Abstract
Sickle cell disease (SCD) has evolved into a debilitating disorder with emerging end-organ damage. One of the organs affected is the liver, causing “sickle hepatopathy,” an umbrella term for a variety of acute and chronic pathologies. Prevalence of liver dysfunction in SCD is unknown, with estimates of 10%. Dominant etiologies include gallstones, hepatic sequestration, viral hepatitis, and sickle cell intrahepatic cholestasis (SCIC). In addition, causes of liver disease outside SCD must be identified and managed. SCIC is an uncommon, severe subtype, with outcome of its acute form having vastly improved with exchange blood transfusion (EBT). In its chronic form, there is limited evidence for EBT programs as a therapeutic option. Liver transplantation may have a role in a subset of patients with minimal SCD-related other organ damage. In the transplantation setting, EBT is important to maintain a low hemoglobin S fraction peri- and posttransplantation. Liver dysfunction in SCD is likely to escalate as life span increases and patients incur incremental transfusional iron overload. Future work must concentrate on not only investigating the underlying pathogenesis, but also identifying in whom and when to intervene with the 2 treatment modalities available: EBT and liver transplantation.
Case presentation
A black British man of African-Caribbean descent was diagnosed with sickle cell disease (SCD; genotype HbSS) as a child and followed up in our institution. His medical history comprised common complications of SCD: gallstones followed by laparoscopic cholecystectomy, priapism, and multiple admissions with acute pain crises. He was reluctant and irregular in taking hydroxyurea. He had no history of alcohol excess, and his body mass index (BMI) was normal.
He developed abnormal steady-state liver function at age 23 years, with hyperbilirubinemia, which peaked at >500 µmol/L (30 mg/dL, 68% conjugated bilirubin) during an acute hospital admission. This was followed by recurrent admissions each characterized by right upper quadrant abdominal pain, fever, and acute worsening of liver enzymes, accompanied by extreme hyperbilirubinemia. A laparoscopic cholecystectomy at age 25 years did not improve symptoms. A regular program of simple (additive) blood transfusions was initiated in an attempt to control the frequency of acute pain and a presumed diagnosis of sickle cell intrahepatic cholestasis (SCIC).
Further liver investigations showed no other obvious cause for liver disease: liver imaging and abdominal computed tomography (CT) demonstrated hepatomegaly with right and left intrahepatic duct dilatation, suggestive of a cholangiopathy, a normally enhancing liver, and patent vessels; laboratory results revealed negative autoimmune screen, normal α-feto-protein levels, and negative viral serology (for hepatitis B, hepatitis C, hepatitis A, and HIV). Ferritin levels in steady state were markedly raised at 3000-4000 µg/L. An R2 magnetic resonance imaging (MRI) liver showed a liver iron concentration of 7.9 mg Fe/g dry weight, consistent with transfusion-related iron overload. At this stage, he has had a lifetime red cell transfusion of 796 units, the large majority being exchange units to maintain a hemoglobin S fraction (HbS%) target of <30% to 40%. His bilirubin and liver enzymes continued to increase despite the supportive treatment, although there was no evidence of impaired hepatic synthetic function.
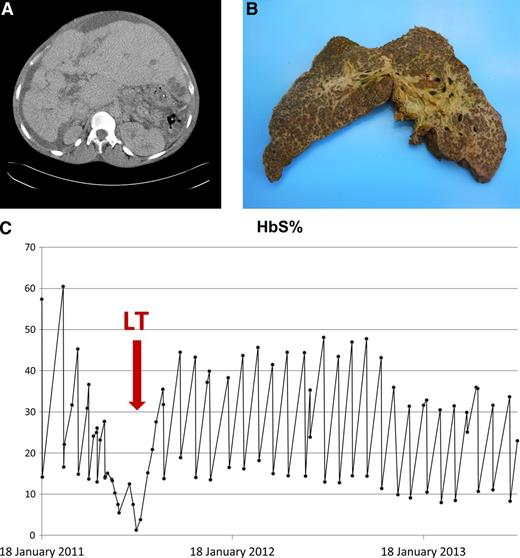
In early 2011, aged 32 years, he developed decompensated liver failure with portal hypertension, ascites, and impaired synthetic function (see Figure 1A for CT liver with contrast). At this stage, evaluation of other vital organs revealed, at worse, only mildly impaired function (echocardiogram with left ventricular ejection fraction 58% and normal tricuspid regurgitant jet velocities; mild reduction in pulmonary function testing (forced expiratory volume in 1 second 67% predicted, vital capacity 65% predicted); no evidence of sickle lung disease on chest CT; and mildly reduced creatinine clearance on 24-hour urinary testing at 50 mL/min). He underwent formal hepatological assessment for liver transplantation (LT) using the standard protocol; his United Kingdom End-Stage Liver Disease score was 61, which indicated a 50% chance of 1-year survival. The situation was that of an uncommon hepatological complication of a common hematologic condition. We should emphasize that end-stage chronic liver disease in SCD is not an accepted indication for LT in the United Kingdom. His case was accepted on appeal to the National Appeals Committee.
CT scan of liver, explanted liver, and response to exchange blood transfusions. (A) CT liver with contrast pretransplant. CT abdomen with intravenous contrast in July 2011 (just prior to transplant) demonstrated long-standing changes, including a nodular liver with left lobe hypertrophy, biliary dilatation, varices (splenic, umbilical, and retroperitoneal), and moderate ascites. A low-density area in liver segment 3 (probable biliary abscess) was also noted. (B) Explanted liver. (C) HbS% pretransplant, peritransplant, and posttransplant.
CT scan of liver, explanted liver, and response to exchange blood transfusions. (A) CT liver with contrast pretransplant. CT abdomen with intravenous contrast in July 2011 (just prior to transplant) demonstrated long-standing changes, including a nodular liver with left lobe hypertrophy, biliary dilatation, varices (splenic, umbilical, and retroperitoneal), and moderate ascites. A low-density area in liver segment 3 (probable biliary abscess) was also noted. (B) Explanted liver. (C) HbS% pretransplant, peritransplant, and posttransplant.
He underwent LT on July 15, 2011, aged 33 years. Postoperatively, he developed metabolic acidosis, which was corrected with hemofiltration, and a paralytic ileus that improved spontaneously. He was also treated for an Escherichia coli bacteremia. The explanted liver was nodular and weighed 3700 g (Figure 1B); histology demonstrated liver cirrhosis with predominant sclerosing cholangitis, moderate siderosis, sickle cells in sinusoids, and a biliary abscess.
After transplantation, his liver enzymes normalized within 1 month while being maintained on an exchange blood transfusion (EBT) program (Figure 1C). Immunosuppression was maintained with steroids and tacrolimus. Sickle-related complications after transplantation included: 2 admissions with sepsis, proteinuria treated with candesartan, and priapism. Intractable priapism was not seen pretransplant and may be related to the return of normal liver function and sex hormone synthesis. His priapism did not respond to etilefrine; hydroxyurea therapy was reinitiated, but again, he was noncompliant. His priapism was eventually controlled by increasing the frequency of EBT (maintaining HbS% <30%) to 4-week intervals, and his serum ferritin has normalized. Currently, he has had a lifetime exposure to >1200 red cell units, although he has not developed any detectable red cell allo-antibodies. Two years after his LT, he fathered another child.
Discussion
How do we define “sickle hepatopathy”?
In developed countries, SCD has evolved into a debilitating chronic disorder with significant morbidity because of end-organ damage. The liver is one of the affected organs, resulting in sickle hepatopathy. In SCD, some end-organ damage has a well-recognized natural history with identified treatments: proteinuria and renal impairment, right-sided heart pressures and pulmonary hypertension, and high velocities on transcranial Dopplers and ischemic stroke risk (in children). However, the nature of liver dysfunction in SCD has not been characterized, nor has the natural history and pathogenesis of the liver disease been fully defined. This lack of knowledge prevents specific monitoring and deciding when, how, and in whom to intervene.
Sickle hepatopathy is an umbrella term covering a wide variety of pathologies, both acute and chronic, that occur as a consequence of the sickling process, including gallstone disease, hypoxic liver injury, hepatic sequestration, venous outflow obstruction, viral hepatitis (especially in the multitransfused patient), hepatic crises, and SCIC.1 Some clinicians, however, consider sickle hepatopathy as a term reserved for intrahepatic cholestasis.2,3 The clinicopathological features are aggravated by liver iron overload that results from cumulative red cell transfusions. It is important to look for additional causes of liver disease; in the 2007 King’s College Hospital cohort,1 37% had a second liver comorbidity, including autoimmune liver disease and viral hepatitis. Diagnosis is confounded by problems encountered with obtaining liver tissue for histologic analysis; SCD has been suggested to be a contraindication to liver biopsy in the acute setting.4
SCIC is an uncommon but severe form of sickle hepatopathy, first described in 1953.5 Clinically, it comprises severe right upper quadrant pain, acute hepatomegaly, coagulopathy, extreme hyperbilirubinemia (mainly conjugated in contrast to the typical unconjugated hyperbilirubinemia seen in hemolytic anemias), but moderately elevated liver enzymes, with occasional progression to acute hepatic failure. Pathologically, it involves sickling within hepatic sinusoids leading to vascular stasis and localized hypoxia. The hypoxic injury results in ballooning of the hepatocytes causing a direct back pressure effect, with resultant intracanalicular cholestasis, its defining feature.6 SCIC is most commonly described in its acute form, sometimes presenting as recurrent episodes, and in a subset becoming chronic, eventually evolving into progressive liver failure. This contrasts with the pediatric experience, where cases of extreme hyperbilirubinemia with intrahepatic sickling have been reported to recover spontaneously with supportive care alone.2,3
How common is liver dysfunction in SCD?
Because liver dysfunction in SCD is difficult to define, its prevalence too is difficult to document, with previous reports of ∼10%.7 Using liver function tests to assess liver damage in SCD is confounded by abnormal liver enzymes reflecting not only intrinsic liver disease but also hemolysis. Our experience reveals that patients with SCD have evidence of disrupted liver synthetic function late in the natural history. Our patient developed abnormal coagulopathy and hypoalbuminemia in March 2011, 9 years after he first developed hyperbilirubinemia. Thus, abnormal liver enzymes should prompt a more comprehensive liver workup including laboratory and radiologic assessments, aimed at identifying true liver dysfunction and determining severity and etiology.
As well as looking for causes of liver dysfunction unrelated to SCD, an assessment of iron overload must also be made, especially in view of the increasing use of blood transfusion in SCD.8 The risk of hepatic siderosis is further likely to escalate with the increasing life span in SCD patients and cumulative exposure to transfused red cells.
What is appropriate management of sickle hepatopathy?
The management of sickle hepatopathy relies on accurate identification and treatment of any coexisting cause(s). The diverse etiologies contribute to the extensive variation in natural history and severity of liver disease. Joint management involving a hepatologist with an interest in SCD is useful.
Investigation.
It is important to exclude other causes of liver dysfunction in SCD including alcohol, medication (including recreational drugs), and BMI evaluation (see Table 1).
Investigations for causes of sickle hepatopathy in SCD
| Investigation . |
|---|
| Clinical history and examination |
| Alcohol history |
| BMI |
| Drug history |
| Imaging |
| Liver USS including assessment of vessel patency and biliary system |
| Other imaging: CT abdomen, MRCP, ERCP |
| Laboratory |
| Viral serology: hepatitis B, hepatitis C, HIV, cytomegalovirus, Epstein-Barr virus, hepatitis A |
| Autoimmune screen: anti-nuclear antibody, anti–smooth muscle antibody, anti–liver/kidney antibody, anti–soluble liver antigen, anti-mitochondrial antibody |
| α-Feto-protein levels |
| Ceruloplasmin |
| α1-Antitrypsin |
| Liver biopsy |
| Usually not indicated unless genuine diagnostic dilemma |
| Iron status |
| Ferritin |
| MRI for liver iron concentration |
| Investigation . |
|---|
| Clinical history and examination |
| Alcohol history |
| BMI |
| Drug history |
| Imaging |
| Liver USS including assessment of vessel patency and biliary system |
| Other imaging: CT abdomen, MRCP, ERCP |
| Laboratory |
| Viral serology: hepatitis B, hepatitis C, HIV, cytomegalovirus, Epstein-Barr virus, hepatitis A |
| Autoimmune screen: anti-nuclear antibody, anti–smooth muscle antibody, anti–liver/kidney antibody, anti–soluble liver antigen, anti-mitochondrial antibody |
| α-Feto-protein levels |
| Ceruloplasmin |
| α1-Antitrypsin |
| Liver biopsy |
| Usually not indicated unless genuine diagnostic dilemma |
| Iron status |
| Ferritin |
| MRI for liver iron concentration |
ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic resonance cholangiopancreatography; USS, ultrasound scan.
In any liver disease, the first imaging investigation is an abdominal ultrasound; the technique is accurate in assessment of the presence of cirrhosis, ascites, portal vein anatomy, biliary calculi, and biliary dilatation. Ultrasound is also the most sensitive imaging technique for assessment of acute calculous cholecystitis with a positive predictive value of 95% when gallbladder wall thickening is more than 3.5 mm and gallstones are present. Abdominal CT is useful in diagnosing complications of biliary disease, perforation, emphysematous cholecystitis, cholangitis, and liver abscess. MRCP is the imaging technique of choice in the diagnosis of cholangiopathy; it is noninvasive, accurate, and predicts the need for ERCP to allow intervention regarding common bile duct calculi and dominant stricture. The accuracy of MRCP for detecting biliary calculi is 98%.9 MRCP is comparable with ERCP for the diagnosis of primary sclerosing cholangitis but less effective in diagnosis of dominant strictures.10
Using ferritin as a marker of iron burden in SCD is complicated by ferritin itself being increased in both liver disease and inflammation. Baseline MRI, such as R2MRI measurements, is recommended to detect liver iron load.
Liver biopsy is a relative contraindication because of the high risk of bleeding and liver rupture. We recommend that it is only performed after specialist hepatology assessment and when results will significantly influence management, such as with a diagnostic dilemma, for example with intrahepatic cholestasis and autoimmune disease in the differential diagnosis. If a liver biopsy is conducted, a transjugular route is recommended.
Treatment options for specific complications.
Iron chelation for iron overload is recommended.11 Quantitatively, chelation is considered appropriate when liver iron concentration exceeds 7 mg Fe/g dry weight, roughly equivalent to transfusion of more than 20 units of red cells.12
The role of elective cholecystectomy for asymptomatic cholelithiasis remains controversial. For example, in a recent longitudinal study of 26 pediatric SCD patients with radiologic evidence of cholelithiasis, 25 remained asymptomatic over a 13-year follow-up period.13 In contrast, in a 2007 Italian series of 30 pediatric patients, significantly increased morbidity was reported in patients who were not electively cholecystectomized and who subsequently proceeded to symptomatic cholecystectomy.14 It is also debatable if patients who have coinherited Gilbert syndrome (polymorphism in promoter region of UGT1A gene) and thus are genetically predisposed to further increases in serum bilirubin levels, and gallstones,15 should have elective cholecystectomy.
In early reports of SCIC, mortality was high, caused by fulminant hepatic failure or bleeding. Outcome has since improved dramatically with EBT (first described by Sheehy in 198016 ). EBT rather than simple blood transfusion is recommended for managing both acute and chronic SCIC for ease of achieving HbS% target and to reduce the risk of secondary iron overload. Early implementation of an EBT program is critical; suggested thresholds of HbS% have been reported as <20% and <30%.17-19
In cholestasis, use of the bile acid ursodeoxycholic acid can improve biliary flow where sludging is a feature. Hydroxyurea, beneficial in patients with frequent painful crises and acute chest syndrome, has not been shown to reduce the frequency of hepatic sequestration crises.20 Further work is needed on exploring the preventative effects of hydroxyurea on organ damage including sickle hepatopathy, especially SCIC.
When do we intervene?
In general, assessment of the severity of liver disease and likely predominant etiology(ies) can be made after clinical assessment and appropriate laboratory and radiologic investigation.
In the severe acute liver dysfunction syndromes, and progressive liver cholestasis, anecdotal experience is accruing for the use of EBT, although this has not been rigorously scrutinized. There are several case reports demonstrating that acute SCIC can be reversed with prompt EBT.3,16,21-24 With progressive disease, it is not clear when EBT should be commenced or what the aims of treatment should be. In addition to our patient, there are 2 examples in the literature of the use of a program of EBT to manage chronic SCIC in the nontransplant setting.19,25 Maintaining HbS levels at <20% to 30% has been proposed, but again this is based on pragmatism rather than evidence. Future work must explore the natural history of SCIC in order to identify which patients are at risk of disease progression and which would benefit from regular EBT. We suggest a pragmatic schema (Figure 2) for managing those with sickle hepatopathy.
Management pathway for sickle hepatopathy. Note:1) Exercise caution in bilirubin interpretation in those with Gilbert's syndrome or G6PD deficiency; 2) At any stage, if patient is in fulminant failure for whatever cause, consideration of LT is dependent on patient and vital organ function; 3) For patients with non-SCD causes (eg, autoimmune hepatitis) and refractory to treatment, consider a regular EBT program; 4) Monitoring and management of iron overload essential as hepatic siderosis may exacerbate other causes of hepatic dysfunction; 5) Liver biopsy not indicated unless a genuine diagnostic dilemma; 6) Ursodeoxycholic acid may be considered to improve biliary sludging in cholestasis.
Management pathway for sickle hepatopathy. Note:1) Exercise caution in bilirubin interpretation in those with Gilbert's syndrome or G6PD deficiency; 2) At any stage, if patient is in fulminant failure for whatever cause, consideration of LT is dependent on patient and vital organ function; 3) For patients with non-SCD causes (eg, autoimmune hepatitis) and refractory to treatment, consider a regular EBT program; 4) Monitoring and management of iron overload essential as hepatic siderosis may exacerbate other causes of hepatic dysfunction; 5) Liver biopsy not indicated unless a genuine diagnostic dilemma; 6) Ursodeoxycholic acid may be considered to improve biliary sludging in cholestasis.
Are there any predisposing factors?
Patients with sickle hepatopathy frequently have multiple causes of liver disease. Whether these are independent pathological processes or represent a predisposing factor to SCIC is unknown; the pathogenesis of SCIC is not clear. What we do note is the high prevalence of gallstone disease, which may represent a predisposing factor to intrahepatic cholestasis prompting elective cholesystectomy for asymptomatic cholelithiasis. We further note in the documented cases of SCIC that there is a gender disparity in disease incidence, with only 26% female. In SCD, transfusional iron overload is linked with liver fibrosis.26 It is likely that nontransferrin bound iron induces reactive oxygen species, which not only directly causes cellular damage but also depletes nitric oxide levels leading to endothelial dysfunction. These additional pathologies are likely to contribute to the disease process of SCIC and therefore represent “predisposing factors,” but they could also represent coincidental diagnoses; either way, it is important to make an early diagnosis and begin management of other hepatic/biliary complications in SCD.
What is the role of LT?
Worldwide experience of LT as a therapeutic modality for end-stage liver disease as a consequence of sickle hepatopathy is slowly developing, with 22 cases reported in the literature.1,17,18,27-37 However, several questions remain unanswered. The poor results reported in early case series have improved, to some degree, in later studies. This is likely a consequence of better patient selection and improved perioperative management of SCD.
What can be extrapolated from the data? Good vital organ function is crucial for LT surgery itself; this precludes many SCD patients who have already developed significant sickle-related complications of the heart, lungs, kidneys, and brain, concomitant with their sickle hepatopathy. Two clinical phenotypes that benefit most from LT can be identified: the first with end-stage liver disease attributable to SCD, but without significant sickle-related damage of other organs, and the second with liver disease coincident to SCD (for example autoimmune liver disease) with perhaps a milder SCD phenotype. Specific transplant-related complications in patients with SCD include vascular thrombosis, an increased incidence of sepsis, and neurologic dysfunction. Recurrent sickle hepatopathy in the liver graft has been reported.28 Recommendations for perioperative management of SCD include regular EBT to maintain HbS fraction <30%; again, this is not evidence based and is largely extrapolated from studies involving nontransplant surgery and primary38 and secondary stroke prevention.39,40 One should also note the variable outcomes of LT in the acute setting; there is a high incidence of failure of the liver graft and poor patient survival.27-30,34,36
Currently, end-stage liver disease as a consequence of sickle hepatopathy is not an accepted indication for LT in the United Kingdom. A pilot study is underway to define the role of this treatment modality in this group of patients. We would recommend that patients approaching end-stage liver disease be referred to centers where joint hematologic and hepatological assessment can be performed and patients offered the full range of treatment options.
Our experience in the United Kingdom represents a well-resourced setting with well-established infrastructure within hematology and hepatology. LT is a difficult option for those in poorly resourced settings. Transplantation planning must not only take into account the workup and surgery itself, but also consider postoperative multidisciplinary care involving hematology and EBT, and the logistics and side effects of immunosuppression.
Conclusion
Sickle hepatopathy is a nonspecific term reflecting the heterogeneity of liver dysfunction in SCD. It is important to look for and treat causes of liver disease outside SCD itself. Data from our cohort suggest that hepatitis C and alcohol as causes appear lower than reports from elsewhere. Being able to predict who will progress to end-stage liver disease remains an elusive goal. Medical managements are limited and not evidence based; there is an urgent need to formally evaluate the therapeutic strategies of EBT and LT. Regular EBT should be considered for patients with severe acute hepatic crises and progressive cholestasis. LT may have a role in a subset of patients. A current trial is exploring the possibility of reduced intensity hematopoietic stem cell transplantation in SCD with end-organ damage, but whether this could reverse liver damage is unknown (http://clinicaltrials.gov/ct2/show/NCT01950429). Finally, we anticipate that liver dysfunction will become an increasing problem in an aging SCD population, with more aggressive transfusion programs contributing to increased hepatic siderosis.
Acknowledgments
The authors thank Claire Steward for help in preparation of the manuscript.
This work was supported by the Medical Research Council, United Kingdom (MRC Grant ID:62593. no. G0001249) (S.L.T.).
Authorship
Contribution: K.G., A.S., P.K., A.B., and S.L.T. wrote the manuscript; and all authors reviewed and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Swee Lay Thein, King’s College London School of Medicine, Molecular Haematology, The James Black Centre, 125 Coldharbour Lane, London SE5 9NU, United Kingdom; e-mail: sl.thein@kcl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal