In this issue of Blood, Strati et al provide evidence suggesting that the efficacy of chemoimmunotherapy is maintained in chronic lymphocytic leukemia (CLL) patients who received only 3 treatment cycles but nevertheless attained minimal residual disease (MRD) negativity.1
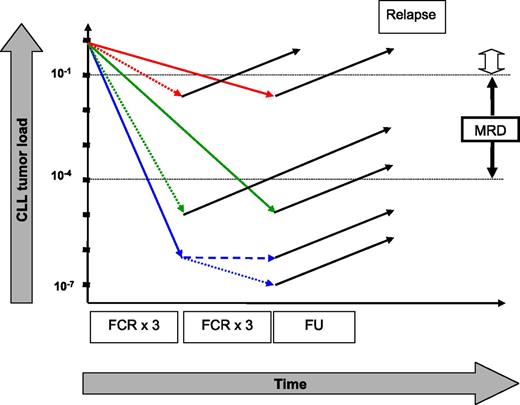
Hypothetical kinetics of tumor load in CLL patients who received 3 or 6 courses of FCR. Patients remaining MRD positive after treatment have a high likelihood of relapse (red arrows), whereas only 1 relapse was observed in MRD-negative patients (green and blue arrows). Three additional courses of FCR for patients who attained MRD negativity after the first 3 courses of FCR might be inefficacious (broken blue line) or might induce an additional reduction in tumor load (dotted blue line) that will translate into a clinical benefit with longer follow-up (FU designates current follow-up). The open arrow symbolizes the sensitivity of clinical staging. The figure is not to scale. See Figure 3A in the article by Strati et al that begins on page 3727.
Hypothetical kinetics of tumor load in CLL patients who received 3 or 6 courses of FCR. Patients remaining MRD positive after treatment have a high likelihood of relapse (red arrows), whereas only 1 relapse was observed in MRD-negative patients (green and blue arrows). Three additional courses of FCR for patients who attained MRD negativity after the first 3 courses of FCR might be inefficacious (broken blue line) or might induce an additional reduction in tumor load (dotted blue line) that will translate into a clinical benefit with longer follow-up (FU designates current follow-up). The open arrow symbolizes the sensitivity of clinical staging. The figure is not to scale. See Figure 3A in the article by Strati et al that begins on page 3727.
The outlook for patients with CLL has considerably improved over the past decade. Standard chemoimmunotherapies like the combination of fludarabine, cyclophosphamide, and rituximab (FCR) induce responses in up to 95% of all patients. These impressive response rates also translate into improved median overall survival and progression free survival (PFS). However, the duration of response remains very variable, and a relapse within 2 to 3 years after chemoimmunotherapy likely designates aggressive disease with shortened overall survival.2 Further improvements in clinical outcome will mainly depend on the prevention of such early relapses. The timely identification of poor-risk patients as candidates for alternative treatment or maintenance strategies therefore gains importance. TP53 defects as best-characterized genetic high-risk lesions can be used as markers for that purpose.3 The quantification of treatment response with a sensitivity of at least 1 CLL cell in 10 000 benign leukocytes (known as MRD4 ) represents an alternative means to predict such an inferior outcome. Patients who remain MRD positive after chemoimmunotherapy have a shortened PFS5-7 and are hence considered eligible for maintenance strategies in clinical trials.8
The research presented by Strati et al now for the first time provides evidence that MRD might also be used to identify candidates for dose deescalations.1 The authors report on a single-institution study of 237 CLL patients scheduled to receive 6 courses of FCR as first-line treatment; 148 of those patients had adequate bone marrow MRD assessments after 3 and again after 6 treatment cycles. Treatment was stopped after a maximum of 3 cycles in 50 patients mainly because of toxicity and comorbidities. With current follow-up, there were no differences in PFS or overall survival in patients who became MRD negative, irrespective of the time point at which this very good response was achieved (after 3 or 6 cycles) and irrespective of the number of treatment cycles. In patients in whom 3 treatment cycles were sufficient to induce MRD negativity, no impact of 3 more courses of FCR on PFS was detectable. If, on the other hand, the last 3 treatment cycles converted MRD-positive patients into MRD-negative patients, the prognosis was still excellent. This observation led to the hypothesis that it is safe to discontinue treatment after 3 courses of FCR provided that MRD negativity in bone marrow had already been attained at that time. The common denominator of good prognosis in the trial was the achievement of an MRD-negative response, irrespective of the number of FCR courses that were required to attain MRD negativity.
The study clearly corroborates the notion that achieving MRD negativity constitutes an important treatment goal in CLL. Although 2 recent investigations5,9 demonstrated that achieving MRD negativity using different treatment regimens is correlated with a similar response duration, the current investigation suggests that the number of treatment cycles also becomes irrelevant as long as MRD negativity can be attained. The findings presented by Strati and coworkers are in keeping with a previous observation showing identical median PFS for patients who became MRD negative in peripheral blood after 3 and after 6 courses of continued treatment with cyclophosphamide/fludarabine or FCR.5 They significantly add to published data by showing that treatment might be safely discontinued after achieving early MRD negativity in bone marrow. Nevertheless, with current follow-up, it remains possible that patients who become MRD negative after the first 3 treatment cycles might show a clinical benefit from an additional 3 courses of FCR when observed longer. Those 3 more courses of FCR might induce a further reduction in tumor load within the range below the currently accepted MRD threshold of 10−4 (1 CLL cell in 10 000 benign leukocytes) in MRD-negative patients (see figure). One can speculate that such an even more profound MRD response might translate into a clinical benefit with longer follow-up.
Patients included in the study were comprehensively characterized for pretherapeutic risk features, including cytogenetic aberrations, IGHV mutational status, and ZAP70.1 MRD negativity was maintained as significant for PFS and overall survival in multivariable analyses that included all those risk features as well as conventional response. These data from a large cohort of patients who received the current standard treatment of FCR are a very important confirmation of 2 previous publications.5,9 Together the 3 publications now clearly prove the independent prognostic significance of MRD in CLL when conventional risk predictors are considered1,5 and even when more recently discovered mutations in TP53, NOTCH1, MYD88, and SF3B19 are additionally considered. The added value of MRD likely stems from the fact that response, when sensitively quantified by MRD assessments, not only reflects the intrinsic apoptosis resistance of the leukemia but also factors such as the composition of the microenvironment, patients’ compliance, pharmacogenetics, and renal and hepatic function, all of which might impact on the efficacy of the treatment.
With an increasing number of interesting new compounds in clinical development for CLL, combination treatment will likely be tailored to achieve an optimal response in individual patients while minimizing toxicity.10 Such an optimal response will equal MRD negativity in many clinical situations. MRD has already been shown to be of prognostic significance after treatment with one of the novel agents, obinutuzumab,7 whereas the impact of MRD after other compounds still awaits clarification. Nevertheless, the possibility to safely discontinue treatment once MRD negativity has been attained in bone marrow, as suggested by the study of Strati et al, will likely impact on the design of future clinical trials and eventually on daily clinical routine.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal