Key Points
BH3-only proteins Bim and Bmf jointly coregulate developmental cell death.
Bim and Bmf act as a barrier against autoimmunity and malignant disease.
Abstract
BH3 domain-only proteins (BH3-only) proteins are members of the Bcl-2 family that play crucial roles in embryogenesis and the maintenance of tissue homeostasis by triggering apoptotic cell death. The BH3-only protein Bim is critical for developmental apoptosis of lymphocytes, securing establishment of tolerance and for the termination of immune responses. Bim is believed to act in concert with other BH3-only proteins or members of the tumor necrosis factor receptor family in getting rid of unwanted cells. Bmf, a related BH3-only protein, was shown to play a role in B-cell homeostasis and to mediate cell death in response to certain apoptotic triggers, including glucocorticoid, histone deacetylase inhibitors, and overexpression of the c-Myc proto-oncogene. Here we show that Bim and Bmf have overlapping functions during mouse development and coregulate lymphocyte homeostasis and apoptosis in a nonredundant manner. Double deficiency of Bim and Bmf caused more B lymphadenopathy than loss of either BH3-only protein alone, and this was associated with autoimmune glomerulonephritis and a range of malignancies in aged mice. Thus, our results demonstrate that Bim and Bmf act in concert to prevent autoimmunity and malignant disease, strengthening the rational for the development of BH3-only protein mimicking therapeutics for the treatment of such disorders.
Introduction
The mitochondrial apoptosis pathway is orchestrated by the interactions between pro- and antiapoptotic members of the Bcl-2 protein family, where proapoptotic members of the BH3 domain-only proteins (BH3-only) protein subgroup induce cell death by neutralizing antiapoptotic members and/or by activating Bax and/or Bak directly to trigger mitochondrial outer membrane permeabilization and subsequent caspase activation.1
The roles of individual BH3-only proteins in normal physiology and stress-induced apoptosis have been addressed by gene targeting studies in mice. Notably, only loss of Bim appears to exert certain nonredundant functions during embryogenesis because loss of the gene causes the death of about half of Bim−/− embryos prior to embryonic day 10.2 Although no other single BH3-only mutant mouse strain shows developmental abnormalities, studies investigating mice lacking Bim plus 1 additional BH3-only protein demonstrate that Bim frequently acts in concert with a subset of BH3-only proteins in a cell type- and context-dependent manner. For example, Bim−/−Bik−/− males have severe defects in spermatogenesis, whereas males lacking either protein are fully fertile.3 Of note, Bim−/−Puma−/− mice develop severe lymphadenopathy that exceeds the one observed in the absence of Bim, although Puma−/− mice have normal leukocyte numbers.4,5 Importantly, BH3-only proteins also exert conserved functions in humans, and deregulation of their expression, most frequently that of BIM, has been documented in different solid, as well as hematopoietic, malignancies,6 where reduced expression correlates with increased disease risk,7 whereas single nucleotide polymorphisms have been associated with impaired responsiveness to frontline anticancer therapies.8,9
We have previously shown that loss of the BH3-only protein Bmf renders mouse embryonic fibroblasts and different lymphocyte subtypes refractory to apoptosis triggered by the inhibition of phosphatidylinositol 3-kinase, impaired cap-dependent protein translation, glucocorticoids, or histone-deacetylase inhibitors (HDACi).10,11 Furthermore, loss of Bmf accelerates c-Myc-driven B lymphomagenesis in mice.12 Notably, Eu-MycBmf−/− lymphomas proved to be refractory to the effects of combined treatment of HDACi and the BH3-mimetic ABT-737.13 Interestingly, Bmf expression was found lost or strongly reduced in primary Burkitt’s lymphoma samples and cell lines, in which it could be restored by 5′Aza-cytidine treatment.12 Furthermore, together with BIM, BMF is defined as a primary response gene in glucocorticoid (GC)-treated children suffering from acute lymphoblastic leukemia (ALL),14 and gene deletions were noted in ETV6/RUNX1-positive ALL where its loss may contribute to GC resistance during relapse.15
In support of functional overlap between Bim and Bmf, some of the effects noted in the absence of Bmf were also previously observed in cells from Bim−/− mice, as well as in human cancer cells lacking BIM expression.6 In addition, both proteins coregulate hematopoietic stem cell dynamics and reconstitution potential in mice, and this role seems conserved in humans.16 Furthermore, Bim and Bmf share a conserved motif near their N termini that allows interaction with cytoskeletal dynein light chain proteins, suggesting similar regulation.17
Here, we investigated the short- and long-term consequences of combined deficiency for Bim and Bmf in double-mutant mice.
Materials and methods
Generation of Bim−/−Bmf−/− mice
All animal experiments were performed according to the guidelines of the Austrian legislation and were approved (BMWF-66.011/0165-II/3b/2010). The generation of Bmf−/−, Bim−/−, and Vav-Bcl-2 transgenic mice has previously been described.2,11,18 All mice were maintained on a C57BL/6J genetic background.
Cell culture and reagents
Cells were cultured in Dulbecco’s modified Eagle medium (PAA Laboratories), 10% fetal calf serum (PAA), 2 mM l-glutamine (Gibco), 50 µM 2-mercaptoethanol, 1× nonessential amino acids (Gibco), 1× penicillin/streptomycin (Sigma-Aldrich), 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and sodium pyruvate (Gibco). Reagents used to induce cell death can be found in the supplemental Methods, available on the Blood Web site.
Antibodies used for flow cytometric analysis and cell sorting
Single cell suspensions were surface-stained with monoclonal antibodies conjugated with fluorescein isothiocyanate, R-phycoerytherin (PE), PE-Cy7, allophycocyanin (APC), or biotin (Molecular Probes). Antibodies and their specificities can be found in the supplemental Methods.
Quantification of total immunoglobulin and autoantibody levels
Ig titers in the serum from 8- to 12-week-old mice were quantified using an Ig clonotyping system (Southern Biotech). Six- to 8-week-old mice were injected intraperitoneally with (4-hydroxy-3-nitrophenyl)acetyl (NP)-ovalbumin (100 µg/mouse adsorbed to alum) to induce a T cell-dependent immune response. To determine the titers of antibodies against NP, the antigen conjugated to bovine serum albumin (Biosearch Technologies) was coated on enzyme-linked immunosorbent assay (ELISA) plates. Serum samples were used in a dilution range from 1:800 to 1:1600. Anti-double-stranded DNA (dsDNA)-specific antibodies were detected as previously described.11
Preparation of histological sections
Organs were fixed in 4% paraformaldehyde in phosphate-buffered saline, processed according to standard procedures, and stained in hematoxylin and eosin using standard procedures. For the detection of renal IgG deposits, an anti-IgG-fluorescein isothiocyanate antibody (eBioscience) diluted 1:10 or 1:100 in phosphate-buffered saline was used on deparaffinized (xylol/ethanol) kidney sections after Pronase K-driven antigen retrieval (0.75 mg/mL in Tris-buffered saline, pH 7.6, 60 minutes at 37°C). Sections were embedded in VectaShield prior to microscopic inspection using a Zeiss Axiovert Fluorescence microscope.
Statistical analysis
Statistical analysis was performed using the unpaired Student t test or analysis of variance (ANOVA) where indicated, applying the Stat-view 4.1 software program. For comparison of survival of mice and tumor onset, the log-rank (Mantel-Cox) test was used. P < .05 was considered statistically significant.
Results
Developmental cell death defects in Bim−/−Bmf−/− double-deficient mice
Due to the close proximity of both genes on chromosome 2, Bim+/−Bmf+/− mice were crossed with single knockouts to identify offspring derived from recombined gametes carrying both modified alleles. Mice deficient for one and heterozygous for the other locus were subsequently crossed with mice of the reciprocal genotype to generate Bim−/−Bmf−/− mice. Consistent with the previously reported partial embryonic lethality of Bim−/− mice,2 only about half of the expected Mendelian number of Bim−/−Bmf−/− mice was born alive.
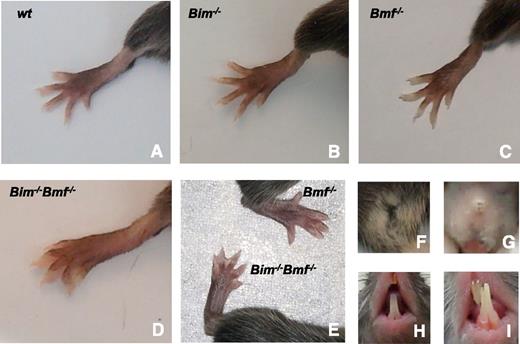
Although none of the mice lacking a single BH3-only protein have webbed feet, this was observed (during their entire lifespan) in all Bim−/−Bmf−/− mice (n = 41) (Figure 1A-E). Vaginal atresia, which is only rarely observed in wild-type (WT) mice, was found in ∼15% of Bim−/− females. The incidence of this developmental defect rose markedly in Bim−/−Bmf+/− (∼50%) and even more so in Bim−/−Bmf−/− (∼65%) mice but remained low (<5%) in Bim+/−Bmf−/− and Bmf−/− mice (Figure 1F-G). In addition, a sizeable fraction of Bim−/−Bmf−/− and Bim−/−Bmf+/− (∼25% of both genotypes) mice also developed malocclusion of the incisors (Figure 1H-I). Together, this indicates that Bim is the rate-limiting and Bmf is the critical auxiliary BH3-only protein required for morphogenesis of digits, the vagina, and in a more redundant manner, the incisors, but these developmental phenotypes were not followed up in more detailed analyses.
Impaired developmentally programmed apoptosis in mice lacking Bim and Bmf. Bim and Bmf mediate the removal of interdigitating mesenchymal cells in (A) WT, (B) Bim−/−, and (C) Bmf−/− mice, whereas (D-E) Bim−/−Bmf−/− mice present with soft tissue syndactyly, (G) vaginal aplasia, and (I) malocclusion of the incisors, (A,F,H) phenotypes that are not or only rarely observed in single knockout mice or WT control animals.
Impaired developmentally programmed apoptosis in mice lacking Bim and Bmf. Bim and Bmf mediate the removal of interdigitating mesenchymal cells in (A) WT, (B) Bim−/−, and (C) Bmf−/− mice, whereas (D-E) Bim−/−Bmf−/− mice present with soft tissue syndactyly, (G) vaginal aplasia, and (I) malocclusion of the incisors, (A,F,H) phenotypes that are not or only rarely observed in single knockout mice or WT control animals.
Thymocyte development is controlled by Bim, but Bmf and Bim can contribute to stress-induced thymocyte apoptosis
Bim and Bmf proteins are coexpressed in many lymphocyte subsets.19 Therefore, we compared the composition of primary and secondary lymphoid organs between 6- to 8-week-old WT, Bmf−/−, Bim−/−, and double-deficient (DKO) animals (Table 1; supplemental Figure 1).
Composition of central and peripheral lymphatic organs
| Cell populations . | WT . | Bmf−/− . | Bim−/− . | DKO . |
|---|---|---|---|---|
| Thymus* | ||||
| Total (×107) | 23.28 ± 2.95 | 31.61 ± 0.90 | 28.95 ± 5.10 | 24.39 ± 2.46 |
| CD4−8- | 0.71 ± 0.02 | 0.95 ± 0.10† | 2.85 ± 0.66†‡ | 2.36 ± 0.23†‡ |
| CD4+8+ | 19.71 ± 3.78 | 27.5 ± 0.77 | 18.5 ± 2.98 | 16.34 ± 1.92‡ |
| CD4+8- | 2.17 ± 0.29 | 2.20 ± 0.08 | 5.58 ± 1.15†‡ | 3.96 ± 0.46†‡ |
| CD4−8+ | 0.70 ± 0.10 | 0.92 ± 0.06 | 1.54 ± 0.20†‡ | 1.74 ± 0.23†‡ |
| Spleen | ||||
| Total (×107) | 13.89 ± 0.87 | 21.21 ± 2.52† | 27.9 ± 4.64† | 41.64 ± 4.14†‡§ |
| B cells | ||||
| B220+ | 8.12 ± 0.88 | 14.5 ± 1.98† | 14.91 ± 2.21† | 26.45 ± 3.48†‡§ |
| T1 | 0.95 ± 0.23 | 1.44 ± 0.3 | 1.85 ± 0.44 | 3.31 ± 0.58†‡ |
| T2 | 0.93 ± 0.07 | 1.88 ± 0.3† | 1.98 ± 0.4† | 3.58 ± 0.38†‡§ |
| FO | 5.19 ± 0.59 | 10.4 ± 1.54† | 9.87 ± 1.56† | 15.09 ± 1.76†§ |
| MZ | 1.08 ± 0.16 | 1.53 ± 0.28 | 1.39 ± 0.25 | 2.35 ± 0.49† |
| PC/PB | 1.37 ± 0.19 | 2.24 ± 0.35† | 3.97 ± 0.6† | 8.4 ± 1.24†‡§ |
| T cells | ||||
| TCRβ+ | 3.98 ± 0.39 | 4.62 ± 0.41 | 7.88 ± 1.39† | 8.04 ± 0.78†‡ |
| Naïve CD4 | 1.42 ± 0.19 | 1.48 ± 0.09 | 1.84 ± 0.49 | 1.38 ± 0.2 |
| TCM CD4 | 0.51 ± 0.11 | 0.56 ± 0.13 | 1.12 ± 0.32 | 1.14 ± 0.26 |
| TEM CD4 | 0.41 ± 0.03 | 0.63 ± 0.12 | 1.05 ± 0.22† | 1.06 ± 0.18† |
| Naïve CD8 | 0.78 ± 0.13 | 0.87 ± 0.18 | 1.3 ± 0.36 | 1.34 ± 0.2 |
| TCM CD8 | 0.46 ± 0.06 | 0.51 ± 0.11 | 1.01 ± 0.18† | 1.13 ± 0.18†‡ |
| TEM CD8 | 0.24 ± 0.06 | 0.39 ± 0.15 | 0.63 ± 0.12† | 0.86 ± 0.19† |
| Ratio CD4/CD8 | 1.66 ± 0.08 | 1.54 ± 0.09 | 1.39 ± 0.08† | 1.13 ± 0.06†‡§ |
| Ratio B220/TCRβ | 2.06 ± 0.16 | 3.12 ± 0.25† | 1.96 ± 0.1‡ | 3.27 ± 0.22†§ |
| Myeloid cells | ||||
| Granulocytes | 0.087 ± 0.02 | 0.16 ± 0.06 | 0.08 ± 0.03 | 0.12 ± 0.01 |
| Monocytes | 0.28 ± 0.1 | 0.4 ± 0.15 | 0.53 ± 0.17 | 0.56 ± 0.21 |
| Ter119+ | 0.99 ± 0.15 | 2.46 ± 0.56† | 4.07 ± 1.05† | 5.43 ± 1.07†‡ |
| Bone marrow | ||||
| Total (×106) | 33.45 ± 4.76 | 36.44 ± 6.77 | 29.45 ± 4.71 | 35.03 ± 4.06 |
| B cells | ||||
| B220+ | 8.7 ± 1.28 | 9.87 ± 1.46 | 10.06 ± 1.59 | 14.49 ± 1.73† |
| Pro-B cells | 0.71 ± 0.1 | 0.73 ± 0.14 | 0.92 ± 0.19 | 1.02 ± 0.08† |
| Pre-B cells | 4.62 ± 0.61 | 5.74 ± 1.13 | 4.27 ± 0.85 | 5.08 ± 0.94 |
| IM B cells | 2.41 ± 0.58 | 2.52 ± 0.42 | 2.7 ± 0.51 | 3.63 ± 0.77 |
| M B cells | 2.15 ± 0.31 | 3.21 ± 0.79 | 4 ± 0.73† | 7.63 ± 1.08†‡§ |
| Percentage small pre-B | 65.14 ± 4.35 | 67.88 ± 1.93 | 71.65 ± 2.45 | 80.07 ± 3.87†‡ |
| Myeloid cells | ||||
| Granulocytes | 6.17 ± 0.77 | 6.76 ± 0.99 | 4.17 ± 0.66 | 3.65 ± 0.62†‡ |
| Monocytes | 3.36 ± 0.45 | 3.44 ± 0.54 | 3 ± 0.48 | 3.03 ± 0.85 |
| Ter119+ | 6.96 ± 1.56 | 9.56 ± 2.82 | 6.32 ± 1.71 | 7.05 ± 1.63 |
| PBL | ||||
| WBC/μL¶ | 7116 ± 808 | 15 180 ± 2 446† | 27 557 ± 4 016†‡ | 33 725 ± 4 938†‡ |
| B220+ | 4867 ± 796 | 11 959 ± 2 020† | 21 207 ± 3 543†‡ | 25 577 ± 4 358†‡ |
| CD4+ | 742 ± 248 | 920 ± 263 | 1 808 ± 573 | 1 338 ± 365 |
| CD8+ | 564 ± 145 | 908 ± 221 | 2 308 ± 606† | 1 780 ± 307† |
| Monocytes | 564 ± 166 | 855 ± 180 | 1 201 ± 354 | 2 494 ± 1007 |
| Granulocytes | 621 ± 108 | 684 ± 139 | 709 ± 123 | 1 784 ± 383†§ |
| Cell populations . | WT . | Bmf−/− . | Bim−/− . | DKO . |
|---|---|---|---|---|
| Thymus* | ||||
| Total (×107) | 23.28 ± 2.95 | 31.61 ± 0.90 | 28.95 ± 5.10 | 24.39 ± 2.46 |
| CD4−8- | 0.71 ± 0.02 | 0.95 ± 0.10† | 2.85 ± 0.66†‡ | 2.36 ± 0.23†‡ |
| CD4+8+ | 19.71 ± 3.78 | 27.5 ± 0.77 | 18.5 ± 2.98 | 16.34 ± 1.92‡ |
| CD4+8- | 2.17 ± 0.29 | 2.20 ± 0.08 | 5.58 ± 1.15†‡ | 3.96 ± 0.46†‡ |
| CD4−8+ | 0.70 ± 0.10 | 0.92 ± 0.06 | 1.54 ± 0.20†‡ | 1.74 ± 0.23†‡ |
| Spleen | ||||
| Total (×107) | 13.89 ± 0.87 | 21.21 ± 2.52† | 27.9 ± 4.64† | 41.64 ± 4.14†‡§ |
| B cells | ||||
| B220+ | 8.12 ± 0.88 | 14.5 ± 1.98† | 14.91 ± 2.21† | 26.45 ± 3.48†‡§ |
| T1 | 0.95 ± 0.23 | 1.44 ± 0.3 | 1.85 ± 0.44 | 3.31 ± 0.58†‡ |
| T2 | 0.93 ± 0.07 | 1.88 ± 0.3† | 1.98 ± 0.4† | 3.58 ± 0.38†‡§ |
| FO | 5.19 ± 0.59 | 10.4 ± 1.54† | 9.87 ± 1.56† | 15.09 ± 1.76†§ |
| MZ | 1.08 ± 0.16 | 1.53 ± 0.28 | 1.39 ± 0.25 | 2.35 ± 0.49† |
| PC/PB | 1.37 ± 0.19 | 2.24 ± 0.35† | 3.97 ± 0.6† | 8.4 ± 1.24†‡§ |
| T cells | ||||
| TCRβ+ | 3.98 ± 0.39 | 4.62 ± 0.41 | 7.88 ± 1.39† | 8.04 ± 0.78†‡ |
| Naïve CD4 | 1.42 ± 0.19 | 1.48 ± 0.09 | 1.84 ± 0.49 | 1.38 ± 0.2 |
| TCM CD4 | 0.51 ± 0.11 | 0.56 ± 0.13 | 1.12 ± 0.32 | 1.14 ± 0.26 |
| TEM CD4 | 0.41 ± 0.03 | 0.63 ± 0.12 | 1.05 ± 0.22† | 1.06 ± 0.18† |
| Naïve CD8 | 0.78 ± 0.13 | 0.87 ± 0.18 | 1.3 ± 0.36 | 1.34 ± 0.2 |
| TCM CD8 | 0.46 ± 0.06 | 0.51 ± 0.11 | 1.01 ± 0.18† | 1.13 ± 0.18†‡ |
| TEM CD8 | 0.24 ± 0.06 | 0.39 ± 0.15 | 0.63 ± 0.12† | 0.86 ± 0.19† |
| Ratio CD4/CD8 | 1.66 ± 0.08 | 1.54 ± 0.09 | 1.39 ± 0.08† | 1.13 ± 0.06†‡§ |
| Ratio B220/TCRβ | 2.06 ± 0.16 | 3.12 ± 0.25† | 1.96 ± 0.1‡ | 3.27 ± 0.22†§ |
| Myeloid cells | ||||
| Granulocytes | 0.087 ± 0.02 | 0.16 ± 0.06 | 0.08 ± 0.03 | 0.12 ± 0.01 |
| Monocytes | 0.28 ± 0.1 | 0.4 ± 0.15 | 0.53 ± 0.17 | 0.56 ± 0.21 |
| Ter119+ | 0.99 ± 0.15 | 2.46 ± 0.56† | 4.07 ± 1.05† | 5.43 ± 1.07†‡ |
| Bone marrow | ||||
| Total (×106) | 33.45 ± 4.76 | 36.44 ± 6.77 | 29.45 ± 4.71 | 35.03 ± 4.06 |
| B cells | ||||
| B220+ | 8.7 ± 1.28 | 9.87 ± 1.46 | 10.06 ± 1.59 | 14.49 ± 1.73† |
| Pro-B cells | 0.71 ± 0.1 | 0.73 ± 0.14 | 0.92 ± 0.19 | 1.02 ± 0.08† |
| Pre-B cells | 4.62 ± 0.61 | 5.74 ± 1.13 | 4.27 ± 0.85 | 5.08 ± 0.94 |
| IM B cells | 2.41 ± 0.58 | 2.52 ± 0.42 | 2.7 ± 0.51 | 3.63 ± 0.77 |
| M B cells | 2.15 ± 0.31 | 3.21 ± 0.79 | 4 ± 0.73† | 7.63 ± 1.08†‡§ |
| Percentage small pre-B | 65.14 ± 4.35 | 67.88 ± 1.93 | 71.65 ± 2.45 | 80.07 ± 3.87†‡ |
| Myeloid cells | ||||
| Granulocytes | 6.17 ± 0.77 | 6.76 ± 0.99 | 4.17 ± 0.66 | 3.65 ± 0.62†‡ |
| Monocytes | 3.36 ± 0.45 | 3.44 ± 0.54 | 3 ± 0.48 | 3.03 ± 0.85 |
| Ter119+ | 6.96 ± 1.56 | 9.56 ± 2.82 | 6.32 ± 1.71 | 7.05 ± 1.63 |
| PBL | ||||
| WBC/μL¶ | 7116 ± 808 | 15 180 ± 2 446† | 27 557 ± 4 016†‡ | 33 725 ± 4 938†‡ |
| B220+ | 4867 ± 796 | 11 959 ± 2 020† | 21 207 ± 3 543†‡ | 25 577 ± 4 358†‡ |
| CD4+ | 742 ± 248 | 920 ± 263 | 1 808 ± 573 | 1 338 ± 365 |
| CD8+ | 564 ± 145 | 908 ± 221 | 2 308 ± 606† | 1 780 ± 307† |
| Monocytes | 564 ± 166 | 855 ± 180 | 1 201 ± 354 | 2 494 ± 1007 |
| Granulocytes | 621 ± 108 | 684 ± 139 | 709 ± 123 | 1 784 ± 383†§ |
Total numbers of hemopoietic cells from 6- to 8-week-old animals were calculated by counting single-cell suspensions derived from the indicated tissues. Cells were subsequently analyzed by flow cytometry after staining for cell surface markers: T1 B cells (B220+sIgMhighCD21low), T2 B cells (B220+sIgMhighCD21high), FO B cells (B220+CD21+CD23+), MZ B cells (B220+CD21+CD23−), PC/PBs (B220+/lowCD138+), naïve CD4+ or CD8+ T cells (CD62LhighCD44low), effector/memory (TEM) CD4+ or CD8+ T cells (CD62LlowCD44high), central memory (TCM) CD4+ or CD8+ T cells (CD62LhighCD44high), granulocytes (Gr-1+CD11b+), monocytes (Gr-1−CD11b+), pro-B cells (B220lowIgM−CD43+), pre-B cells (B220lowIgM−CD43−), immature (IM) B cells (B220lowIgM+), and mature (M) B cells (B220highIgM+). Numbers represent the mean ± standard error of the mean (SEM) of 5 to 8 mice per genotype. Total cellularity of both femora is presented.
Only female animals were analyzed.
P < .05 compared with WT mice.
P < .05 compared with Bmf−/− mice.
P < .05 compared with Bim−/− mice.
Cell number assessed in a Vet-ABC blood sample analyzer.
The percentages and overall numbers of all 4 major thymocyte subsets, ie, CD4−8− double-negative (DN), CD4+8+ double positive (DP), and CD4+8− and CD4−8+ single positive (SP) cells were comparable between Bim−/− and DKO mice (Table 1). Loss of Bim caused the previously described abnormal thymic cell subset composition, characterized by reduced percentages and overall numbers of DP thymocytes and an accumulation of T-cell receptor (TCR)α/β+ DN and SP thymocytes.2 Additional loss of Bmf did not cause any additional alterations (Figure 2A). As noted before, thymic cell subset composition was comparable between WT and Bmf−/− mice,11 and mice of all genotypes displayed no significant differences in total thymic cellularity (Table 1).
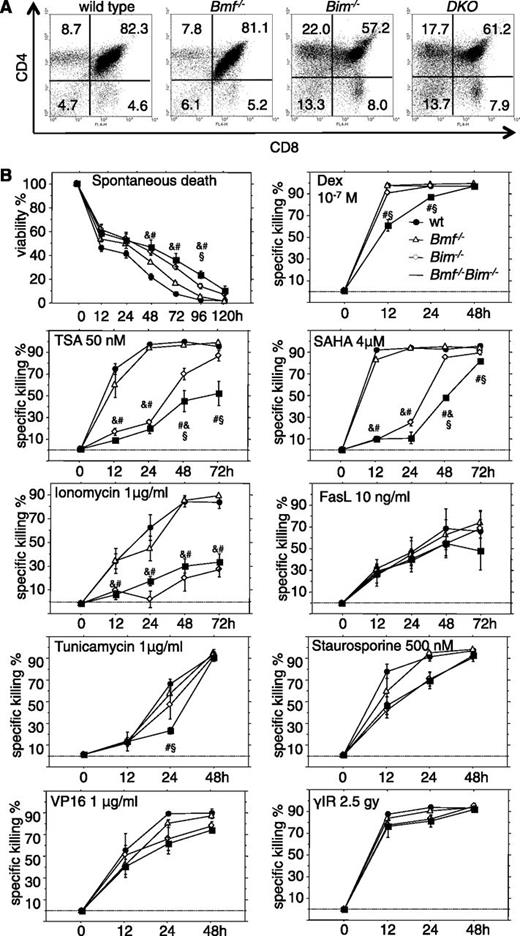
Bim and Bmf coregulate thymocyte apoptosis in response to stress, but only Bim appears critical for the developmentally programmed death of thymocytes. (A) Representative dot plots from flow cytometric analysis of single cell suspensions of thymocytes from 7- to 9-week-old mice of the indicated genotypes stained with CD4- and CD8-specific antibodies. (B) Sorted CD4+CD8+ thymocytes from 7- to 9-week-old mice of the indicated genotypes were placed in culture and incubated in the absence or presence of the indicated cell death inducers. Cell viability was assessed over time by AnnexinV/propidium iodide (PI) staining and flow cytometric analysis (Annexin V−/PI− cells were considered alive). Data are presented as means ± SEM of ≥4 independent experiments and 4 to 8 animals per genotype. Significant differences by ANOVA (P < .05) at individual time points &between WT and Bim−/−, #between WT and DKO, and §between Bim−/− and DKO cells. Due to differences in spontaneous cell death in culture, specific drug-induced killing was calculated using the formula (induced apoptosis − spontaneous cell death)/(100 − spontaneous cell death) (%).
Bim and Bmf coregulate thymocyte apoptosis in response to stress, but only Bim appears critical for the developmentally programmed death of thymocytes. (A) Representative dot plots from flow cytometric analysis of single cell suspensions of thymocytes from 7- to 9-week-old mice of the indicated genotypes stained with CD4- and CD8-specific antibodies. (B) Sorted CD4+CD8+ thymocytes from 7- to 9-week-old mice of the indicated genotypes were placed in culture and incubated in the absence or presence of the indicated cell death inducers. Cell viability was assessed over time by AnnexinV/propidium iodide (PI) staining and flow cytometric analysis (Annexin V−/PI− cells were considered alive). Data are presented as means ± SEM of ≥4 independent experiments and 4 to 8 animals per genotype. Significant differences by ANOVA (P < .05) at individual time points &between WT and Bim−/−, #between WT and DKO, and §between Bim−/− and DKO cells. Due to differences in spontaneous cell death in culture, specific drug-induced killing was calculated using the formula (induced apoptosis − spontaneous cell death)/(100 − spontaneous cell death) (%).
To investigate possible nonredundant functions of Bim and Bmf in stress-induced apoptosis, sorted DP thymocytes from WT, Bim−/−, Bmf−/−, and DKO animals were placed in culture without further treatment (spontaneous death) or were exposed to γ-radiation, the calcium ionophore ionomycin, the DNA damage-inducing drug etoposide/VP16, the glucocorticoid dexamethasone, the broad-spectrum kinase inhibitor staurosporine, the glycosylation-inhibitor and endoplasmic reticulum (ER) stressor tunicamycin, and the histone-deacetylase inhibitors trichostatin A, m-carboxycinnamic acid bis-hydroxamide, suberoylanilide hydroxamic acid (SAHA/Vorinostat), or FasL (Figure 2B; data not shown).
Combined loss of Bim and Bmf rendered thymocytes more resistant than those from single knockout mice to dexamethasone, HDAC inhibitors, and at early time points, tunicamycin (Figure 2B). Spontaneous death was mainly Bim dependent, as was death induced by ionomycin or staurosporine (Figure 2B). Apoptosis triggered by DNA damage or FasL was not affected by either single or combined loss of Bim and Bmf (Figure 2B; data not shown). Notably, in mature T cells, apoptosis resistance was strictly associated with loss of Bim. Additional loss of Bmf failed to enhance resistance toward spontaneous death or death induced by dexamethasone, m-carboxycinnamic acid bis-hydroxamide, or staurosporine that was afforded by loss of Bim. Apoptosis triggered by ER stress, DNA damage, or proteasome inhibition occurred normally in T cells from mice of all genotypes examined (supplemental Figure 2).
Bim and Bmf coregulate B lymphopoiesis
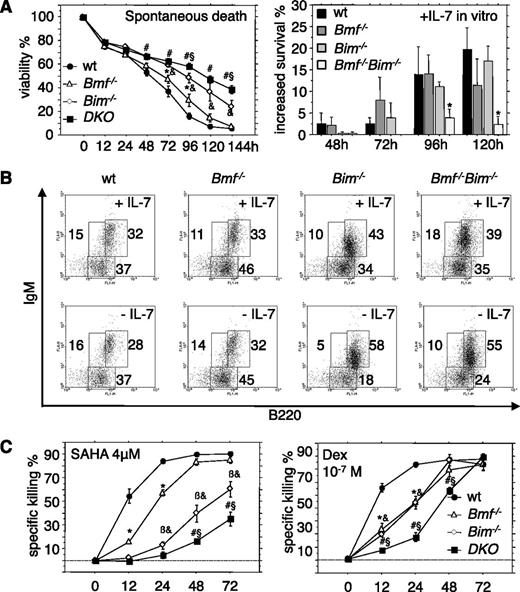
We next quantified the numbers of pro-B, pre-B, and immature B cells in the bone marrow from WT, Bim−/−, Bmf−/−, and DKO mice. The numbers of pro-B and pre-B cells were comparable in mice of all genotypes examined with the exception of a minor but significant increase in pro-B cells in DKO mice (Table 1). However, double deficiency failed to delay spontaneous cell death of pro-B cells in culture (data not shown), consistent with barely detectable protein expression in these cells.19 In contrast, when pre-B cells that express significant amounts of both proteins19 were placed in culture and apoptosis was followed over time, we noted a significant delay of death in the absence of Bim. Additional loss of Bmf further augmented this resistance to spontaneous cell death in culture, although this effect was not very pronounced (Figure 3A). In the presence of interleukin (IL)-7, double-deficient pre-B cells did not show increased survival, suggesting that they are largely factor independent (Figure 3A), yet cells of all genotypes tested eventually died in culture over time. When pro-B/pre-B cells (B220+IgM−) were sorted and cultured for 4 days, a significantly higher percentage of cells developed into sIgM-expressing naive B cells when Bim or both Bim plus Bmf were lacking. This enhanced differentiation potential, most likely was based on increased survival of cells lacking Bim (or Bim plus Bmf) and was even more pronounced when cells were cultured in the absence of IL-7 (Figure 3B).
Overlapping roles of Bim and Bmf in the control of pre-B-cell survival. (A) Pre-B cells (B220+CD43−IgM−) were isolated from the bone marrow of 7- to 9-week-old mice of the indicated genotypes and placed in culture (left) without additional treatment or in the presence of 10 ng/mL recombinant IL-7. (Right) Relative survival advantages induced by IL-7 were assessed over time. (B) B220+IgM− pro/pre-B cells were sorted from the bone marrow of 7- to 9-week-old mice of the indicated genotypes and cultured for 96 hours in the presence or absence of IL-7. sIgM expression was monitored by flow cytometric analysis. Representative dot plots from 1 of 3 independent experiments are shown. (C) Fluorescence-activated cell sorter-sorted pre-B cells from the bone marrow of 7- to 9-week-old mice of the indicated genotypes were treated in culture with the HDAC inhibitor SAHA or the glucocorticoid dexamethasone, and cell survival was assessed over time by flow cytometric analysis. Data are presented as means ± SEM of ≥3 independent experiments and 3 to 6 animals per genotype. *Significant differences by ANOVA (P < .05) at individual time points between WT and Bmf−/− derived cells; &between WT and Bim−/−, βbetween Bim−/− and Bmf−/−, #between WT and DKO, and §between Bim−/− and DKO cells. Due to differences in spontaneous cell death in culture, specific drug-induced killing was calculated using the formula (induced apoptosis − spontaneous cell death)/(100 − spontaneous cell death) (%).
Overlapping roles of Bim and Bmf in the control of pre-B-cell survival. (A) Pre-B cells (B220+CD43−IgM−) were isolated from the bone marrow of 7- to 9-week-old mice of the indicated genotypes and placed in culture (left) without additional treatment or in the presence of 10 ng/mL recombinant IL-7. (Right) Relative survival advantages induced by IL-7 were assessed over time. (B) B220+IgM− pro/pre-B cells were sorted from the bone marrow of 7- to 9-week-old mice of the indicated genotypes and cultured for 96 hours in the presence or absence of IL-7. sIgM expression was monitored by flow cytometric analysis. Representative dot plots from 1 of 3 independent experiments are shown. (C) Fluorescence-activated cell sorter-sorted pre-B cells from the bone marrow of 7- to 9-week-old mice of the indicated genotypes were treated in culture with the HDAC inhibitor SAHA or the glucocorticoid dexamethasone, and cell survival was assessed over time by flow cytometric analysis. Data are presented as means ± SEM of ≥3 independent experiments and 3 to 6 animals per genotype. *Significant differences by ANOVA (P < .05) at individual time points between WT and Bmf−/− derived cells; &between WT and Bim−/−, βbetween Bim−/− and Bmf−/−, #between WT and DKO, and §between Bim−/− and DKO cells. Due to differences in spontaneous cell death in culture, specific drug-induced killing was calculated using the formula (induced apoptosis − spontaneous cell death)/(100 − spontaneous cell death) (%).
Cell survival assays in vitro revealed that stress-induced apoptosis of pre-B cells triggered by the HDAC inhibitor SAHA was clearly dependent on Bim and Bmf, as was cell death triggered by dexamethasone (Figure 3C). Of note, immature B cells that are auditioning for positive and negative selection were found to be mildly elevated in the bone marrow of Bim−/−Bmf−/− mice. Mature recirculating IgM+IgD+ B cells were previously shown increased in Bim−/− and Bmf−/− mice11 ; interestingly, this accumulation was significantly (P < .01) augmented in the bone marrow of Bim−/−Bmf−/− mice compared with WT or singly mutant animals (Table 1).
Combined loss of Bim and Bmf causes enhanced B-cell lymphadenopathy
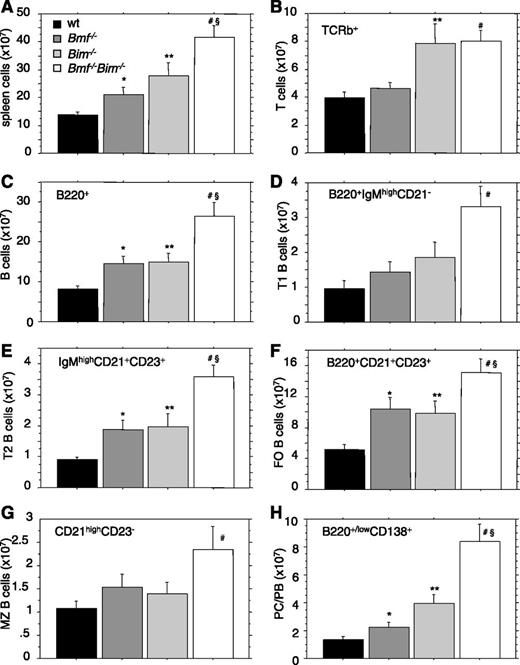
Assessment of splenic cellularity in Bim−/−Bmf−/− mice revealed a nearly fourfold increase compared with WT animals (Figure 4A; Table 1). Of note, splenic cellularity was also significantly increased over that of Bim and Bmf single knockout mice (P < .05). Lymphoid cell subset analysis by flow cytometry showed that combined loss of Bmf and Bim did not increase the number of mature T cells beyond the increase observed in Bim-deficient animals (Figure 4B; data not shown). In contrast, the numbers of B220+ B cells were significantly increased in Bim−/−Bmf−/− mice compared with the single knockout controls (Figure 4C; Table 1). A more detailed flow cytometric analysis of splenocytes revealed that the numbers of transitional type 1 (T1) and T2 transitional B cells were increased in the DKO mice (Figure 4D-E). Mature follicular (FO) B cells were also significantly increased in DKO mice above the numbers observed in either Bmf−/− or Bim−/− mice (Figure 4F). However, the marginal zone (MZ) B cells were elevated only mildly compared with WT mice but not compared with the single mutant mice, suggesting minor impact of gene ablation on this specialized B-cell compartment producing natural immunoglobulines (Figure 4G). Notably, the numbers of B220+/lowCD138+ plasma cells/plasmablasts (PC/PBs) were significantly increased in the DKO mice above the levels found in Bim−/− animals (Figure 4H).
Loss of Bmf enhances the B cell-restricted lymphadenopathy caused by loss of Bim. (A-H) Spleens were harvested from 7- to 9-week-old mice of the indicated genotypes and single cell suspensions were counted to assess total leukocyte cellularity. Cell suspensions were stained using different combinations or fluorochrome-labeled antibodies for identification of total splenic T and B cells, as well as T1, T2, MZ, and FO B cells and PC/PBs. Data are presented as means ± SEM of ≥4 independent experiments and 4 to 8 animals per genotype. Significant differences by unpaired Student t test (P < .05) are indicated *between WT and Bmf−/−, **between WT and Bim−/−, #between WT and DKO, and §between Bim−/− and Bim/Bmf DKO mice.
Loss of Bmf enhances the B cell-restricted lymphadenopathy caused by loss of Bim. (A-H) Spleens were harvested from 7- to 9-week-old mice of the indicated genotypes and single cell suspensions were counted to assess total leukocyte cellularity. Cell suspensions were stained using different combinations or fluorochrome-labeled antibodies for identification of total splenic T and B cells, as well as T1, T2, MZ, and FO B cells and PC/PBs. Data are presented as means ± SEM of ≥4 independent experiments and 4 to 8 animals per genotype. Significant differences by unpaired Student t test (P < .05) are indicated *between WT and Bmf−/−, **between WT and Bim−/−, #between WT and DKO, and §between Bim−/− and Bim/Bmf DKO mice.
Similar to findings made in mature T cells, cell death triggered in culture by cytokine deprivation or different apoptosis inducers was mainly Bim dependent in B220+ splenic B cells (supplemental Figure 3). A significant contribution of Bmf to the killing of these cells was only recognized at certain time points in response to the HDAC inhibitor trichostatin A, dexamethasone, the ER stressor tunicamycin, or the calcium ionophore ionomycin, which is considered to trigger Bim-dependent apoptosis in immature thymocytes and mature T cells (Figure 2B; supplemental Figure 2). Apoptosis triggered by DNA damage or proteasome inhibition progressed normally in B cells from all genotypes tested (supplemental Figure 3).
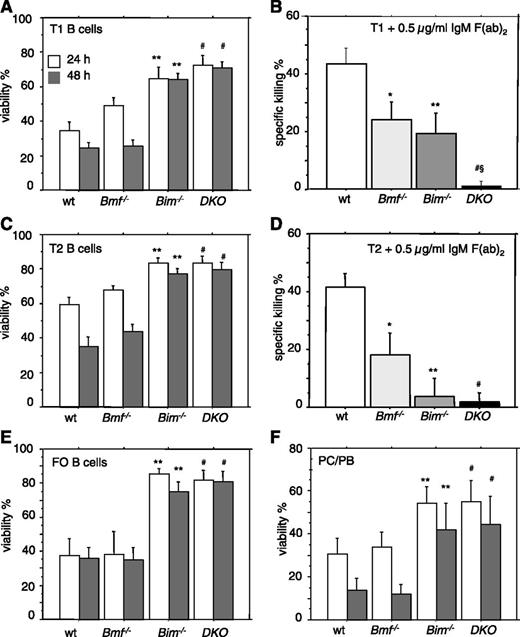
The accumulation of transitional B cells in DKO mice and resistance to apoptosis triggered by increased calcium flux suggested that both BH3-only proteins might be required for B-cell receptor (BCR) ligation-induced killing (a mimic of negative selection). To explore this hypothesis, fluorescence-activated cell sorter-sorted splenic transitional T1 and T2 B cells, which are susceptible to negative selection signals,20 were placed in culture and either left untreated or exposed to anti-IgM Fab2 antibody fragments, causing BCR ligation. Although the spontaneous death of these cells was strictly Bim dependent (Figure 5A,C), BCR ligation-induced killing was most effectively inhibited in T1 B cells from Bim−/−Bmf−/− mice (Figure 5B,D). Spontaneous cell death of FO B-cells and PC/PBs was again exclusively dependent on Bim (Figure 5E-F).
Combined loss of Bim and Bmf renders B cells independent of survival-promoting cytokines and refractory to BCR ligation-induced apoptosis. Transitional T1 (sIgMhighCD21low) or T2 (sIgMhighCD21high) B cells were sorted from spleens of 8- to 10-week-old mice of the indicated genotypes and placed in culture. Cells were either (A,C) left untreated or (B,D) stimulated with plate-bound anti-IgM F(ab)2 fragments for 48 hours. (E-F) Follicular (FO) B cells (CD21+CD23+) or plasma cells/plasmablasts (PC/PB) (B220+/lowCD138+) were sorted from spleens of mice of the indicated genotypes and placed untreated in culture. Cell viability was assessed after 24 and/or 48 hours by flow cytometric analysis. Data are presented as means ± SEM of ≥3 independent experiments and 3 to 5 animals per genotype. Significant differences (P < .05) are indicated *between WT and Bmf−/−, **between WT and Bim−/−, #between WT and DKO, and §between Bim−/− and Bim/Bmf DKO mice.
Combined loss of Bim and Bmf renders B cells independent of survival-promoting cytokines and refractory to BCR ligation-induced apoptosis. Transitional T1 (sIgMhighCD21low) or T2 (sIgMhighCD21high) B cells were sorted from spleens of 8- to 10-week-old mice of the indicated genotypes and placed in culture. Cells were either (A,C) left untreated or (B,D) stimulated with plate-bound anti-IgM F(ab)2 fragments for 48 hours. (E-F) Follicular (FO) B cells (CD21+CD23+) or plasma cells/plasmablasts (PC/PB) (B220+/lowCD138+) were sorted from spleens of mice of the indicated genotypes and placed untreated in culture. Cell viability was assessed after 24 and/or 48 hours by flow cytometric analysis. Data are presented as means ± SEM of ≥3 independent experiments and 3 to 5 animals per genotype. Significant differences (P < .05) are indicated *between WT and Bmf−/−, **between WT and Bim−/−, #between WT and DKO, and §between Bim−/− and Bim/Bmf DKO mice.
Combined loss of Bim and Bmf favors development of λ light-chain secreting plasma cells
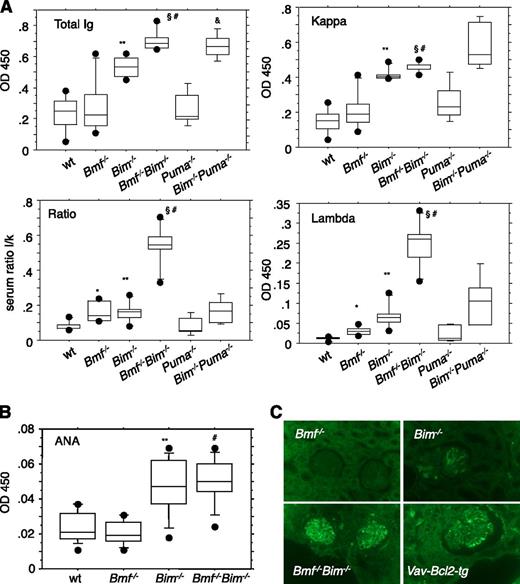
To investigate possible consequences of the B-cell excess in Bim−/−Bmf−/− mice, we measured serum Ig levels in unchallenged mice and in animals immunized with a T cell-dependent antigen. Serum Ig levels were significantly elevated in DKO mice compared with the levels found in single mutant or WT animals (Figure 6A). Remarkably, we observed a pronounced increase in the levels of λ light chain-containing immunoglobulins, leading to a shift in the κ/λ ratio in the sera of DKO mice. This phenomenon may be due to an increased frequency of rearrangements at the λ light chain locus in long-lived small resting pre-B or prolonged receptor editing in potentially autoreactive IgM+ immature B cells. In support of a specific contribution of Bmf to this process, accumulation of Igλ was not observed in sera from Bim−/−Puma−/− mice (Figure 6A). Furthermore, assessment of BCR expression demonstrated a significant increase in Igλ+ immature IgM+ B cells in bone marrow, as well as Igλ+ T1 and FO B cells in the spleen of Bim−/−Bmf−/− mice, demonstrating that both proteins coregulate the lifespan of B cells that undergo receptor editing (supplemental Figure 4).
Hypergammaglobulinemia in Bim−/−Bmf−/−mice is associated with increased levels of λ light chain-containing antibodies. (A) Sera were collected from 8- to 10-week-old mice of the indicated genotypes and total Ig, Igλ, and Igκ titers were quantified by ELISA. Overall quantities and λ/κ ratio are depicted based on OD450 values. Data from OD values are represented as box plots (n = 5-6 animals per genotype). Box length equals interquartile range. Circles represent minimal and maximal values. Significant differences (P < .05) are indicated *between WT and Bmf−/−, **between WT and Bim−/−, #between WT and Bim−/−Bmf−/−, §between Bim−/− and Bim−/−Bmf−/−, and &between WT and Bim−/−Puma−/− mice (n = 4). (B) Autoantibodies to dsDNA were quantified by ELISA using calf thymus dsDNA for coating of plates and serum samples from 8- to 10-week-old mice of the indicated genotypes. (C) Immunofluorescence staining of paraffin-embedded kidney sections reveals the presence of IgG deposits in glomeruli of female morbid tumor-free Bim−/−, DKO, and Vav-Bcl-2 transgenic mice (age range, 8-14 months). Healthy Bmf−/− mice, 18 months of age, were included as control. Magnification, ×400.
Hypergammaglobulinemia in Bim−/−Bmf−/−mice is associated with increased levels of λ light chain-containing antibodies. (A) Sera were collected from 8- to 10-week-old mice of the indicated genotypes and total Ig, Igλ, and Igκ titers were quantified by ELISA. Overall quantities and λ/κ ratio are depicted based on OD450 values. Data from OD values are represented as box plots (n = 5-6 animals per genotype). Box length equals interquartile range. Circles represent minimal and maximal values. Significant differences (P < .05) are indicated *between WT and Bmf−/−, **between WT and Bim−/−, #between WT and Bim−/−Bmf−/−, §between Bim−/− and Bim−/−Bmf−/−, and &between WT and Bim−/−Puma−/− mice (n = 4). (B) Autoantibodies to dsDNA were quantified by ELISA using calf thymus dsDNA for coating of plates and serum samples from 8- to 10-week-old mice of the indicated genotypes. (C) Immunofluorescence staining of paraffin-embedded kidney sections reveals the presence of IgG deposits in glomeruli of female morbid tumor-free Bim−/−, DKO, and Vav-Bcl-2 transgenic mice (age range, 8-14 months). Healthy Bmf−/− mice, 18 months of age, were included as control. Magnification, ×400.
Therefore, and based on the abnormal accumulation of transitional B cells in Bim−/−Bmf−/− mice that resist BCR ligation-induced apoptosis, we reasoned that an increased number of autoreactive B cells might be present that can differentiate into autoantibody-secreting plasma cells in these animals. However, quantification of antinuclear autoantibodies by ELISA revealed, as previously reported,2 increased levels in the absence of Bim but no further increase due to additional loss of Bmf (Figure 6B). In line with the increased overall Ig titers, IgG deposits were detected in the kidneys of aged Bim−/− and DKO mice. Kidney sections from Vav-Bcl2 transgenic animals served as positive control (Figure 6C).
Immunization with the T cell-dependent antigen, NP-ovalbumin, caused increased antigen-specific Ig titers in Bim-deficient mice, as previously reported21 ; this was, however, not increased significantly further by additional loss of Bmf (supplemental Figure 5).
Combined loss of Bim and Bmf function causes premature lethality
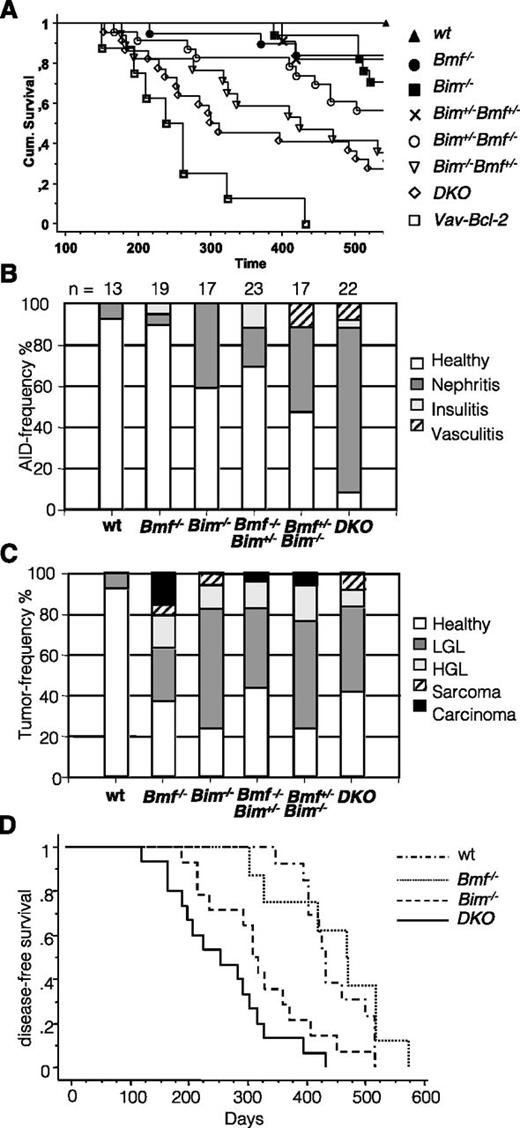
To investigate whether the severe lymphadenophathy and increased Ig titers observed in DKO mice cause pathology later in life, cohorts of WT, Bim−/−, Bim−/−Bmf+/−, Bim+/−Bmf−/−, and Bim−/−Bmf−/− mice were monitored over a period of up to 18 months. Interestingly, although none of the WT animals (0/13) and only 1 of 16 Bim−/− (6%) and 3 of 19 (15%) Bmf−/− mice had to be killed within this period, a significant portion of Bim+/−Bmf−/− (10/23; ∼43%), Bim−/−Bmf+/− (10/17; ∼58%), and Bim−/−Bmf−/− (15/22; ∼68%) mice developed signs of morbidity and had to be killed before the defined cutoff of 18 months (Figure 7A). Histopathological assessment of kidneys revealed features of immune complex-mediated glomerulonephritis with highest incidence (and levels of deposits) in DKO animals (20/22), whereas only 1 of 19 Bmf-deficient and 7 of 16 Bim-deficient mice showed similar changes (supplemental Figure 6). Other signs of autoimmunity included vasculitis and insulitis that were sometimes also found to occur simultaneously with glomerulonephritis in these mice (Figure 7B).
Combined loss of Bim and Bmf causes premature lethality. Cohorts of mice of the indicated genotypes were followed over 18 months. Mice developing overt signs of illness (cachexia, short breath, fuzzy fur, and enlarged lymph nodes or spleen) were killed, and organs were subjected to histopathological assessment in a blinded fashion revealing evidence for autoimmunity and tumor formation in a significant portion of double mutant mice. (A) Kaplan-Meier analysis of disease-free survival. Significant differences of interest (log-rank Mantle-Cox): WT vs Bim−/− mice (P = .036); WT vs Bim+/−Bmf−/− (P = .0074); WT vs Bim−/−Bmf+/− (P = .0001); WT vs DKO (P < .0001); WT vs Vav-Bcl2 (P < .0001); Bmf−/− vs DKO (P = .0009); Bim−/− vs Bim−/−Bmf+/− (P = .0065); Bim−/− vs DKO (P = .0055); and DKO vs Vav-Bcl2 (P = .032). The relative frequency of autoimmune pathologies and different types of malignancies observed are shown in B and C, respectively. (D) Kaplan-Meier analysis of disease-free survival of C57BL/6 Ly5.1+ coisogenic recipients reconstituted with 2 × 106 bone marrow cells of mice of the indicated genotypes (all C57BL/6 Ly5.2+) after a single dose of γ-irradiation (9.5 Gy). Significant differences (log-rank Mantle-Cox) were observed between WT vs Bim−/− (P = .022); WT vs DKO (P < .0001); Bmf−/− vs Bim−/− (P = .0262); Bmf−/− vs DKO (P = .0003); and Bim−/− vs DKO (P = .0423).
Combined loss of Bim and Bmf causes premature lethality. Cohorts of mice of the indicated genotypes were followed over 18 months. Mice developing overt signs of illness (cachexia, short breath, fuzzy fur, and enlarged lymph nodes or spleen) were killed, and organs were subjected to histopathological assessment in a blinded fashion revealing evidence for autoimmunity and tumor formation in a significant portion of double mutant mice. (A) Kaplan-Meier analysis of disease-free survival. Significant differences of interest (log-rank Mantle-Cox): WT vs Bim−/− mice (P = .036); WT vs Bim+/−Bmf−/− (P = .0074); WT vs Bim−/−Bmf+/− (P = .0001); WT vs DKO (P < .0001); WT vs Vav-Bcl2 (P < .0001); Bmf−/− vs DKO (P = .0009); Bim−/− vs Bim−/−Bmf+/− (P = .0065); Bim−/− vs DKO (P = .0055); and DKO vs Vav-Bcl2 (P = .032). The relative frequency of autoimmune pathologies and different types of malignancies observed are shown in B and C, respectively. (D) Kaplan-Meier analysis of disease-free survival of C57BL/6 Ly5.1+ coisogenic recipients reconstituted with 2 × 106 bone marrow cells of mice of the indicated genotypes (all C57BL/6 Ly5.2+) after a single dose of γ-irradiation (9.5 Gy). Significant differences (log-rank Mantle-Cox) were observed between WT vs Bim−/− (P = .022); WT vs DKO (P < .0001); Bmf−/− vs Bim−/− (P = .0262); Bmf−/− vs DKO (P = .0003); and Bim−/− vs DKO (P = .0423).
Further histopathological assessment revealed that a significant portion of mutant mice showed signs of malignant disease (supplemental Figure 7). Most frequently observed were low-grade lymphomas that increased in frequency when Bim levels were reduced (Bim+/−) or lost (Bim−/−) (range, 39-58%). The frequency of clearly malignant high-grade diffuse large B-cell lymphomas was comparable between aged Bim−/− (2/16), Bmf−/− (3/19), Bim−/−Bmf+/− (3/17), Bim+/−Bmf−/− (3/23), and Bim−/−Bmf−/− (2/22) mice. A range of solid tumors, including soft tissue sarcomas, angiosarcomas, adenocarcinomas, and hepatocellular carcinomas, was also observed and seemed to preferentially be associated with loss of Bmf, but occurred at too low a frequency to yield a statistically significant correlation (Figure 7C).
When comparing disease-free survival according to genotype and type of disease, ie, autoimmunity (supplemental Figure 6) vs tumors (supplemental Figure 7), we noted that Bim- and Bmf-deficient mice showed a tendency to develop spontaneous tumors more frequently in the second year of life than WT mice (P < .036; log-rank Mantel-Cox). In contrast, neither of the single mutant strains (Bim−/− or Bmf−/−) showed significantly increased frequency of autoimmune disease-associated symptoms. However, when 1 allele of Bim was lost together with loss of both Bmf alleles, disease-free life expectancy dropped significantly (P = .007), and this was mainly associated with increased tumor formation (P = .0027) but only occasionally with autoimmune disease. Similarly, Bim-deficient mice lacking 1 allele of Bmf showed signs of disease significantly earlier than WT animals, and the incidences of both autoimmunity and tumor formation were significantly increased (P < .0001). The same observations were made for DKO animals (P < .0001). Of note, Bmf-deficient mice did not differ significantly in their disease-free survival when 1 allele of Bim was additionally lost (data not shown), but loss of the second allele substantially reduced disease-free survival (P = .009). Notably, although the tumor incidence did increase in Bmf−/− compared with Bim−/−Bmf−/− mice only to a minor extent (P = .045), the increased incidence of autoimmune disease was highly significant (P < .0001). On the other hand, loss of 1 allele of Bmf on top of complete Bim deficiency markedly shortened tumor-free survival (P = .037) and also potently increased incidence of autoimmune disease (P = .0015). This suggests that combined loss of Bim and Bmf preferentially favors development of autoimmunity. Consistently, Bim−/−Bmf−/− mice showed significantly reduced disease-free survival compared with the Bim-deficient mice, but only the incidence of autoimmune disease was significantly increased (P < .0001), whereas the frequency of tumor formation was not affected (P = .24).
To examine whether Bim and Bmf needed to be lost in hematopoietic cells, nonhematopoietic cells, or both, bone marrow reconstitution experiments were performed (Figure 7D). Of note, lethally irradiated WT mice that had received Bim−/− or DKO bone marrow showed a drastically reduced lifespan compared with WT (WT vs Bim−/−: P = .0218; WT vs DKO: P < .0001; WT vs Bmf−/−: not significant; Bim−/− vs DKO: P = .0423), and this was mainly associated with autoimmune kidney disease as verified by histopathology (data not shown).
Discussion
Our results support a prominent overlapping role for Bim and Bmf during developmentally programmed apoptosis of interdigitating mesenchymal cells (Figure 1), a process that depends on bone morphogenic proteins and retinoic acid-mediated signaling.22 Notably, this death is only delayed in mice lacking Apaf-1, a key activator of the caspase cascade in the Bcl-2-regulated apoptotic pathway,23 whereas it is completely blocked in Bax/Bak-double deficient mice.24 Similar developmental defects have been noted in mice simultaneously lacking Bim, Puma, and Bid25 or Bim and Bax,26 the latter suggesting that Bmf may serve to activate Bax in this context.
Our study further demonstrates a significantly overlapping action for both BH3-only proteins in lymphocyte homeostasis, which is most pronounced in the B-cell lineage. This differs in Bim−/−Puma−/− mice that show increased accumulation of B and T lymphocytes that, in contrast to Bim−/−Bmf−/− lymphocytes, were all highly refractory to spontaneous cell death in culture.4 This indicates that Bmf acts as a rather specific cell death mediator activated at certain developmental stages under selected conditions, such as IL-7 deprivation or BCR ligation in developing B cells (Figures 3 and 4).
In contrast to a previous report, which touched only superficially on the consequences of combined loss of Bim and Bmf,27 we did not observe increased thymic cellularity or an increase in the percentages of DP thymocytes in Bim−/−Bmf−/− mice. Consistent with previous studies,2,28 we actually observed abnormally reduced percentages and numbers of DP thymocytes in mice lacking Bim or Bim plus Bmf. We attribute this inconsistency either to a gender bias, excluded in our study, and/or to the more narrow age range of the animals analyzed here (Table 1).
Previous studies using primary mouse lymphocytes or certain human cancer-derived cell lines demonstrated that different anticancer drugs require Bim or Bmf for efficient cell killing.6 We observed that combined loss of Bim and Bmf rendered thymocytes, pre-B, and to a minor extent mature B cells, but not mature T cells, more resistant to glucocorticoids or HDACi compared with cells lacking Bim alone (Figure 2; supplemental Figure 3). This finding fits the rather poor expression of Bmf in mature T cells.19 Notably, lymphocytes lacking Bim and Puma do show a much broader spectrum of resistance phenotypes and are refractory to cytokine withdrawal, DNA damage, ER stress, pan-kinase inhibition, or glucocorticoid treatment.4 This defines Bim, Puma, and Bmf as mediators of glucocorticoid-induced apoptosis, at least in mouse thymocytes and B cells. Whether Puma, alone or together with Bim or Bmf, also contributes to HDACi killing of lymphocytes remains to be tested. These findings may become important considering application of such drugs for therapy in humans as loss or reduced expression of these BH3-only protein has been documented in several cancers, including Burkitt’s lymphoma,12,29 mantle cell lymphoma,29 or renal cell carcinoma.30 Furthermore, an intronic polymorphism in the human BIM gene was shown to impair responses of chronic myeloid leukemia and non-small cell lung cancer to inhibitors of oncogenic kinases.8 It will be interesting to test whether these single nucleotide polymorphisms can also impair the responses of cancer cells to glucocorticoid treatment or HDAC inhibition. Although loss or mutations in BMF have thus far not been reported to contribute to drug resistance in human cancers, c-Myc-driven murine B-cell lymphomas depend selectively on Bmf to efficiently undergo apoptosis on Vorinostat (SAHA) plus ABT-737 combination therapy.13 Increased resistance of DKO thymocytes and pre-B cells to glucocorticoids is also in line with studies that showed that both BIM and BMF are induced early in response to glucocorticoids in childhood ALL and constitute a predictor of therapeutic outcome.14,15 Of note, loss of BMF appears to be a recurrent event in childhood ALL carrying ETV6/Runx-1 translocations where it also associates with glucocorticoid resistance during relapse.15 Hence, functional availability of both BH3-only proteins appears critical for therapeutic response and supports the concept of mimicking their action pharmacologically in cases where these genes are repressed.
Consistent with the B lymphadenopathy observed in Bim−/−Bmf−/− mice, we noted that developing pre-B cells became independent of their key survival cytokine, IL-7 (Figure 3). This suggests that Bim and Bmf exert critical roles in apoptosis as part of checkpoints during Ig-gene rearrangement. Accordingly, loss of Bim can partially rescue developing B cells in the bone marrow from death caused by the absence of IL-7, although they are unable to proceed further in differentiation.31 Bmf has not been implicated in this process thus far. However, given our observations that Bim−/−Bmf−/− mice show higher numbers of λ light chain-expressing B cells and increased levels of λ light chain-containing immunoglobulins (Figure 6), we hypothesize that developing Bim−/−Bmf−/− B cells can undergo abnormally prolonged Ig light chain gene rearrangement for receptor editing; this may allow escape from death by neglect or negative selection.32 Along that line, we also noted that transitional B cells, which are subject to negative selection based on the specificity of their BCR,20 were increased in number in Bim−/−Bmf−/− mice compared with Bim-deficient mice (Figure 4) and refractory to BCR ligation-mediated apoptosis (Figure 5). Together these observations suggest a possible contribution of Bmf (alongside Bim) to B-cell selection that awaits detailed validation in Ig transgenic mouse models. Our long-term follow-up of DKO mice that showed increased incidence of autoimmune disease actually supports this hypothesis (Figure 7).
Although DKO mice had significantly increased serum Ig levels compared with Bim−/− mice, no further increase in antinuclear antibodies was detected in these animals. We therefore hypothesize that the DKO animals have increased numbers of anergic B cells or that the plasma cells produce mainly low affinity antibodies. Consistent with this notion, immunization with a T-dependent antigen revealed no further increase in NP-specific antibody titers in the DKO animals compared with Bim−/− mice (supplemental Figure 4). This suggests that the overall increase in serum Ig levels in the DKO mice (Figure 6A) may be due to an increase in Ig production by MZ B cells and/or B1 B cells.
In line with the abnormally high serum Ig levels, our histophathological assessment revealed a significant increase in autoimmune kidney disease incidence in the DKO mice compared with the Bim−/− animals. Although this disease was mainly driven by loss of Bim, it was strongly exacerbated by additional loss of Bmf, in line with its impact on enhanced B-cell accumulation and antibody secretion. This finding is reminiscent of observations made in Vav-Bcl-2 transgenic mice, which also develop severe systemic autoimmune disease even on the relatively nonautoimmune-prone C57BL/6 background.33 In contrast, although Bim deficiency caused fatal autoimmune kidney disease on a mixed C57BL/6 × 129SV background, disease severity was substantially reduced after backcrossing onto a C57BL/6 background (our study and ref. 34). It is therefore interesting that additional loss of Bmf can revert this and cause severe autoimmune disease in conjunction with loss of Bim. Of note, this appears unique to the combined loss of Bim and Bmf, because neither combined loss of Bim plus Bad,35 Bim plus Puma,4 nor combined loss of Bmf plus Bad19 caused such morbidity. Hence, it will be interesting to test whether BMF levels are deregulated, either alone or in conjunction with those of BIM, in B cell-dependent autoimmune pathologies in humans.
Although the frequency of spontaneously arising malignant high-grade lymphomas was comparable between Bim−/−, Bmf−/− and DKO mice, more low-grade lymphomas developed in mice that lacked Bim. Most strikingly, however, is that loss of a single allele of Bmf together with complete loss of Bim markedly shortened tumor-free survival in these mice. This provides evidence for a critical role of Bmf in tumor suppression in conjunction with Bim. Notably, reduced Bmf levels facilitated the emergence of nonlymphatic tumors, reminiscent of what was previously seen in mice deficient for both Bmf and Bad.19 Interestingly, although a significant percentage of Bim−/−Puma−/− mice developed signs of malignant disease within the first year of life, we never observed nonhematopoietic tumors,4 suggesting a role for Bmf in limiting transformation of epithelial and mesenchymal cells. Although a critical tumor suppressive role for BMF in solid cancers in humans has not yet been directly confirmed, miR221, which targets BMF, is overexpressed in hepatocellular carcinoma, and the genomic region harboring BMF (15q14) is frequently deleted in metastatic, but not primary, carcinomas.36
In conclusion, we demonstrated that Bim and Bmf are essential mediators of developmentally programmed cell death, critical for killing lymphocytes by a range of anticancer therapeutics, and constitute a barrier against cancer and autoimmunity. This strongly supports the ongoing effort to mimic the function of these proteins by small-molecular-weight compounds for the treatment of cancer and autoimmune diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Rossi, B. Rieder, C. Soratroi, R. Pfeilschifter, and I. Gaggl for technical assistance and/or animal care; G. Böck for cell sorting; R. Johnstone and M. Ausserlechner for reagents; and S. Herzog and E. Derudder for discussion.
This work was supported by Austrian Science Fund grants P23510-B19 and Y212-B13 (to A.V.), the National Health and Medical Research Council Australia, Leukemia and Lymphoma Society (Specialized Center of Research grant) and the Cancer Council of Victoria (A.S. and P.B.), the Tyrolean Science Fund (V.L.), and “Österreichische Krebshilfe–Tirol” (V.L. and C.W.). C.W. was supported by a Doc-fFORTE doctoral fellowship from the Austrian Academy of Science.
Authorship
Contribution: V.L. performed experiments, analyzed data, contributed to writing, and prepared figures; C.W. and S.T. performed experiments and analyzed data; M.E. performed experiments, analyzed data, and prepared figures; P.B. and A.S. contributed valuable reagents, analyzed data, and edited the manuscript; A.T. performed histopathological analysis; and A.V. designed the research, analyzed data, wrote the manuscript, and conceived the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for V.L. is Immune Regulation and Cancer, Max-Delbrück-Center for Molecular Medicine, Berlin, Germany.
The current affiliation for C.W. is Institute for Forensic Medicine, Medical University Innsbruck, Innsbruck, Austria.
Correspondence: Andreas Villunger, Division of Developmental Immunology, Biocenter, Innrain 80-82, Innsbruck Medical University, A-6020 Innsbruck, Austria; e-mail: andreas.villunger@i-med.ac.at.