To the editor:
Follicular lymphoma is typically a disseminated disease with frequent bone marrow infiltration at the time of diagnosis,1 but only a few patients manifest detectable circulating peripheral blood lymphoma cells (CLC).2-5 We recently described the poor prognosis of patients with follicular lymphoma presenting with this feature. The present report evaluates the effect of rituximab maintenance on the outcome of such patients.
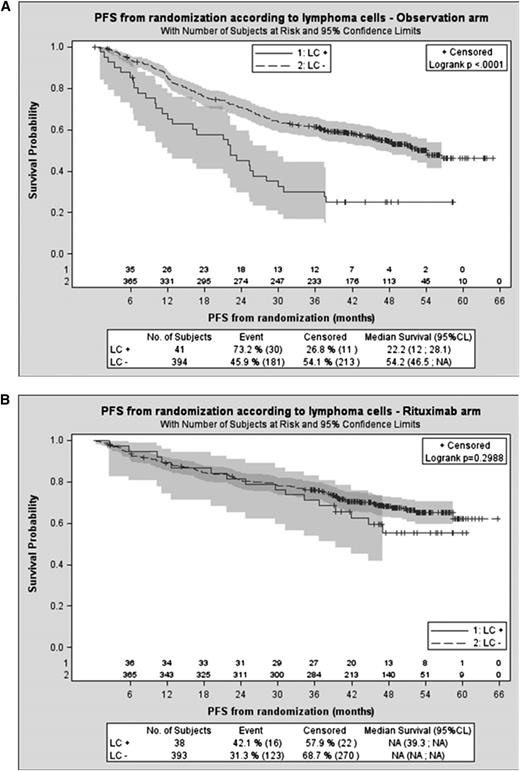
At Primary Rituximab and Maintenance (PRIMA) study (NCT00140582)6 entry, 92 patients were identified with CLC assessed by local laboratories and documented in case reports forms, with a mean lymphoma cell count of 14.4 × 109/L (median, 2 × 109/L; range, 0.1 - 176 × 109/L). These patients with CLC were significantly younger and presented more frequently with advanced-stage disease, extranodal involvement, anemia, and high serum levels of lactate dehydrogenase or β2-microglobulin than those without CLC (each P < .05; data not shown). Response rates at the end of rituximab chemotherapy induction were similar in patients with and without CLC (complete response/complete response unconfirmed [CR/CRu], 68.5% and 64.9%, and partial response [PR], 25% and 25%, respectively).7 Considering all patients from randomization (13 patients with CLC failed to reach randomization), progression-free survival (PFS) and time to next lymphoma treatment (TTNT) both were shorter in patients with CLC (respectively, 36.8 months vs not reached [log-rank P = .0003] and 45.6 months vs not reached [P = .0005]). However, a striking difference was observed between the 2 PRIMA study groups. In patients from the observation group, the presence of CLC at study entry was associated with a significantly reduced PFS (median, 22 months vs 54 months for patients without CLC; P < .0001; Figure 1A) and TTNT (median, 30 months vs not reached; P < .0001); whereas in the rituximab maintenance group, PFS and TTNT for patients with or without CLC did not significantly differ (P = .3 and .9, respectively; Figure 1B). A trend for a shorter overall survival for patients with CLC was also observed in the observation group (P = .07; hazard ratio [HR], 2.4; 95% confidence interval [CI], 0.9 - 6.34), but not in the maintenance group (P = .61; HR, 0.7; 95% CI, 0.16 - 2.9). Only 5 of 44 patients with CLC experiencing disease progression presented with CLC again at that time, as assessed at local laboratories. Of note, the PFS for patients without CLC according to the randomization group remained highly significant (P < .0001; HR, 0.615; 95% CI, 0.49 - 0.77), indicating that rituximab maintenance benefit was not restricted to patients with CLC.
PRIMA study: PFS according to CLC and maintenance group. (A) Patients with follicular lymphoma and leukemic cells (CLC) treated without rituximab maintenance after induction regimen presented a worse PFS than patients with follicular lymphoma without CLC who were treated without rituximab maintenance. LC+/−, presence/absence of circulating lymphoma cells. (B) With rituximab maintenance therapy, patients with and without CLC presented similar PFS.
PRIMA study: PFS according to CLC and maintenance group. (A) Patients with follicular lymphoma and leukemic cells (CLC) treated without rituximab maintenance after induction regimen presented a worse PFS than patients with follicular lymphoma without CLC who were treated without rituximab maintenance. LC+/−, presence/absence of circulating lymphoma cells. (B) With rituximab maintenance therapy, patients with and without CLC presented similar PFS.
These results indicate that 2-year rituximab maintenance is able to obviate the poor prognosis associated with CLC but also raise several questions. Despite similar CR/CRu rates, shorter PFS estimates in the observation group for patients with CLC demonstrate the persistence of residual lymphoma cells responsible for early progression. Monitoring minimal residual disease at the end of induction, using quantitative methods, could be valuable in this context.8 Moreover, it may be hypothesized that different mechanisms of action of the antibody, including induction of immune specific mechanisms,9 may account for the clinical efficacy of rituximab maintenance against the pool of these residual lymphoma cells. Overall, achieving a sustained control of tumor cells in these patients with high circulating tumor burden at diagnosis appears to require a prolonged treatment, even if an apparent CR is achieved after induction.
Authorship
Contribution: C.S. and G.S. designed and performed research, analyzed data, and wrote the paper; J.F.S, C.F., D.C., H.G., S.L., R.D., L.M.P., C.M., M.G.S., C.C.-C., and M.M. performed research and contributed data ; and all authors edited and approved the manuscript.
Conflict-of-interest disclosure: C.S. and M.G.S. received honoraria from Roche. R.D. received honorarium and travel support from Roche. G.S. participated on Roche advisory boards, and received honoraria and research support from Roche. The remaining authors declare no competing financial interests.
Correspondence: Gilles Salles, Centre Hospitalier Lyon Sud, 165, Chemin du Grand Revoyet, 69495 Pierre-Bénite, France; email: gilles.salles@chu-lyon.fr.