The same venules were reexamined for stasis on days 1 and 2 in steady state without any additional stimulus. Untreated HbSS mice had 8% to 10% stasis in steady state on days 1 and 2, compared with 0% to 1% stasis in HbAA mice (P < .05, Figure 1D). Steady-state stasis in HbSS mice was abrogated after infusion of haptoglobin or hemopexin (P < .05), indicating that chronic hemolysis is continually driving stasis in HbSS mice. On day 3, infusion of Hb (0.32 µmol heme/kg) into HbSS mice increased stasis to 30% (data not shown), suggesting that vaso-occlusion occurs during steady state in HbSS mice and small increases in hemolysis can induce additional vaso-occlusion.
Blocking antibodies to EC adhesion molecules inhibit heme-induced stasis
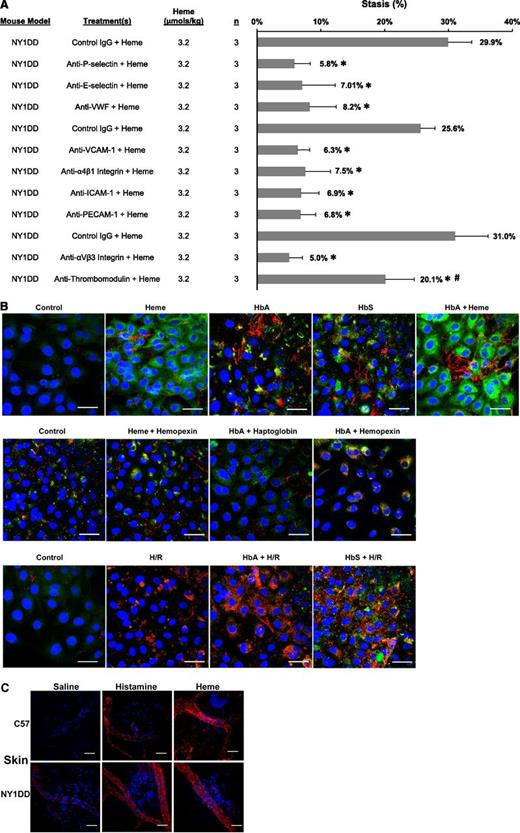
Multiple adhesion molecules have been previously implicated in the adhesion of blood cells to the endothelium and vaso-occlusion.5,22,27-31 We examined the role of adhesion molecules in heme-induced stasis (Figure 2A). Infusion of IgG against P-selectin, E-selectin, VWF, VCAM-1, α4β1 integrin, ICAM-1, PECAM-1, or αVβ3 integrin inhibited heme-induced stasis compared with control IgG (P < .001). In contrast, an antibody to thrombomodulin on endothelium had less stasis than control IgG but more stasis than anti–adhesion molecule IgG (P < .002).
Multiple EC adhesion molecules are required for stasis including mobilization of WPB constituents P-selectin and VWF to the EC surface. (A) Blocking antibodies to adhesion molecules P-selectin, E-selectin, VWF, VCAM-1, α4β1 integrin, ICAM-1, PECAM-1, αVβ3 integrin, thrombomodulin, or control IgG (30 µg per mouse, IV) were infused into NY1DD sickle mice with DSFCs 5 minutes prior to infusion of heme (3.2 µmol/kg). Percent stasis was measured at 1 hour after heme infusion as described in Figure 1. *P < .001 vs control IgG and #P < .001 antithrombomodulin vs anti-P-selectin, E-selectin, VWF, VCAM-1, α4β1 integrin, ICAM-1, PECAM-1, or αVβ3 integrin. (B) HUVECs were fixed in 4% paraformaldehyde and stained for surface P-selectin (green) or VWF (red) after the indicated incubations. Nuclei were counterstained with DAPI (blue). White bars represent 40 µm. Representative cells are shown. Top row: HUVECs in 0.1% FBS were incubated with heme (20 µM) for 15 minutes, HbA or HbS (20 µM heme) for 24 hours, or HbA for 24 hours followed by heme for 15 minutes. Middle row: HUVECs were incubated with heme + hemopexin (20 μM), HbA + haptoglobin (20 µM), or HbA + hemopexin for 24 hours. Bottom row: HUVECs were incubated for 24 hours in 0.1% FBS with or without HbA or HbS followed by H/R (5% CO2/95% N2 for 3 hours and 5% CO2/95% air for 1 hour). Control cells were incubated in 0.1% FBS for 24 hours. Cells were not permeabilized; all pictures represent cell-surface expression. (C) Normal C57BL/6 and NY1DD sickle mice were infused with saline (12 mL/kg, negative control), histamine (1200 µmol/kg, positive control), or heme (3.2 µmol/kg). Fifteen minutes after infusion, mice were sacrificed in CO2 and dorsal skin (n = 1/treatment) was removed and fixed in Zamboni’s for immunofluorescence staining of VWF (red) and counterstaining of nuclei with DAPI (blue). White bars represent 30 µm. Representative venules are shown.
Multiple EC adhesion molecules are required for stasis including mobilization of WPB constituents P-selectin and VWF to the EC surface. (A) Blocking antibodies to adhesion molecules P-selectin, E-selectin, VWF, VCAM-1, α4β1 integrin, ICAM-1, PECAM-1, αVβ3 integrin, thrombomodulin, or control IgG (30 µg per mouse, IV) were infused into NY1DD sickle mice with DSFCs 5 minutes prior to infusion of heme (3.2 µmol/kg). Percent stasis was measured at 1 hour after heme infusion as described in Figure 1. *P < .001 vs control IgG and #P < .001 antithrombomodulin vs anti-P-selectin, E-selectin, VWF, VCAM-1, α4β1 integrin, ICAM-1, PECAM-1, or αVβ3 integrin. (B) HUVECs were fixed in 4% paraformaldehyde and stained for surface P-selectin (green) or VWF (red) after the indicated incubations. Nuclei were counterstained with DAPI (blue). White bars represent 40 µm. Representative cells are shown. Top row: HUVECs in 0.1% FBS were incubated with heme (20 µM) for 15 minutes, HbA or HbS (20 µM heme) for 24 hours, or HbA for 24 hours followed by heme for 15 minutes. Middle row: HUVECs were incubated with heme + hemopexin (20 μM), HbA + haptoglobin (20 µM), or HbA + hemopexin for 24 hours. Bottom row: HUVECs were incubated for 24 hours in 0.1% FBS with or without HbA or HbS followed by H/R (5% CO2/95% N2 for 3 hours and 5% CO2/95% air for 1 hour). Control cells were incubated in 0.1% FBS for 24 hours. Cells were not permeabilized; all pictures represent cell-surface expression. (C) Normal C57BL/6 and NY1DD sickle mice were infused with saline (12 mL/kg, negative control), histamine (1200 µmol/kg, positive control), or heme (3.2 µmol/kg). Fifteen minutes after infusion, mice were sacrificed in CO2 and dorsal skin (n = 1/treatment) was removed and fixed in Zamboni’s for immunofluorescence staining of VWF (red) and counterstaining of nuclei with DAPI (blue). White bars represent 30 µm. Representative venules are shown.
Hb, heme, or H/R induces the rapid expression of P-selectin and VWF on HUVEC surfaces
Two of the adhesion molecules required for stasis, P-selectin and VWF, are stored in EC WPBs.32 Agonists of WPB mobilization, such as histamine and thrombin, induce transport of WPBs to EC surfaces within minutes. Since stasis occurs less than 30 minutes after heme infusion (data not shown), we examined P-selectin and VWF expression on the cell surface of HUVECs treated with Hb, heme, and/or H/R. Heme stimulated the expression of P-selectin and VWF “strings” on the cell surface within 15 minutes of heme exposure (Figure 2B) and as early as 5 minutes after heme addition (data not shown). Heme-mediated expression was inhibited by hemopexin. Treatment of HUVECs with HbA or HbS for 24 hours stimulated P-selectin and VWF expression that was inhibited by haptoglobin or hemopexin, supporting a role for free-heme in Hb-mediated WPB degranulation. Treatment of HUVECs with Hb for 24 hours followed by heme for 15 minutes enhanced P-selectin and VWF expression on the cell surface to levels higher than with heme or Hb alone. H/R stimulated VWF expression that was enhanced in ECs pretreated with HbA or HbS for 24 hours. P-selectin and VWF surface expression was greater in ECs pretreated with HbS + H/R vs HbA + H/R, suggesting greater release of heme from HbS after H/R.
Heme induces rapid P-selectin and VWF expression on vessel walls in vivo
Heme was infused into C57BL/6 and NY1DD mice to examine P-selectin and VWF expression in vivo. Infusion with histamine (WPB agonist) or saline provided positive and negative controls, respectively. After 15 minutes, mice were sacrificed and organs examined for expression of P-selectin and VWF on vessel surfaces. Varying degrees of P-selectin and VWF expression occurred on venules in the skin (Figure 2C, only VWF shown), lungs (supplemental Figure 2A), brain (supplemental Figure 2B), liver (supplemental Figure 2C), and kidneys (supplemental Figure 2D) of heme- and histamine-treated mice. Expression of P-selectin and VWF in saline-treated mice was visibly lower but higher in saline-treated NY1DD than in C57BL/6 mice. Thus, heme markedly induces expression of P-selectin and VWF on vessel walls in both normal and sickle mice in vivo.
Heme-mediated stasis and P-selectin and VWF expression requires NOX, PKC, and oxidants
NADPH oxidase (NOX) is a major source of oxidants in ECs and has been implicated in vessel wall adhesion in SCD mice.33 NOX inhibitors apocynin or DPI inhibited heme-induced stasis in NY1DD mice (P < .05, Figure 3A). Inhibition of NOX activator protein kinase C (PKC) with calphostin C or treatment with the iron chelator S-DFO or the antioxidant NAC inhibited stasis (P < .05; Figure 3A). Also, heme-mediated VWF expression on HUVECs was blocked by apocynin or calphostin C treatment (Figure 3B). Moreover, apocynin, DPI, calphostin C, and the antioxidants NAC, quercetin, or DFO prevented heme-mediated ROS production in HUVECs (P < .025; Figure 3C). These data indicate that PKC, NOX, and oxidants are required for heme-induced WPB degranulation and stasis.
Heme-mediated stasis and P-selectin and VWF expression require NOX, PKC, and oxidants. (A) Thirty minutes prior to infusion of heme (3.2 µmol/kg), NY1DD mice (n = 3 per treatment) were injected with NOX inhibitors apocynin (10 mg/kg, IP) or DPI (30 mg/kg, IP), the PKC inhibitor calphostin C (100µg/kg, IP), the antioxidant NAC (2.4 mg/kg, IP), or the iron chelator S-DFO (6 mL/kg, IV, every third day × 4). Controls include vehicle (0.012 mL/g dimethylsulfoxide [DMSO], IP) or hetastarch (6 mL/kg, IV, every third day × 4). Percent stasis was measured using intravital microscopy 1 hour after heme infusion. Bars are mean percent stasis + SD with mean stasis values written above the bars. *P < .05 vs control. (B) HUVECs were pretreated 30 minutes with apocynin (1 mM) or calphostin C (300 nm) followed by the addition of heme (10 µM) for 15 minutes. Control cells were untreated. Cells were fixed and immunostained for VWF (red) on the cell surface. Nuclei were counterstained with DAPI (blue). (C) Heme-induced oxidative stress was measured in HUVECs. Confluent HUVECs in 1% FBS were pretreated for 1 hour with vehicle (DMSO), apocynin (10 mM), DPI (20 µM), calphostin C (200 nM), NAC (10 mM), quercetin (50 µM), or DFO (100 µM). After 1 hour, cells were washed and loaded with the cell-permeable ROS probe DCFH-DA (100 µM) for 30 minutes. After 30 minutes, cells were washed and incubated with heme (20 µM) or LPS (20 ng/mL) in 0.1% FBS media for 4 hours. After 4 hours, cells were washed and ROS production was measured as accumulated cell fluorescence from oxidized DCFH-DA. Values are means + SD (n = 4 or 5 wells per treatment).
Heme-mediated stasis and P-selectin and VWF expression require NOX, PKC, and oxidants. (A) Thirty minutes prior to infusion of heme (3.2 µmol/kg), NY1DD mice (n = 3 per treatment) were injected with NOX inhibitors apocynin (10 mg/kg, IP) or DPI (30 mg/kg, IP), the PKC inhibitor calphostin C (100µg/kg, IP), the antioxidant NAC (2.4 mg/kg, IP), or the iron chelator S-DFO (6 mL/kg, IV, every third day × 4). Controls include vehicle (0.012 mL/g dimethylsulfoxide [DMSO], IP) or hetastarch (6 mL/kg, IV, every third day × 4). Percent stasis was measured using intravital microscopy 1 hour after heme infusion. Bars are mean percent stasis + SD with mean stasis values written above the bars. *P < .05 vs control. (B) HUVECs were pretreated 30 minutes with apocynin (1 mM) or calphostin C (300 nm) followed by the addition of heme (10 µM) for 15 minutes. Control cells were untreated. Cells were fixed and immunostained for VWF (red) on the cell surface. Nuclei were counterstained with DAPI (blue). (C) Heme-induced oxidative stress was measured in HUVECs. Confluent HUVECs in 1% FBS were pretreated for 1 hour with vehicle (DMSO), apocynin (10 mM), DPI (20 µM), calphostin C (200 nM), NAC (10 mM), quercetin (50 µM), or DFO (100 µM). After 1 hour, cells were washed and loaded with the cell-permeable ROS probe DCFH-DA (100 µM) for 30 minutes. After 30 minutes, cells were washed and incubated with heme (20 µM) or LPS (20 ng/mL) in 0.1% FBS media for 4 hours. After 4 hours, cells were washed and ROS production was measured as accumulated cell fluorescence from oxidized DCFH-DA. Values are means + SD (n = 4 or 5 wells per treatment).
Heme triggers stasis via TLR4-mediated P-selectin and VWF expression on ECs
Heme activates TLR4 signaling and tumor necrosis factor α (TNF-α) production in macrophages.4 TLR4 signaling was examined in heme-treated HUVECs. We inhibited TLR4 signaling with TAK-242 (resatorvid), a specific small-molecule inhibitor of TLR4 signaling that selectively binds to Cys747 on the intracellular domain of TLR4 and interrupts interactions between TLR4 and its adaptor molecules.34 TAK-242 inhibited heme-induced P-selectin and VWF expression on the surface of HUVECs (Figure 4A), indicating that heme induces the release of P-selectin and VWF via TLR4 signaling.
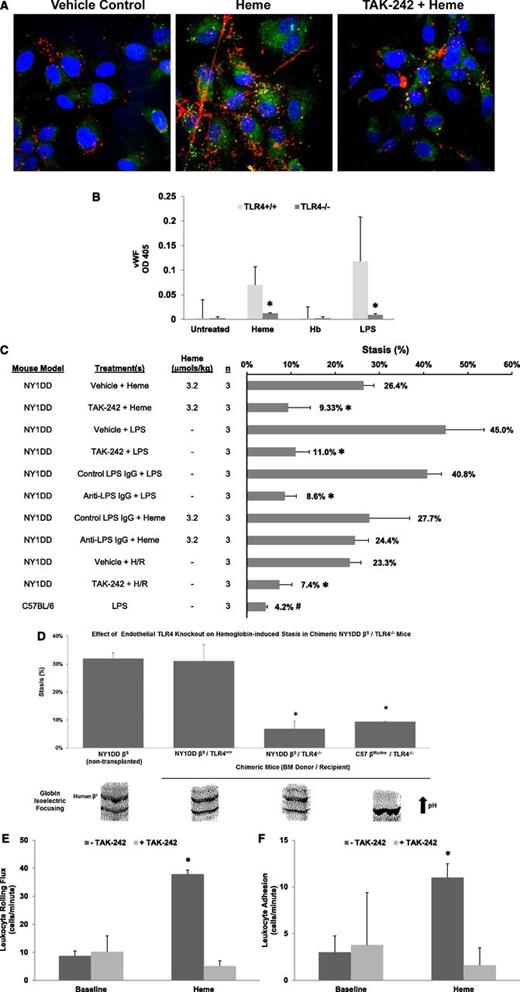
Endothelial TLR4 signaling is required for WPB activation and vascular stasis induced by heme. (A) HUVECs were incubated with vehicle (DMSO), heme (10 µM), or heme plus the TLR4-signaling inhibitor TAK-242 (400 nM). After a 15-minute incubation, cells were fixed and stained for surface P-selectin (green) and VWF (red). Nuclei were counterstained with DAPI (blue). Data are representative of at least 3 experiments. (B) mPVECs were isolated and cultured from TLR4+/+ and TLR4−/− mice. mPVECs were incubated with heme (10 µM), Hb (10 µM heme), or LPS (10 ng/ml) for 15 minutes (n = 6 wells per treatment). Cells were fixed and surface VWF was measured by enzyme immunoassay (*P < .05 for TLR4+/+ vs TLR4−/−). (C) Percent stasis was measured in the subcutaneous venules of NY1DD and C57BL/6 mice with DSFCs as described in Figure 1. Mice were given a bolus infusion (0.012 mL/g) of the indicated treatments at the specified heme doses: vehicle (DMSO/saline, 1:9 vol/vol) + heme (3.2 µmol/kg), TAK-242 (2 mg/kg, IP 30 minutes before heme) + heme, vehicle + LPS (1 mg/kg), TAK-242 + LPS, isotype control IgG (30 µg/mouse) + LPS, anti-LPS IgG (30 µg per mouse) + LPS, isotype control IgG + heme, anti-LPS IgG + heme, vehicle + H/R, TAK-242 + H/R, or LPS alone. The numbers of mice (n) in each treatment group are indicated. Bars are mean % stasis + SD with mean stasis values written above the bars. *P < .01 vs control; #P < .001 C57 + LPS vs NY1DD vehicle + LPS. (D) Chimeric NY1DD βS/TLR4−/− and NY1DD βS/TLR4+/+ mice were generated by transplanting BM from NY1DD βS sickle mice into TLR4−/− or TLR4+/+ mice (n = 3 recipients per group). C57BL/6 mice were used as TLR4+/+ mice. Negative control chimeric C57 βMurine/TLR4−/− mice were generated by transplanting BM from C57BL/6 mice into TLR4−/− mice. The presence of human βS was confirmed by isoelectric focusing (IEF) at 3 months posttransplant. Representative IEF bands for each group are shown (bottom). Stasis was measured in transplanted mice 1 to 2 weeks after IEF determination after IV infusion of Hb (0.32 µmol heme/kg) (*P < .01 NY1DD βS/TLR4−/− vs NY1DD βS/TLR4+/+). Nontransplanted NY1DD βS mice (n = 2) served as positive controls. All stasis values are mean percent stasis + SD. (E) Leukocyte rolling flux was measured in venules of NY1DD mice with DSFCs before (baseline) and 1 hour after infusion of heme (3.2 µmol/kg). Half of the mice (n = 4) were treated with TAK-242 after baseline (+TAK-242, 2 mg/kg, IP, 30 minutes before heme). Control mice (n = 4) were untreated with TAK-242 (−TAK-242). Values are mean number of rolling cells per minute + SD. (F) Leukocyte adhesion was measured in the same venules as described in panel C. Values are mean number of adherent cells per 100 µm + SD.
Endothelial TLR4 signaling is required for WPB activation and vascular stasis induced by heme. (A) HUVECs were incubated with vehicle (DMSO), heme (10 µM), or heme plus the TLR4-signaling inhibitor TAK-242 (400 nM). After a 15-minute incubation, cells were fixed and stained for surface P-selectin (green) and VWF (red). Nuclei were counterstained with DAPI (blue). Data are representative of at least 3 experiments. (B) mPVECs were isolated and cultured from TLR4+/+ and TLR4−/− mice. mPVECs were incubated with heme (10 µM), Hb (10 µM heme), or LPS (10 ng/ml) for 15 minutes (n = 6 wells per treatment). Cells were fixed and surface VWF was measured by enzyme immunoassay (*P < .05 for TLR4+/+ vs TLR4−/−). (C) Percent stasis was measured in the subcutaneous venules of NY1DD and C57BL/6 mice with DSFCs as described in Figure 1. Mice were given a bolus infusion (0.012 mL/g) of the indicated treatments at the specified heme doses: vehicle (DMSO/saline, 1:9 vol/vol) + heme (3.2 µmol/kg), TAK-242 (2 mg/kg, IP 30 minutes before heme) + heme, vehicle + LPS (1 mg/kg), TAK-242 + LPS, isotype control IgG (30 µg/mouse) + LPS, anti-LPS IgG (30 µg per mouse) + LPS, isotype control IgG + heme, anti-LPS IgG + heme, vehicle + H/R, TAK-242 + H/R, or LPS alone. The numbers of mice (n) in each treatment group are indicated. Bars are mean % stasis + SD with mean stasis values written above the bars. *P < .01 vs control; #P < .001 C57 + LPS vs NY1DD vehicle + LPS. (D) Chimeric NY1DD βS/TLR4−/− and NY1DD βS/TLR4+/+ mice were generated by transplanting BM from NY1DD βS sickle mice into TLR4−/− or TLR4+/+ mice (n = 3 recipients per group). C57BL/6 mice were used as TLR4+/+ mice. Negative control chimeric C57 βMurine/TLR4−/− mice were generated by transplanting BM from C57BL/6 mice into TLR4−/− mice. The presence of human βS was confirmed by isoelectric focusing (IEF) at 3 months posttransplant. Representative IEF bands for each group are shown (bottom). Stasis was measured in transplanted mice 1 to 2 weeks after IEF determination after IV infusion of Hb (0.32 µmol heme/kg) (*P < .01 NY1DD βS/TLR4−/− vs NY1DD βS/TLR4+/+). Nontransplanted NY1DD βS mice (n = 2) served as positive controls. All stasis values are mean percent stasis + SD. (E) Leukocyte rolling flux was measured in venules of NY1DD mice with DSFCs before (baseline) and 1 hour after infusion of heme (3.2 µmol/kg). Half of the mice (n = 4) were treated with TAK-242 after baseline (+TAK-242, 2 mg/kg, IP, 30 minutes before heme). Control mice (n = 4) were untreated with TAK-242 (−TAK-242). Values are mean number of rolling cells per minute + SD. (F) Leukocyte adhesion was measured in the same venules as described in panel C. Values are mean number of adherent cells per 100 µm + SD.
Heme does not induce VWF expression on mPVECs from TLR4−/− mice
We quantified VWF expression on mPVECs in primary culture from TLR4+/+ and TLR4−/− mice by enzyme immunoassay. LPS, the classic TLR4 ligand, or heme induced VWF expression within 15 minutes on TLR4+/+, but not TLR4−/−, mPVEC cell surfaces (Figure 4B). This finding confirms that TLR4 is required for heme- and LPS-mediated release of VWF to the EC surface. Treatment of cells with Hb for 15 minutes did not induce VWF expression on either TLR4+/+ or TLR4−/− mPVECs, indicating that Hb does not release significant amounts of heme in the first 15 minutes of cell culture.
Heme and LPS induce stasis via TLR4 signaling
We examined whether TLR4 signaling is required for stasis. TAK-242 inhibited heme-induced stasis in NY1DD mice at 1 hour (Figure 4C; P < .01, 26.4% stasis with vehicle + heme vs 9.3% stasis with TAK-242 + heme).
LPS also induced stasis in NY1DD mice through TLR4. Mice were treated with vehicle + LPS or TAK-242 + LPS (Figure 4C). LPS induced stasis (45.0%), but pretreatment with TAK-242 inhibited LPS-induced stasis (11.0%, P < .001). Thus, both heme and LPS initiate TLR4 signaling, P-selectin and VWF expression, and vaso-occlusion in sickle mice. LPS did not induce stasis in C57BL/6 mice (4.2%; Figure 4C), indicating the βS globin is required for stasis.
Heme-induced TLR4 signaling is not due to LPS contamination
All Hb/heme preparations contained <0.1 EU/mL. Anti-LPS IgG directed against the LPS core did not inhibit heme-induced stasis (27.7% stasis with isotype control IgG + heme vs 24.4% stasis with anti-LPS IgG + heme) but significantly inhibited LPS-induced stasis (40.8% stasis with isotype control IgG + LPS vs 8.6% stasis with anti-LPS IgG + LPS, P < .001) (Figure 4C), confirming that heme-specific TLR4 activation is not caused by LPS contamination.
TAK-242 inhibits H/R-induced stasis
TAK-242 also inhibited H/R-induced stasis in NY1DD mice at 1 hour (23.3% stasis with vehicle + H/R vs 7.4% stasis with TAK-242 + H/R, P < .01) (Figure 4C). Thus, H/R-induced stasis requires TLR4 signaling that may be initiated by increased plasma heme levels. In corroboration, H/R-induced stasis in sickle mice was accompanied by an increase in total plasma heme (Figure 1C).
Heme does not induce vaso-occlusion in TLR4−/− mice transplanted with BM from TLR4+/+ sickle mice
To confirm that heme-induced stasis requires endothelial TLR4 signaling, we generated chimeric NY1DD sickle mice lacking endothelial TLR4 expression by transplanting BM from NY1DD sickle mice into lethally irradiated TLR4−/− or TLR4+/+ mice. Three months posttransplant, the presence of the βS globin in blood was confirmed by isoelectric focusing (Figure 4D, bottom), DSFCs were implanted, and stasis was measured 1 hour after heme infusion. Nontransplanted NY1DD mice and TLR−/− mice transplanted with BM from C57BL/6 mice served as positive and negative controls, respectively. Heme-induced stasis was prevented in NY1DD βS/TLR4−/− chimeric mice, but not in NY1DD βS/TLR4+/+ chimeric mice or nontransplanted NY1DD mice (Figure 4D, top). These results confirm that heme induces stasis in sickle mice through endothelial TLR4.
Heme induces leukocyte rolling and adhesion that is inhibited by TAK-242
Leukocyte rolling and adhesion were measured in subcutaneous venules in NY1DD with DSFCs at baseline and after infusion of heme ± TAK-242. Leukocyte rolling flux (Figure 4E) and adhesion (Figure 4F) increased significantly after heme infusion in mice not pretreated with TAK-242. Pretreatment with TAK-242 blocked the heme-mediated increase in rolling and adhesion.
Heme activates endothelial NF-κB through TLR4 signaling
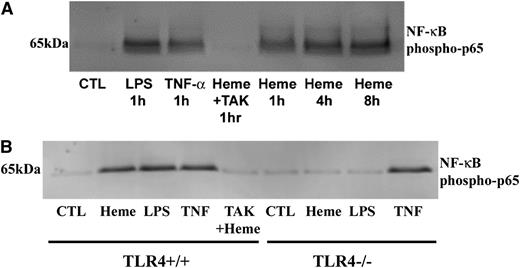
We previously demonstrated that NF-κB is activated and adhesion molecules are overexpressed in transgenic sickle mice.7 To determine whether heme activates NF-κB via TLR4 signaling, we used TAK-242 to inhibit TLR4 signaling in HUVECs (Figure 5A). LPS, TNF-α, and heme increased nuclear NF-κB phospho-p65, a specific marker of NF-κB activation.23 TAK-242 blocked nuclear NF-κB phospho-p65 induction by heme. Similar findings were observed in mPVECs isolated from TLR4+/+ and TLR4−/− mice (Figure 5B). Heme, LPS, and TNF-α activated nuclear NF-κB phospho-p65 in TLR4+/+ mPVECs and TAK-242 inhibited heme-mediated activation. In TLR4−/− cells, NF-κB was activated by TNF-α, but not by heme or LPS. These findings demonstrate that heme and LPS activate nuclear NF-κB phospho-p65 in ECs via TLR4 signaling.
Heme and LPS induce EC NF-κB activation via TLR4 signaling. (A) HUVECs were incubated with LPS (10 ng/ml), TNF-α (10 ng/ml), heme (10 µM), or TAK-242 (400 nM) + heme for the indicated times (1-8 hours). At the end of the incubation period, nuclear extracts were prepared, run on a western blot, and stained for NF-κB phospho-p65. (B) mPVECs from TLR4+/+ or TLR4−/− mice were incubated with heme (10 µM), LPS (10 ng/ml), TNF-α (10 ng/ml), or TAK-242 (400 nM) + heme (10 µM) for 1 hour. At the end of the incubation period, nuclear extracts were prepared, run on western blot, and stained for NF-κB phospho-p65.
Heme and LPS induce EC NF-κB activation via TLR4 signaling. (A) HUVECs were incubated with LPS (10 ng/ml), TNF-α (10 ng/ml), heme (10 µM), or TAK-242 (400 nM) + heme for the indicated times (1-8 hours). At the end of the incubation period, nuclear extracts were prepared, run on a western blot, and stained for NF-κB phospho-p65. (B) mPVECs from TLR4+/+ or TLR4−/− mice were incubated with heme (10 µM), LPS (10 ng/ml), TNF-α (10 ng/ml), or TAK-242 (400 nM) + heme (10 µM) for 1 hour. At the end of the incubation period, nuclear extracts were prepared, run on western blot, and stained for NF-κB phospho-p65.
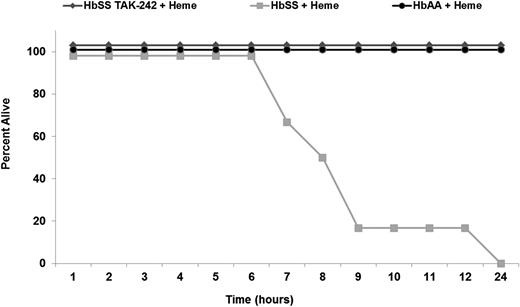
TAK-242 rescues HbSS-Townes mice from heme-induced lethality
In studies in HbSS-Townes mice, we found that heme infusion was lethal within 24 hours (Figure 6). Autopsies could not determine a definitive cause of death. Heme was not lethal in HbAA-Townes mice, suggesting the βS mutation is needed for heme lethality. Pretreatment with TAK-242 completely prevented heme-induced lethality, indicating a role for TLR4 signaling in heme-induced lethality.
Intravenous heme is lethal in HbSS, but not HbAA, mice and inhibition of TLR4 signaling with TAK-242 rescues HbSS mice. All HbSS and HbAA mice (n = 6 mice per treatment) were given a bolus infusion of heme (32 µmol/kg) at time zero. One treatment group received TAK-242 (2 mg/kg, IP) 30 minutes before heme infusion. Time of death after heme infusion was recorded.
Intravenous heme is lethal in HbSS, but not HbAA, mice and inhibition of TLR4 signaling with TAK-242 rescues HbSS mice. All HbSS and HbAA mice (n = 6 mice per treatment) were given a bolus infusion of heme (32 µmol/kg) at time zero. One treatment group received TAK-242 (2 mg/kg, IP) 30 minutes before heme infusion. Time of death after heme infusion was recorded.
Discussion
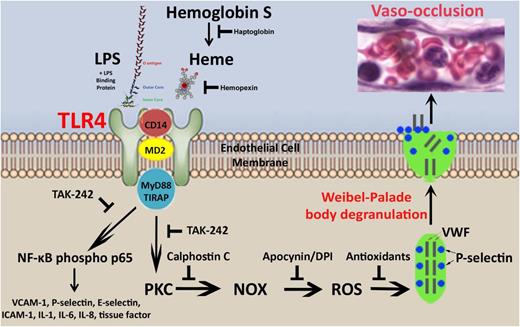
Heme, released during hemolysis, activates TLR4 signaling on ECs leading to delivery of WPB constituents P-selectin and VWF to the vessel wall and NF-κB activation. These events trigger vaso-occlusion in sickle, but not normal, mice. The effects of heme are mimicked by TLR4 ligand LPS, thereby supporting a model that links hemolysis- and infection-induced vaso-occlusion in SCD through TLR4 signaling leading to NF-κB, PKC, and NOX activation; ROS production; and WPB degranulation (Figure 7).
Proposed model of heme-induced stasis. Intravascular hemolysis in SCD leads to release of heme from methemoglobin S. Heme release can be blocked by haptoglobin or the liberated heme can be bound and removed by hemopexin. When plasma levels of haptoglobin and hemopexin are depleted, heme can activate TLR4 signaling at the EC membrane independently of LPS, but in manner similar to LPS + LPS binding protein that is dependent on cofactors CD14 and MyD88.4 TLR4 signaling, which can be inhibited by TAK-242, leads to the activation of NF-κB phospho-p65 and the transcription of NF-κB–responsive genes in ECs including VCAM-1, P-selectin, E-selectin, interleukin-1, interleukin-6, interleukin-8, and tissue factor. TLR4 signaling also activates an acute stress sentinel pathway that involves PKC activation leading to the production of ROS by endothelial NOX and EC degranulation of WPB P-selectin and VWF to the cell surface. Rapid mobilization of P-selectin and VWF to the cell surface, triggers vaso-occlusion. Vaso-occlusion also requires the expression of NF-κB–responsive adhesion molecules VCAM-1, ICAM-1, and E-selectin that are chronically upregulated on the endothelium of transgenic sickle mice in steady state.7
Proposed model of heme-induced stasis. Intravascular hemolysis in SCD leads to release of heme from methemoglobin S. Heme release can be blocked by haptoglobin or the liberated heme can be bound and removed by hemopexin. When plasma levels of haptoglobin and hemopexin are depleted, heme can activate TLR4 signaling at the EC membrane independently of LPS, but in manner similar to LPS + LPS binding protein that is dependent on cofactors CD14 and MyD88.4 TLR4 signaling, which can be inhibited by TAK-242, leads to the activation of NF-κB phospho-p65 and the transcription of NF-κB–responsive genes in ECs including VCAM-1, P-selectin, E-selectin, interleukin-1, interleukin-6, interleukin-8, and tissue factor. TLR4 signaling also activates an acute stress sentinel pathway that involves PKC activation leading to the production of ROS by endothelial NOX and EC degranulation of WPB P-selectin and VWF to the cell surface. Rapid mobilization of P-selectin and VWF to the cell surface, triggers vaso-occlusion. Vaso-occlusion also requires the expression of NF-κB–responsive adhesion molecules VCAM-1, ICAM-1, and E-selectin that are chronically upregulated on the endothelium of transgenic sickle mice in steady state.7
Heme induces TNF-α secretion in monocyte/macrophages through TLR4 signaling.4 Our studies expand this finding by demonstrating that heme activates EC WPB degranulation, NF-κB, and vaso-occlusion in SCD mice. Heme, not ferrous Hb, was the active molecule in TLR4 induction of vaso-occlusion. MetHb, which gives up heme, induced stasis, whereas cyanometHb, which tightly binds heme,25 did not. Neither heme nor Hb was able to induce stasis or WPB degranulation in the presence of the high-affinity heme-binding protein hemopexin. Our data suggest that generation of metHb is a necessary step in the release of heme. The metHb reducing agent methylene blue at clinically relevant concentrations also inhibited Hb-induced stasis. MetHb can be generated in vivo when oxyhemoglobin reacts with NO. Plasma metHb levels are elevated in SCD10 and are further increased by inhaled NO.35 Thus, plasma oxyhemoglobin S in the pro-oxidative environment of SCD11 may react with NO or other oxidants to generate methemoglobin S, which can release heme.
Plasma heme in SCD patients is approximately 4 μM compared with 0.2 μM in normal volunteers.10 However, plasma heme levels in SCD patients can exceed 20 µM.10,36,37 In our studies, plasma heme is elevated in sickle mice and positively correlated with stasis. Endothelial TLR4 signaling and vascular stasis were induced at plasma heme levels that are in the pathophysiologic range (20-60 µM). Steady-state plasma heme levels reflect increased heme release during hemolysis and degradation by heme oxygenase but do not completely reveal the excessive heme throughput in SCD. Sustained extravascular hemolysis in SCD and shortened RBC lifespans, especially during crisis,9 continually deliver heme from sickle RBC to the vessel wall.
Although heme and Hb can activate endothelium in wild-type C57BL/6 mice and human and mouse ECs in culture, the βS mutation was necessary for stasis. In C57BL/6 mice, supraphysiologic infusions (32 µmol heme/kg) of Hb or heme that overwhelm plasma haptoglobin or hemopexin induced similar amounts of stasis (5.9% to 6.1%) as saline. Stasis was dose responsive to βS expression as heterozygous HbAS mice had stasis intermediate between HbAA and HbSS mice.
Stasis (∼10%) in untreated HbSS mice in steady state, but not HbAA mice or in HbSS mice treated with haptoglobin or hemopexin, supports a model in which hemolysis-driven vaso-occlusion is occurring continuously in SCD. HUVECs treated with Hb for 24 hours to mimic chronic hemolysis had increased P-selectin and VWF on the cell surface relative to untreated HUVECs. The addition of heme or H/R to the Hb-treated cells, simulating enhanced local hemolysis or hypoxic venules, elicited an exaggerated P-selectin/VWF response. Unstable HbS38 elicited even greater inflammatory responses and stasis than HbA. The WPB agonists heme and histamine stimulated expression of P-selectin and VWF on vessel walls of sickle and normal mice in all tissues examined (skin, lung, liver, brain, and kidney). However, in tissues, there were variable responses (eg, EC activation and vaso-occlusion) that may depend on local factors in different vascular beds including EC heterogeneity, hemolysis, cytokines, haptoglobin/hemopexin levels, heme oxygenase activity, infections, and O2 gradients. We hypothesize that local or global changes in hemolytic rates, infection, or hypoxia may precipitate vaso-occlusive crises.
Hemolytic rates in SCD correlate with the quantity of “activated” VWF in plasma.39 Large VWF multimers promote thrombosis in SCD and increase adhesion of SS-RBC to ECs.31 In SCD plasma, the ADAMTS13 proteolysis site in VWF can be blocked by Hb bound to the A2-domain of these VWF multimers,40 fostering an adhesive milieu. Our data demonstrate that heme induces VWF expression on endothelium and provide an explanation for the correlation among hemolytic rates, plasma VWF levels, and vaso-occlusive crises.
Haptoglobin minimizes heme loss from Hb by transporting Hb to CD163 on macrophages for catabolism.41 SCD patients have low plasma haptoglobin levels.10 Plasma haptoglobin levels were lower in sickle mice compared with normal control mice (supplemental Figure 3). Infusion of human haptoglobin blocked Hb-induced stasis in sickle mice. These observations provide a rationale for the use of haptoglobin as a new therapeutic approach to SCD.42
When haptoglobin is overwhelmed by excess plasma Hb, Hb can be oxidized to metHb with subsequent liberation of heme. The released heme can bind to plasma hemopexin (Kd < 1 pM) or albumin (Kd ∼10 nM) from which it is normally transferred to hemopexin.43 However, when haptoglobin and hemopexin are depleted, albumin becomes a reservoir for heme.44 Normally, hemopexin safely transports heme away from the vessel wall to CD91 on hepatocytes where the heme is degraded and hemopexin recycled back to the plasma.45-47 Thus, hemopexin protects the endothelium from heme-induced oxidative stress, inflammation, and cellular injury.47-49 In SCD patients37 and sickle mice (supplemental Figure 3), hemopexin levels are lower than in normal subjects. Despite the shorter half-life of RBCs in HbSS-Townes mice compared with NY1DD mice, plasma haptoglobin and hemopexin levels appear similar, perhaps explaining the similar amounts of stasis (30%) seen when infused with low doses of Hb (0.32 µmol/kg). However, it is noteworthy that stasis plateaued at supraphysiological heme doses (32 µmol/kg) and was approximately 10% higher in HbSS-Townes (50%) vs NY1DD (40%) mice (data not shown). These data suggest that hemopexin supplementation might blunt EC dysfunction in haptoglobin/hemopexin deficiency states such as SCD.42
Infusion of antibodies to WPB P-selectin or VWF inhibited stasis. Adhesion molecules that are not found in WPBs but are found on activated endothelium are also required for vaso-occlusion. Antibody blockade of adhesion molecules VCAM-1, α4β1 integrin, ICAM-1, PECAM-1, E-selectin, or αVβ3 integrin inhibited heme-induced stasis. Remarkably, blockade of only one adhesion molecule inhibited stasis. Isotype control antibodies or antibodies to thrombomodulin on the EC membrane had limited effects on stasis. PECAM-1 is required for leukocyte extravasation and for the first time has been implicated in vaso-occlusion. Endothelial αVβ3 integrin mediates adhesion of sickle RBCs via activated intercellular adhesion molecule 4 ligands on SS-RBC.29 In addition, α4β1 integrin on leukocytes and SS-reticulocytes is a counter-receptor for VCAM-1 and can form multicellular aggregates in plasma linked by fibronectin.27 Thus, multiple cell adhesion molecules participate in vaso-occlusion. Further studies are warranted to examine the effects of TLR4 signaling on the expression and activation state of these adhesion molecules.
NOX may be involved in TLR4 signaling. LPS can activate WPB degranulation and NF-κB activation in ECs that requires oxidant production by NOX.50,51 In our studies, inhibition of NOX, its upstream activator PKC, or antioxidants inhibited heme-mediated stasis, WPB degranulation, and oxidant production. NOX in sickle RBCs and leukocytes and xanthine oxidase are also possible sources of oxidants at the vessel wall.52 Further studies in chimeric HbSS/NOX−/− mice are warranted.33
Autopsies were performed on the HbSS mice after they succumbed to intravenous heme. RBC congestion was present in the lungs, but the cause of death could not be definitively determined. Intravenous heme was recently shown to induce acute lung injury and death in sickle mice that can be averted by TLR4 inhibition, TLR4 knockout in nonhematopoietic tissues, or hemopexin replacement therapy.53 Suppression of TLR4-induced vaso-occlusion in the pulmonary vasculature may explain the survival of HbSS mice treated with TAK-242.
TNF-α, which is produced in response to heme-induced TLR4 signaling in monocytes,4 can induce P-selectin–dependent vaso-occlusion in SCD mice.30 TNF-α signaling, which is independent of TLR4, induces WPB degranulation at the vessel wall.54 Thus, multiple agonists (eg, TNF-α, LPS, interleukin-1β, thrombin, and histamine) in ECs can activate WPB exocytosis and vaso-occlusion in SCD. However, the defining and chronic nature of hemolysis in SCD suggests that heme-induced TLR4 signaling on monocytes, platelets, and ECs may be a primary driver of cell activation, inflammation, cytokine production, and vaso-occlusion.
EC TLR4 plays a critical role during sepsis and hemolytic states.55 Chronic hemolysis in SCD may promote a proinflammatory tone mediated by TLR4 signaling and oxidant production leading to WPB degranulation, activation of NF-κB, and increases in adhesion molecules and proinflammatory cytokines and chemokines.6 Thus, heme may be central to the evolution of EC dysfunction and vasculopathy in SCD. These studies uncover new pharmacologic modalities that interrupt heme-mediated inflammation and vaso-occlusion in SCD, including haptoglobin and hemopexin and inhibitors of TLR4 and adhesion molecules. Improved understanding of the regulatory elements of TLR4 signaling pathways in ECs may facilitate the development of novel therapies for SCD and sepsis. These data add support for the therapeutic impact of therapies that decrease hemolysis in SCD, scavenge Hb or heme (haptoglobin or hemopexin), detoxify heme, or modulate TLR4 activation. Furthermore, these therapies would likely impact the pathobiology of disseminated intravascular coagulation, transfusion reactions, crush injuries, and sepsis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (P01HL55552; R.P.H. and G.M.V.), (HL115467-01; G.M.V. and J.D.B.), (HL1109000-01A1; A.I.A.), and by a research grant award from Sangart (G.M.V. and J.D.B.). Dr Ann Smith thanks the University of Missouri Research Board and School of Biological Sciences at University of Missouri–Kansas City Research Incentive Funds for support. K.A.N. received support from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK47060). We would like to thank Graham Brown for preparation of metHb and cyanometHb, Dr Heather Bechtel for assistance with the graphics in Figure 7, Dr Mark Young and Ashok Malavalli at Sangart for the preparation and generous gift of stroma-free Hb, Bio Products Laboratory for the preparation and generous donation of human haptoglobin, and Bo Hedlund for the generous gift of S-DFO. Michael J. Franklin read the manuscript and was extremely helpful in making editorial suggestions.
Authorship
Contribution: J.D.B. designed the experiments, analyzed data, and wrote the paper; C.C. collected stasis, leukocyte rolling/adhesion, NF-κB, lethality, and plasma haptoglobin/hemopexin data; J.N. collected and analyzed cell culture, plasma heme, and immunohistochemistry data; L.M. and F.A. bred and phenotyped mice and performed transplants; A.I.A. designed the haptoglobin experiments and wrote the paper; A.S. designed the hemopexin experiments and wrote the paper; K.A.N. discussed the data and wrote the paper; R.P.H. analyzed data, designed experiments, and wrote the paper; and G.M.V. designed the experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.B., G.V., and R.H. receive research funding from and are consultants for Sangart.
Correspondence: John Belcher, University of Minnesota, 420 Delaware St SE, MMC 480, Minneapolis, MN 55455; e-mail: belcher@umn.edu.

![Figure 3. Heme-mediated stasis and P-selectin and VWF expression require NOX, PKC, and oxidants. (A) Thirty minutes prior to infusion of heme (3.2 µmol/kg), NY1DD mice (n = 3 per treatment) were injected with NOX inhibitors apocynin (10 mg/kg, IP) or DPI (30 mg/kg, IP), the PKC inhibitor calphostin C (100µg/kg, IP), the antioxidant NAC (2.4 mg/kg, IP), or the iron chelator S-DFO (6 mL/kg, IV, every third day × 4). Controls include vehicle (0.012 mL/g dimethylsulfoxide [DMSO], IP) or hetastarch (6 mL/kg, IV, every third day × 4). Percent stasis was measured using intravital microscopy 1 hour after heme infusion. Bars are mean percent stasis + SD with mean stasis values written above the bars. *P < .05 vs control. (B) HUVECs were pretreated 30 minutes with apocynin (1 mM) or calphostin C (300 nm) followed by the addition of heme (10 µM) for 15 minutes. Control cells were untreated. Cells were fixed and immunostained for VWF (red) on the cell surface. Nuclei were counterstained with DAPI (blue). (C) Heme-induced oxidative stress was measured in HUVECs. Confluent HUVECs in 1% FBS were pretreated for 1 hour with vehicle (DMSO), apocynin (10 mM), DPI (20 µM), calphostin C (200 nM), NAC (10 mM), quercetin (50 µM), or DFO (100 µM). After 1 hour, cells were washed and loaded with the cell-permeable ROS probe DCFH-DA (100 µM) for 30 minutes. After 30 minutes, cells were washed and incubated with heme (20 µM) or LPS (20 ng/mL) in 0.1% FBS media for 4 hours. After 4 hours, cells were washed and ROS production was measured as accumulated cell fluorescence from oxidized DCFH-DA. Values are means + SD (n = 4 or 5 wells per treatment).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/3/10.1182_blood-2013-04-495887/4/m_377f3.jpeg?Expires=1769081486&Signature=keO5EUIphZuRmuq8QbIyD-Blv6FP-FBFa3gkfHkxLv~Lbsa3CpTCATVnPBRKNYxdSG8N32UjGG8MlAO-3V65BGAWBmVE6RPQJDTCZf2rui571hWstUVOeVhcKb~v2s1r2wunJ9JYJiUFATYY8uiesKsTgkSdEnG9SMvbc8aWm~XTrB2Qlwqq3xycEtUNfkluta-SJsfaWA6YQU9gck4HM420TB3jqd0WuT-j0KPjsd4yek9KEFbciWa4Gbu5CcQ1RHGuof5RoWG51lUOO~EuwA6sfd1hO6GVDsI-AJlD5wchxCM~0LTXvGcGHwjmCmuIMcnoW7Sv0XFtLfBHFK61vQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)