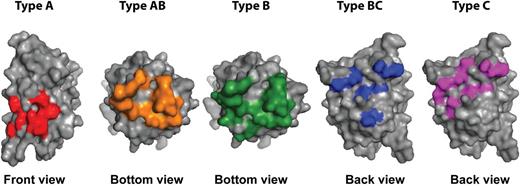
In this issue of Blood, Nguyen et al employ high-resolution mapping to precisely define epitopes on the C2 domain of blood coagulation factor VIII (FVIII).1 Their results nicely complement a recent report in Blood describing the structure of a ternary complex of 2 inhibitory antibodies with the C2 domain.2 Together, these studies reveal fascinating molecular details on the unexpectedly large number of exposed surfaces in the C2 domain that contribute to the binding of inhibitory antibodies. These findings are relevant in the context of neutralizing anti-FVIII antibodies that develop in patients with hemophilia A. Insight into the antigenic properties of the C2 domain is needed to design FVIII variants with decreased antigenicity. Pioneering studies by Dorothea Scandella on the epitope mapping of FVIII inhibitors already pointed toward the C2 domain as a major binding site for FVIII inhibitors.3 The recent studies by Nguyen and Walter, combined with earlier work by Meeks et al, have provided evidence for 3 major binding sites for inhibitory anti-FVIII antibodies within the C2 domain.4 The overall dimensions of the C2 domain are small when compared to antibodies. Nevertheless, at least 2 and probably 3 monoclonal antibodies can simultaneously bind to the C2 domain.2 The mapping studies reported by Nguyen also suggest the presence of 3 distinct clusters of surface-exposed side chains on the FVIII C2 domain (see figure).1
Inhibitor epitopes on the C2 domain. Binding sites of the 5 different types (A, AB, B, BC, and C) of anti-C2 domain antibodies are displayed. The orientation of the C2 domain is depicted below the image. For type AB and B antibodies, a bottom view of the C2 domain is shown. Image was prepared using the crystal structure of B domain-deleted FVIII (3cdz), using PyMOL.
Inhibitor epitopes on the C2 domain. Binding sites of the 5 different types (A, AB, B, BC, and C) of anti-C2 domain antibodies are displayed. The orientation of the C2 domain is depicted below the image. For type AB and B antibodies, a bottom view of the C2 domain is shown. Image was prepared using the crystal structure of B domain-deleted FVIII (3cdz), using PyMOL.
The results obtained raise the issue of why so many distinct antigenic sites are present within the C2 domain. The currently available structures of antibodies in complex with the C2 domain reveal that positively charged surfaces contribute to the binding of anti-C2 antibodies.2,5 These positively charged clusters contribute to the binding of FVIII to negatively charged phospholipids. Conversely, these patches of positively charged amino acids may also direct the immune response toward the C2 domain by promoting the selection of B-cell clones expressing antibody molecules with negatively charged residues in their variable domains.
It should be noted that the majority of monoclonal antibodies analyzed in this study are derived from hemophilia A mice injected with human FVIII. Epstein-Barr virus immortalization and phage display have been employed to isolate human monoclonal anti-C2 antibodies from peripheral blood of inhibitor patients.6,7 Reactivity of only a single human monoclonal antibody (BO2C11) belonging to the type AB group (see figure) was included in this study. Competition experiments have shown that so-called type BC/C antibodies (see figure) are also present in patients with FVIII inhibitors.8 Nevertheless, it would be important to extend the innovative studies reported in this article to a panel of human monoclonal antibodies derived from B cells of inhibitor patients.
In their search for antigenic sites on the C2 domain, Nguyen et al focused on antigenic variants that increased the dissociation rate of antibody–C2 domain complexes. This elegant approach has proven to be highly diagnostic for the identification of residues crucial for the high-affinity binding of antibodies to the C2 domain. Modification of the identified C2 domain residues in conjunction with antigenic loops in other antigenic sites within other domains provides an interesting approach for the development of less-antigenic variants of FVIII.
Apart from the proposed modification of B-cell epitopes on FVIII, a number of other approaches are currently being explored to prevent formation or eradicate preexisting inhibitors in hemophilia A patients.9,10 These efforts, together with the novel half-life-extending bioengineered FVIII molecules and the recent revival of gene therapy approaches, provide exciting new opportunities to further extend the current portfolio of therapeutic options for hemophilia A.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal