Key Points
Activating MYD88 as well as nonsense and frameshift WHIM-like CXCR4 somatic mutations are common in WM.
CXCR4 NS mutations are present in aggressive cases including hyperviscosity syndrome, and MYD88 status is a determinant of survival.
Abstract
Whole genome sequencing has revealed activating somatic mutations in MYD88 (L265P) and CXCR4 in Waldenström macroglobulinemia (WM). CXCR4 somatic mutations in WM are the first ever reported in human cancer and are similar to nonsense (NS) and frameshift (FS) germline mutations found in warts, hypogammaglobulinemia, infections and myelokathexis (WHIM) syndrome. We genotyped lymphoplasmacytic cells from 175 WM patients and observed significantly higher bone marrow (BM) disease involvement, serum immunoglobulin-M levels, and symptomatic disease requiring therapy, including hyperviscosity syndrome in those patients with MYD88L265PCXCR4WHIM/NS mutations (P < .03). Patients with MYD88L265PCXCR4WHIM/FS or MYD88L265PCXCR4WILDTYPE (WT) had intermediate BM and serum immunoglobulin-M levels; those with MYD88WTCXCR4WT showed lowest BM disease burden. Fewer patients with MYD88L265P and CXCR4WHIM/FS or NS vs MYD88L265PCXCR4WT presented with adenopathy (P < .01), further delineating differences in disease tropism based on CXCR4 status. Neither MYD88 nor CXCR4 mutations correlated with SDF-1a (RS1801157) polymorphisms in 54 patients who were genotyped for these variants. Unexpectedly, risk of death was not impacted by CXCR4 mutation status, but by MYD88WT status (hazard ratio 10.54; 95% confidence interval 2.4-46.2, P = .0018). Somatic mutations in MYD88 and CXCR4 are important determinants of clinical presentation and impact overall survival in WM. Targeted therapies directed against MYD88 and/or CXCR4 signaling may provide a personalized treatment approach to WM.
Introduction
Waldenström macroglobulinemia (WM) is an incurable B-cell neoplasm characterized by accumulation of malignant lymphoplasmacytic cells (LPC) in the bone marrow (BM), lymph nodes, and spleen, and excess production of serum immunoglobulin-M (IgM) can produce symptoms related to hyperviscosity, tissue infiltration, and autoimmune-related pathology.1 The most striking findings from recent whole genome sequencing in WM is the discovery of 2 activating somatic mutations affecting Toll-like receptor and CXCR4 receptor signaling.2,3 Approximately 90% to 95% of WM patients carry MYD88L265P, an activating mutation that triggers interleukin-1 receptor–associated kinase (IRAK) and Bruton’s tyrosine kinase (BTK) that in turn activate nuclear factor-κB-p65–dependent nuclear translocation and malignant cell growth. The first reported somatic mutations in CXCR4 in cancer were identified by Hunter et al3 in 30% of WM patients and involve the carboxyl terminus (C terminus), which contains serine phosphorylation sites that regulate signaling of CXCR4 by its only known ligand SDF-1a (CXCL12). The location of somatic mutations in the C-terminal domain of WM patients are similar to those observed in the germline of patients with warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome, a congenital immunodeficiency disorder characterized by chronic noncyclic neutropenia.4,5 Germline mutations in the C terminus of CXCR4 in WHIM patients block receptor internalization after SDF-1a stimulation in myeloid cells that results in persistent CXCR4 activation and BM myeloid cell trafficking.6
In WM patients, 2 classes of CXCR4 mutations occur in the C terminus. These include nonsense (CXCR4WHIM/NS) mutations that truncate the distal 15- to 20-amino acid region and frameshift (CXCR4WHIM/FS) mutations that comprise a region of up to 40 amino acids in the C-terminal domain.3 Nonsense (NS) and frameshift (FS) mutations are almost equally divided among WM patients with CXCR4 somatic mutations. Preclinical studies with the most common CXCR4WHIM/NS mutation in WM (S338X) have shown enhanced and sustained AKT, extracellular signal-regulated kinase, and BTK signaling following SDF-1a relative to CXCR4WT as well increased cell migration, adhesion, growth, and survival of WM cells.7-9 The clinical implications for MYD88 and CXCR4 somatic mutations in WM remain to be delineated and are the focus of this report.
Patients and methods
BM aspirates and peripheral blood samples were collected after informed written consent from 175 untreated patients with the clinicopathological diagnosis of WM as defined by the Second International Workshop on WM.10 Consensus criteria were used to delineate patients as having symptomatic vs smoldering disease.11 The study was approved by the Dana Farber Cancer Institute/Harvard Cancer Center Institutional Review Board. This study was conducted in accordance with the Declaration of Helsinki. MYD88L265P status was determined using allele-specific polymerase chain reaction, and Sanger sequencing of the C-terminal domain of CXCR4 was performed using sorted (CD19+) lymphoplasmacytic cells (LPC) from BM aspirates as previously reported.2,11 The somatic nature of these mutations was confirmed by sequencing CD19-depleted peripheral blood cells.2,11 SDF-1a tumor sequencing and germline polymorphisms at RS1801157 were available for 54 patients in this study who underwent whole genome sequencing using previously reported methods.3
Statistical analysis
Data sets were analyzed by analysis of variance and nonparametric comparisons made by the Fisher’s exact probability test. Tukey’s honest significant difference test was used for pairwise analysis. Ordinal data were analyzed using pairwise Wilcoxon rank sum test with Holm Bonferroni correction. A P value < .05 was deemed to be significant. A multivariate analysis to estimate risk of death was performed using the Cox proportional hazard regression model. The Fleming-Harrington test was used to assess differences in Kaplan-Meier survival curves. Calculations were performed with R (R Foundation for Statistical Computing, Vienna, Austria).
Results
MYD88 and CXCR4 status in WM patients
MYD88L265P was detected in BM LPC from 158/175 (90.3%) patients, and mutations in the C terminus of CXCR4 were present in 51/175 (29.1%) patients. Fifty of 51 patients (98%) who were CXCR4WHIM-mutated also exhibited the MYD88L265P mutation (Table 1). Among the 51 CXCR4WHIM-mutated patients, 25 (49%) and 26 (51%) had NS and FS mutations, respectively. One patient with a CXCR4WHIM/FS mutation was MYD88WT. Two patients with CXCR4WHIM/NS demonstrated subclonal populations with other CXCR4 C-terminal mutations. One patient who had a K333X NS mutation had a subclonal population with a 1013C>G (S338X) NS mutation. Another patient with a S338X NS mutation demonstrated a subclonal population with 993_995insA (G332fs) FS mutation. All mutations were confirmed to be somatic in nature by sequencing of corresponding normal paired tissues.2 The CXCR4 somatic mutations that were identified after Sanger sequencing are annotated for the first time in this report and presented in Table 1.
Somatic mutations in the C terminus of CXCR4 identified by Sanger sequencing and stratified by MYD88 L265P mutation status in WM patients
| n . | MYD88 status . | Mutation type . | Nucleotide change . | Amino acid change . |
|---|---|---|---|---|
| 1 | L265P | NS | r.997 A>T* | K333X* |
| 3 | L265P | NS | r.1000C>T | R334X |
| 7 | L265P | NS | r.1013C>A | S338X |
| 15 | L265P | NS | r.1013C>G† | S338X† |
| 1 | WT | FS | r.931_933insT | T311fs |
| 3 | L265P | FS | r.952_954insA | T318fs |
| 2 | L265P | FS | r.951_953delACCTC | T318fs |
| 1 | L265P | FS | r.954_956insC | S319fs |
| 1 | L265P | FS | r.958_960delTG | V320fs |
| 1 | L265P | FS | r.963_965insC | R322fs |
| 1 | L265P | FS | r.969_971insG | S324fs |
| 1 | L265P | FS | r.978_980insT | K327fs |
| 1 | L265P | FS | r.984_986insT | L329fs |
| 1 | L265P | FS | r.993_995insA | G332fs |
| 1 | L265P | FS | r.1005_1007insT | G336fs |
| 2 | L265P | FS | r.1013_1015delATCT | S338fs |
| 1 | L265P | FS | r.1013_1015delATCTGTTTCCACTGAGT | S338fs |
| 3 | L265P | FS | r.1012_1014insT | S338fs |
| 1 | L265P | FS | r.1015_1017delCT | S339fs |
| 1 | L265P | FS | r.1020_1022delT | S341fs |
| 1 | L265P | FS | r.1024_1026delCT | S342fs |
| 1 | L265P | FS | r.1030_1041CTGAGTCTTC>GT | S344fs |
| 1 | L265P | FS | r.1033_1035delAG | E345fs |
| n . | MYD88 status . | Mutation type . | Nucleotide change . | Amino acid change . |
|---|---|---|---|---|
| 1 | L265P | NS | r.997 A>T* | K333X* |
| 3 | L265P | NS | r.1000C>T | R334X |
| 7 | L265P | NS | r.1013C>A | S338X |
| 15 | L265P | NS | r.1013C>G† | S338X† |
| 1 | WT | FS | r.931_933insT | T311fs |
| 3 | L265P | FS | r.952_954insA | T318fs |
| 2 | L265P | FS | r.951_953delACCTC | T318fs |
| 1 | L265P | FS | r.954_956insC | S319fs |
| 1 | L265P | FS | r.958_960delTG | V320fs |
| 1 | L265P | FS | r.963_965insC | R322fs |
| 1 | L265P | FS | r.969_971insG | S324fs |
| 1 | L265P | FS | r.978_980insT | K327fs |
| 1 | L265P | FS | r.984_986insT | L329fs |
| 1 | L265P | FS | r.993_995insA | G332fs |
| 1 | L265P | FS | r.1005_1007insT | G336fs |
| 2 | L265P | FS | r.1013_1015delATCT | S338fs |
| 1 | L265P | FS | r.1013_1015delATCTGTTTCCACTGAGT | S338fs |
| 3 | L265P | FS | r.1012_1014insT | S338fs |
| 1 | L265P | FS | r.1015_1017delCT | S339fs |
| 1 | L265P | FS | r.1020_1022delT | S341fs |
| 1 | L265P | FS | r.1024_1026delCT | S342fs |
| 1 | L265P | FS | r.1030_1041CTGAGTCTTC>GT | S344fs |
| 1 | L265P | FS | r.1033_1035delAG | E345fs |
Patient also had subclonal population with 1013C>G (S338X) NS mutation.
One patient had subclonal population with 993_995insA (G332fs) FS mutation.
MYD88 and CXCR4 mutation status and clinical presentation of WM patients
We first sought to delineate the impact of MYD88 and CXCR4 mutation status on the initial clinical presentation of WM patients. We limited our analysis to 3 subgroups of patients: MYD88WTCXCR4WT (n = 15); MYD88L265PCXCR4WT (n = 109); and those with MYD88L265PCXCR4WHIM mutations, which included 50 patients with MYD88L265PCXCR4WHIM. One patient with MYD88WTCXCR4WHIM who had an FS mutation in CXCR4 was not included in the initial analysis because of the singular nature of the presentation. This patient presented with 50% BM disease involvement, a serum IgM level of 550 mg/dL, adenopathy, and renal failure attributed to symptomatic WM disease.
Among the 174 patients included in the analysis, those with MYD88WT presented at an older age and had less BM involvement vs patients with the MYD88L265P mutation, regardless of CXCR4 mutation status. For age comparisons, this was P = .01 and .08 vs MYD88L265PCXCR4WT and MYD88L265PCXCR4WHIM, and for BM comparisons, P = .03 and 0.01 vs MYD88L265PCXCR4WT and MYD88L265PCXCR4WHIM patients (Table 2). By multivariate analysis, a higher percentage (72.7%) of patients with MYD88WT also had a B2-microglobulin (B2M) level of >3.0 mg/L vs the 2 other subgroups (P = .003). Patients with MYD88WT also showed differences in absolute lymphocyte and platelet count vs the 2 other subgroups, but these differences were not clinically meaningful. Of interest, patients with MYD88L265PCXCR4WHIM mutations exhibited a lower incidence of adenopathy vs patients with MYD88L265PCXCR4WT (P < .01). No differences in gender; absolute neutrophil counts; hemoglobin levels; serum IgA, IgG, and IgM levels; B2M levels; presence of splenomegaly; familial history for B-cell malignancies; prior history of monoclonal gammopathy of unknown significance (MGUS); or symptomatic status at diagnosis was observed among these 3 subgroups.
Disease characteristics at presentation for WM patients stratified by MYD88 and CXCR4 mutation status
| . | MYD88WTCXCR4WT . | MYD88L265PCXCR4WT . | MYD88L265PCXCR4WHIM . | P . |
|---|---|---|---|---|
| n | 15 | 109 | 50 | |
| Gender (M/F) | 7/8 | 73/36 | 31/19 | – |
| Age (years) | 66 (42-82) | 59 (40-88) | 60 (34-88) | .02 |
| BM involvement (%) | 15 (5-65) | 40 (5-95) | 50 (5-95) | .02 |
| Abs neutrophil (k/μL) | 2.93 (0.72-3.7) | 3.5 (1.04-8.94) | 3.2 (1.52-8.2) | – |
| Abs lymphocyte (k/μL) | 1.61 (0.8-17.3) | 1.73 (0.48-11.1) | 1.69 (0.51-3.98) | .03 |
| Hemoglobin (g/dL) | 11.3 (8-14.4) | 11.5 (6-15.5) | 11.6 (4.8-15.6) | – |
| Platelet (k/μL) | 218 (105-378) | 274 (75-512) | 251 (42-441) | .01 |
| Serum IgA (mg/dL) | 140 (27-324) | 61 (0-1240) | 45 (7-864) | – |
| Serum IgG (mg/dL) | 734 (441-1480) | 686 (138-2920) | 595 (168-1330) | – |
| Serum IgM (mg/dL) | 2720 (134-5810) | 3190 (345-8720) | 3490 (416-8767) | – |
| B2M (mg/L) | 3.1 (2-5.5) | 3.1 (1.4-10.4) | 2.3 (1-9.2) | – |
| Adenopathy | 6 (40%) | 59 (54.1%) | 15 (30%) | .01 |
| Splenomegaly | 6 (40%) | 18 (16.5%) | 8 (16%) | – |
| Family history | 4 (26.7%) | 31(28.4%) | 20 (40%) | – |
| Prior MGUS history | 4 (26.7%) | 18 (16.5%) | 8 (16%) | – |
| Symptomatic disease | 9 (60%) | 69 (63.3%) | 34 (66.6%) | – |
| . | MYD88WTCXCR4WT . | MYD88L265PCXCR4WT . | MYD88L265PCXCR4WHIM . | P . |
|---|---|---|---|---|
| n | 15 | 109 | 50 | |
| Gender (M/F) | 7/8 | 73/36 | 31/19 | – |
| Age (years) | 66 (42-82) | 59 (40-88) | 60 (34-88) | .02 |
| BM involvement (%) | 15 (5-65) | 40 (5-95) | 50 (5-95) | .02 |
| Abs neutrophil (k/μL) | 2.93 (0.72-3.7) | 3.5 (1.04-8.94) | 3.2 (1.52-8.2) | – |
| Abs lymphocyte (k/μL) | 1.61 (0.8-17.3) | 1.73 (0.48-11.1) | 1.69 (0.51-3.98) | .03 |
| Hemoglobin (g/dL) | 11.3 (8-14.4) | 11.5 (6-15.5) | 11.6 (4.8-15.6) | – |
| Platelet (k/μL) | 218 (105-378) | 274 (75-512) | 251 (42-441) | .01 |
| Serum IgA (mg/dL) | 140 (27-324) | 61 (0-1240) | 45 (7-864) | – |
| Serum IgG (mg/dL) | 734 (441-1480) | 686 (138-2920) | 595 (168-1330) | – |
| Serum IgM (mg/dL) | 2720 (134-5810) | 3190 (345-8720) | 3490 (416-8767) | – |
| B2M (mg/L) | 3.1 (2-5.5) | 3.1 (1.4-10.4) | 2.3 (1-9.2) | – |
| Adenopathy | 6 (40%) | 59 (54.1%) | 15 (30%) | .01 |
| Splenomegaly | 6 (40%) | 18 (16.5%) | 8 (16%) | – |
| Family history | 4 (26.7%) | 31(28.4%) | 20 (40%) | – |
| Prior MGUS history | 4 (26.7%) | 18 (16.5%) | 8 (16%) | – |
| Symptomatic disease | 9 (60%) | 69 (63.3%) | 34 (66.6%) | – |
This table denotes comparisons of patient characteristics at diagnosis stratified by all CXCRWHIM-mutated patients.
Abs, absolute; –, not significant.
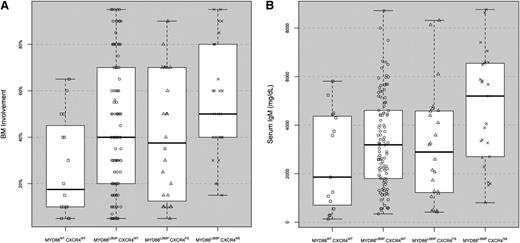
We next examined if differences in clinical presentation existed when NS vs FS CXCR4WHIM mutation status was delineated (Table 3). As shown in Figure 1, patients with CXCR4WHIM/NS demonstrated significantly higher levels of BM disease vs CXCR4WHIM/FS and MYD88WT patients (P = .05 and .005, respectively). Patients with MYD88L265PCXCR4WHIM/NS also had significantly higher serum IgM levels vs MYD88L265PCXCR4WHIM/FS, MYD88L265PCXCR4WT, and MYD88WTpatients; P = .05, .01, and .01, respectively (Figure 1). As before, fewer patients with MYD88L265PCXCR4WHIM, regardless of FS or NS mutation status, exhibited adenopathy when compared with patients who were MYD88L265PCXCR4WT-mutated (P = .04 and .05, respectively). As shown in Table 3, more patients with MYD88L265PCXCR4WHIM/NS also presented with symptomatic disease requiring therapy at diagnosis, which contrasted particularly against MYD88L265PCXCR4WHIM/FS patients (P = .007). Patients with MYD88L265PCXCR4WHIM/NS were also more likely to present with symptomatic hyperviscosity, reflecting the higher serum IgM levels observed in these patients at diagnosis (Table 3). No differences in gender, absolute lymphocyte counts, hemoglobin levels, serum IgA and IgG levels, B2M levels, presence of splenomegaly, familial history for B-cell malignancies, or prior history of MGUS were observed among the 4 subgroups. Clinically not meaningful differences in absolute neutrophil and platelet counts were also observed (Table 3). For 54 patients, both tumor and germline sequencing data for SDF-1a were available. No somatic mutations in SDF-1a were observed in these patients. The distribution for SDF-1a polymorphisms at RS RS1801157, which are associated with increased SDF-1a transcriptional activity, did not vary from those reported in healthy donors,12,13 nor did MYD88 (P = 1.0) or CXCR4 mutations (P = .763) show any correlation with RS1801157 SDF-1a polymorphisms in these patients.
Disease characteristics at presentation for WM patients stratified by MYD88 and NS and FS CXCR4 mutation status
| . | MYD88WTCXCR4WT . | MYD88L265PCXCR4WT . | MYD88L265PCXCR4WHIM/FS . | MYD88L265PCXCR4WHIM/NS . | P . |
|---|---|---|---|---|---|
| n | 15 | 109 | 24 | 26 | |
| Gender (M/F) | 7/8 | 73/36 | 14/10 | 17/9 | – |
| Age (years) | 66 (42-82) | 59 (40-88) | 62 (34-88) | 59 (41-75) | .05 |
| BM involvement (%) | 15 (5-65) | 40 (5-95) | 37 (5-90) | 50 (15-95) | <.01 |
| Abs neutrophil (k/μL) | 2.93 (0.72-3.7) | 3.5 (1.04-8.94) | 3.54 (1.52-8.2) | 3.1 (1.56-6.14) | .01 |
| Abs lymphocyte (k/μL) | 1.61 (0.8-17.3) | 1.73 (0.48-11.1) | 1.77 (0.96-3.55) | 1.63 (0.51-3.88) | – |
| Hemoglobin (g/dL) | 11.3 (8-14.4) | 11.5 (6-15.5) | 11.8 (4.8-15.6) | 11.5 (6.2-14.2) | – |
| Platelet (k/μL) | 218 (105-378) | 274 (75-512) | 275 (101-441) | 208 (42-314) | <.01 |
| Serum IgA (mg/dL) | 140 (27-324) | 61 (0-1240) | 45 (20-202) | 45 (7-864) | – |
| Serum IgG (mg/dL) | 734 (441-1480) | 686 (138-2920) | 612 (267-978) | 569 (168-1330) | – |
| Serum IgM (mg/dL) | 2720 (134-5810) | 3190 (345-8720) | 2895 (416-8320) | 5200 (804-8767) | <.01 |
| B2M (mg/L) | 3.1 (2-5.5) | 3.1 (1.4-10.4) | 2.45 (1.4-9.2) | 2.4 (1-5) | – |
| Adenopathy | 6 (40%) | 59 (54.1%) | 7 (29.2%) | 8 (30.1%) | .04 |
| Splenomegaly | 6 (40%) | 18 (16.5%) | 2 (8.3%) | 6 (23.1%) | – |
| Family history | 4 (26.7%) | 31(28.4%) | 9 (37.5%) | 11 (42.3%) | – |
| Prior MGUS history | 4 (26.6%) | 18 (16.5%) | 5 (20.8%) | 3 (11.5%) | – |
| Symptomatic disease | 9 (60%) | 69 (63.3%) | 11 (45.8%) | 22 (84.6%) | .03 |
| Hyperviscosity syndrome | 0 (0%) | 10 (9.2%) | 2 (8.3%) | 8 (30.7%) | .01 |
| . | MYD88WTCXCR4WT . | MYD88L265PCXCR4WT . | MYD88L265PCXCR4WHIM/FS . | MYD88L265PCXCR4WHIM/NS . | P . |
|---|---|---|---|---|---|
| n | 15 | 109 | 24 | 26 | |
| Gender (M/F) | 7/8 | 73/36 | 14/10 | 17/9 | – |
| Age (years) | 66 (42-82) | 59 (40-88) | 62 (34-88) | 59 (41-75) | .05 |
| BM involvement (%) | 15 (5-65) | 40 (5-95) | 37 (5-90) | 50 (15-95) | <.01 |
| Abs neutrophil (k/μL) | 2.93 (0.72-3.7) | 3.5 (1.04-8.94) | 3.54 (1.52-8.2) | 3.1 (1.56-6.14) | .01 |
| Abs lymphocyte (k/μL) | 1.61 (0.8-17.3) | 1.73 (0.48-11.1) | 1.77 (0.96-3.55) | 1.63 (0.51-3.88) | – |
| Hemoglobin (g/dL) | 11.3 (8-14.4) | 11.5 (6-15.5) | 11.8 (4.8-15.6) | 11.5 (6.2-14.2) | – |
| Platelet (k/μL) | 218 (105-378) | 274 (75-512) | 275 (101-441) | 208 (42-314) | <.01 |
| Serum IgA (mg/dL) | 140 (27-324) | 61 (0-1240) | 45 (20-202) | 45 (7-864) | – |
| Serum IgG (mg/dL) | 734 (441-1480) | 686 (138-2920) | 612 (267-978) | 569 (168-1330) | – |
| Serum IgM (mg/dL) | 2720 (134-5810) | 3190 (345-8720) | 2895 (416-8320) | 5200 (804-8767) | <.01 |
| B2M (mg/L) | 3.1 (2-5.5) | 3.1 (1.4-10.4) | 2.45 (1.4-9.2) | 2.4 (1-5) | – |
| Adenopathy | 6 (40%) | 59 (54.1%) | 7 (29.2%) | 8 (30.1%) | .04 |
| Splenomegaly | 6 (40%) | 18 (16.5%) | 2 (8.3%) | 6 (23.1%) | – |
| Family history | 4 (26.7%) | 31(28.4%) | 9 (37.5%) | 11 (42.3%) | – |
| Prior MGUS history | 4 (26.6%) | 18 (16.5%) | 5 (20.8%) | 3 (11.5%) | – |
| Symptomatic disease | 9 (60%) | 69 (63.3%) | 11 (45.8%) | 22 (84.6%) | .03 |
| Hyperviscosity syndrome | 0 (0%) | 10 (9.2%) | 2 (8.3%) | 8 (30.7%) | .01 |
This table denotes comparisons of patient characteristics at diagnosis delineated by NS and FS CXCR4 mutation status.
Abs, absolute; –, not significant.
BM disease involvement and serum IgM levels at diagnosis for WM patients stratified by MYD88 and CXCR4 mutation status. MYD88WTCXCR4WT (n = 15), MYD88L265PCXCR4WT (n = 109), MYD88L265PCXCR4WHIM/FS (n = 24), and MYD88L265PCXCR4WHIM/NS (n = 26). Box plots with interquartile ranges are shown with an overlay of the individual data points.
BM disease involvement and serum IgM levels at diagnosis for WM patients stratified by MYD88 and CXCR4 mutation status. MYD88WTCXCR4WT (n = 15), MYD88L265PCXCR4WT (n = 109), MYD88L265PCXCR4WHIM/FS (n = 24), and MYD88L265PCXCR4WHIM/NS (n = 26). Box plots with interquartile ranges are shown with an overlay of the individual data points.
MYD88 and CXCR4 mutation status and overall survival in WM
The median follow-up for all patients was 4.84 (range 0.65-23.45) years from time of diagnosis and did not differ among the 4 subgroups (P = .51). There were no significant differences in the number of therapies or the percentage of patients who received rituximab, alkylators, proteasome inhibitors, or immunomodulatory agents among the 4 subgroups (Table 4). However, intergroup comparison showed that a higher number of patients with MYD88L265PCXCR4WHIM/NS vs MYD88L265PCXCR4WHIM/FS received proteasome inhibitor therapy (P = .009).
Treatment characteristics for studied WM patients stratified by MYD88 and NS and FS CXCR4 mutation status
| . | MYD88WTCXCR4WT . | MYD88L265PCXCR4WT . | MYD88L265PCXCR4WHIM/FS . | MYD88L265PCXCR4WHIM/NS . | P . |
|---|---|---|---|---|---|
| n | 15 | 109 | 24 | 26 | |
| Number of therapies | 2 (0-8) | 2 (0-7) | 1 (0-4) | 2 (1-8) | – |
| Rituximab | 11 (68.8%) | 89 (81.7%) | 18 (75.0%) | 22 (84.6%) | – |
| Alkylator | 7 (43.8%) | 63 (57.8%) | 13 (54.2%) | 13 (50.0%) | – |
| Nucleoside analog | 3 (18.8%) | 20 (18.4%) | 2 (8.3%) | 3 (11.5%) | – |
| Proteasome inhibitor | 6 (37.5%) | 41 (37.6%) | 4 (17.0%) | 14 (53.9%) | – |
| Immunomodulatory | 1 (6.3%) | 6 (5.5%) | 0 (0%) | 2 (7.7%) | – |
| . | MYD88WTCXCR4WT . | MYD88L265PCXCR4WT . | MYD88L265PCXCR4WHIM/FS . | MYD88L265PCXCR4WHIM/NS . | P . |
|---|---|---|---|---|---|
| n | 15 | 109 | 24 | 26 | |
| Number of therapies | 2 (0-8) | 2 (0-7) | 1 (0-4) | 2 (1-8) | – |
| Rituximab | 11 (68.8%) | 89 (81.7%) | 18 (75.0%) | 22 (84.6%) | – |
| Alkylator | 7 (43.8%) | 63 (57.8%) | 13 (54.2%) | 13 (50.0%) | – |
| Nucleoside analog | 3 (18.8%) | 20 (18.4%) | 2 (8.3%) | 3 (11.5%) | – |
| Proteasome inhibitor | 6 (37.5%) | 41 (37.6%) | 4 (17.0%) | 14 (53.9%) | – |
| Immunomodulatory | 1 (6.3%) | 6 (5.5%) | 0 (0%) | 2 (7.7%) | – |
–, not significant.
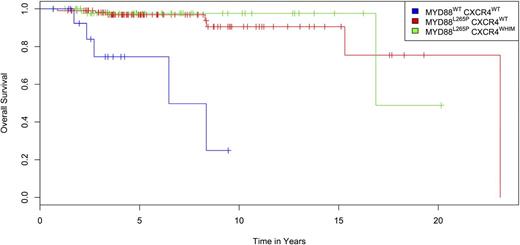
Fifteen patients died during follow-up, with cause of death attributed to progressive or transformed disease (n = 11), other cancers (n = 2), treatment-related myelodysplasia (n = 1), and amyotrophic lateral sclerosis (n = 1). The Kaplan-Meier curve for overall survival for patients with MYD88WTCXCR4WT, MYD88L265PCXCR4WT, and MYD88L265PCXCR4WHIM is shown in Figure 2. Differences in survival curves based on CXCR4 and MYD88 mutation status were significant (P < .0001), as was the analysis based on MYD88 status alone (P < .0001), with the highest mortality (5/15; 30%) observed in the MYD88WTCXCR4WT cohort. One additional patient with MYD88WTCXCR4WHIM/FS also succumbed to progressive disease. Hence 6/16 (38%) of patients with MYD88WT died during the follow-up period, including 5 of 11 (45.5%) patients in the entire cohort who died of progressive disease. By comparison, lower incidences of mortality were observed in patients with MYD88L265P, including 7/109 (6.4%) with CXCR4WT and 2/50 (4%) with CXCR4WHIM mutations (1 FS, 1 NS) (P = .0007) during the follow-up period. By Cox proportional hazards, the age and B2M (>3 mg/L) adjusted risk of death associated with MYD88WT was 10.54 (95% confidence interval [CI] 2.40-46.2; P = .002), whereas the risk of death associated with CXCR4WHIM was 0.96 (95% CI 0.16-6.7; P = .96).
Kaplan-Meier plot for overall survival of 175 WM patients from time of diagnosis stratified by MYD88 and CXCR4 mutation status. Differences in survival curves based on CXCR4 and MYD88 mutation status were significant (P < .0001), as was the analysis based on MYD88 status alone (P < .0001) by Fleming-Harrington log-rank analysis.
Kaplan-Meier plot for overall survival of 175 WM patients from time of diagnosis stratified by MYD88 and CXCR4 mutation status. Differences in survival curves based on CXCR4 and MYD88 mutation status were significant (P < .0001), as was the analysis based on MYD88 status alone (P < .0001) by Fleming-Harrington log-rank analysis.
Discussion
This is the first study to address the clinical findings associated with 2 newly discovered somatic mutations (MYD88 L265P and CXCR4) in WM. A paucity of knowledge for these mutations in cancer exists because of the low prevalence of the MYD88 L265P mutation in other B-cell diseases as well as the fact that CXCR4 somatic mutations have not been previously described in any malignant condition despite the often-reported dysregulation of this gene in other cancers.4 Important strengths of this study are the relatively large genotyped population of WM patients and the long median follow-up (almost 5 years) from diagnosis. The key findings that emerged from this study are that distinct subsets of patients can be identified based on mutational analysis of MYD88 and CXCR4. Patients with MYD88L265PCXCR4WHIM/NS showed higher BM disease burden, higher serum IgM levels, and were more likely to have symptomatic disease requiring therapy at presentation. In contrast, patients with MYD88WTCXCR4WT showed the lowest BM disease burden, whereas patients with either MYD88L265PCXCR4WT or MYD88L265PCXCR4WHIM/FS showed intermediate levels of BM disease involvement. Patients with CXCRWHIM, regardless of whether they had an NS or FS mutation, were also less likely to have adenopathy. These findings suggest an increased tropism for CXCR4WHIM-mutated WM cells for the BM stroma, which promotes homing and adhesion of CXCR4 expressing WM cells by elaboration of SDF-1a.14 Adhesion to the BM stroma promotes malignant cell survival and IgM release, which could account for the more pronounced BM involvement and serum IgM levels that were particularly observed in CXCR4WHIM/NS-mutated patients.
Surprising in this study were the differences observed in the clinical presentation of CXCR4WHIM/NS- and CXCR4WHIM/FS-mutated WM patients. Patients with CXCR4WHIM/NS showed more aggressive disease features at presentation, including higher BM disease burden, serum IgM levels (which were nearly double), and symptomatic status including hyperviscosity syndrome requiring therapy vs CXCR4WHIM/FS-mutated patients. These findings likely contributed to the finding of higher proteasome inhibitor use in CXCR4WHIM/NS-mutated patients, because bortezomib is recommended by consensus and National Comprehensive Cancer Network guidelines in patients presenting with symptomatic hyperviscosity.15,16 The findings also suggest that NS and FS mutations involving the C terminus of CXCR4 behave differently. In WHIM syndrome patients, very few have been identified with FS mutations, and nearly all of these patients have NS mutations (R334X or S338X).4,17 As such, little or no preclinical modeling has been done to understand the activating nature of CXCR4 C-terminal domain FS mutations. In the C terminus, at least 6 serines are phosphorylated in response to SDF-1a, beginning with serine 321.5 Both G protein–coupled receptor kinases (2 and 6) and arrestins (β1 and β2) bind to the C-terminal domain of CXCR4 at different serine residues, resulting in both positive and negative modulations of CXCR4 signaling. Rapid attenuation of CXCR4 signaling follows SDF-1a ligation, which is mediated by recruitment of arrestins, and is dependent on the phosphorylation of the terminal 5 serines that include serines 346/347 that modulate the phosphorylation of more proximal serines.5,18 As such, NS mutations such as S338X and R334X that truncate the more distal portions of the C terminus may affect more proximal serine phosphorylation as well as remove a scaffold critical for arrestin binding, thereby promoting prolonged CXCR4 signaling. Preclinical modeling comparing CXCR4WHIM/NS with CXCR4WHIM/FS will be required to clarify any signaling differences in response to SDF-1a, which might also be amenable to therapeutic exploitation.
The potential to translate the findings of MYD88 and CXCR4 mutations into therapeutic gains for WM patients is noteworthy. Both IRAK 1 and 4 as well as BTK signal for MYD88 L265P, whereas BTK is activated by CXCR4.8,19 Inhibitors to IRAK are in clinical development, whereas the BTK inhibitor ibrutinib has shown impressive activity in relapsed and refractory WM patients.18 Major responses to ibrutinib therapy are higher in MYD88L265P and CXCR4WT patients, the latter being highly associated with ibrutinib response.20 In preclinical studies, WM cells engineered to express the S338X CXCR4 NS mutation show resistance to the suppressive effects of ibrutinib on AKT and extracellular signal-regulated kinase 1/2 signaling, which could be restored by use of the CXCR4-specific inhibitor plerixafor.8 Taken together, these studies highlight the importance of understanding both MYD88 and CXCR4 mutation status in WM and may provide the basis for a more personalized treatment approach, including the use of relevant inhibitors for MYD88-mutated patients and the use of CXCR4 inhibitors in CXCR4-mutated WM patients. In addition to plerixafor, several other antagonists to CXCR4 have been developed and are in clinical trials—including BMS-936564, AMD-070, and TG-0054—and could be investigated for use either alone or in combination treatment strategies for WM patients with CXCR4 mutations.
Despite the aggressive presentation of patients harboring CXCRWHIM/NS mutations, overall survival for these patients was not adversely impacted. A similar number of deaths occurred in both CXCR4 WT (6%) and WHIM-mutated (4%) patients who harbored the MYD88 L265P mutation. Poulain et al13 reported that a germline polymorphism in SDF-1a (-801GG) at RS1801157 was associated with shorter survival after initiation of therapy in WM patients. We observed no somatic mutations in SDF-1a for 54 patients in this study whose tumor cells were sequenced, nor did MYD88 or CXCR4 mutations correlate with RS1801157 SDF-1a polymorphisms determined by germline sequencing in these patients. Prospective studies incorporating SDF-1a polymorphisms and CXCR4 mutation status in assessing treatment response, progression-free, and overall survival in WM patients could nonetheless be illuminating and help better clarify the predictive and prognostic roles of SDF-1a and CXCR4 variants alone and together.
Unexpectedly, patients with MYD88WT showed significantly higher mortality (38%) vs patients with MYD88L265P (6%) during the follow-up period. Patients with MYD88WT were older, and more demonstrated a B2M of >3.0 mg/L, a poor prognosis marker in WM.1 However, accounting for both age and B2M by multivariate analysis, MYD88WT status remained a significant risk for death. Important genomic differences between MYD88WT and MYD88L265P have previously been described, including presence of MLL2 mutations in MYD88WT patients. MLL2 mutations are usually found in follicular and aggressive non-Hodgkin lymphoma, and decreased IGHV3-23 rearrangements and somatic hypermutation are found in MYD88WT patients.2,21,22 These studies highlight the urgency to better understand the pathogenesis underlying MYD88WT WM disease and the development of targeted and more effective treatments for this patient population.
In summary, we report that MYD88 and CXCR4 mutation status denotes important differences in disease presentation for patients with WM, including BM disease burden, presence of extramedullary disease, serum IgM levels, symptomatic status at diagnosis including presentation with hyperviscosity syndrome, and overall survival. In addition, these studies provide the first reporting of clinical differences in cancer patients associated with CXCR4 somatic mutations, including NS and FS mutation status. These studies also provide a framework for the investigation of MYD88 and CXCR4 mutations as prognostic and predictive markers as well as the development of targeted therapeutics for use in personalized treatment approaches to WM.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the WM patients who provided samples.
This study was supported by the Peter and Helen Bing Foundation, the International Waldenström’s Macroglobulinemia Foundation, the Coyote Fund for WM, the D’Amato Family Fund for Genomic Discovery, and the Edward and Linda Nelson Fund for WM Research.
Authorship
Contribution: S.P.T. and Z.R.H. designed the study wrote the manuscript; S.P.T. and Z.R.H. performed the data analysis; Z.R.H. and X.L. designed allele-specific polymerase chain reactions and Sanger sequencing primers; G.Y., Y.C., X.L., and L.X. prepared the samples and performed the sequencing studies; and S.P.T. provided patient care and obtained consent and samples.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström's Macroglobulinemia, Dana Farber Cancer Institute, M548, 450 Brookline Ave, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu