Key Points
Heme oxygenase-1 levels increase during erythroid differentiation.
Heme oxygenase-1 actively participates in maintaining appropriate hemoglobinization rates.
Abstract
Heme is essential for the function of all aerobic cells. However, it can be toxic when it occurs in a non–protein-bound form; cells maintain a fine balance between heme synthesis and catabolism. The only physiological mechanism of heme degradation is by heme oxygenases (HOs). The heme-inducible isoform, HO-1, has been extensively studied in numerous nonerythroid cells, but virtually nothing is known about the expression and potential significance of HO-1 in developing red blood cells. We have demonstrated that HO-1 is present in erythroid cells and that its expression is upregulated during erythroid differentiation. Overexpression of HO-1 in erythroid cells impairs hemoglobin synthesis, whereas HO-1 absence enhances hemoglobinization in cultured erythroid cells. Based on these results, we conclude that HO-1 controls the regulatory heme pool at appropriate levels for any given stage of erythroid differentiation. In summary, our study brings to light the importance of HO-1 expression for erythroid development and expands our knowledge about the fine regulation of hemoglobin synthesis in erythroid cells. Our results indicate that HO-1 plays an important role as a coregulator of the erythroid differentiation process. Moreover, HO-1 expression must be tightly regulated during red blood cell development.
Introduction
Heme is a complex of iron with protoporphyrin IX, which is essential for the function of all aerobic cells. Heme serves as the prosthetic group of numerous hemoproteins (eg, hemoglobin, myoglobin, cytochromes, guanylate cyclase, nitric oxide synthase) and plays an important role in controlling protein synthesis and cell differentiation.1 However, when left unguarded, non–protein-bound heme promotes free radical formation and lipid peroxidation, resulting in cell damage and tissue injury.2,3 Hence, cellular heme levels are tightly controlled; this is achieved by a fine balance between heme biosynthesis and catabolism.
The highest amounts of organismal heme (75% to 80%) are present in circulating red blood cells (RBCs) whose precursors synthesize heme with rates that are at least 1 order of magnitude higher than those in the liver, the second most active heme producer in mammals. Differences in iron metabolism and in the genes encoding 5-aminolevulinic acid synthase (the first enzyme in heme biosynthesis) are responsible for the variation in the regulation and rates of heme synthesis in erythroid and nonerythroid cells.1
Heme is degraded by heme oxygenases (HOs) yielding iron, bilirubin, and carbon monoxide.4,5 Heme from RBCs is fated to be catabolized in splenic and hepatic macrophages after erythrophagocytosis of senescent RBCs. There are 2 isoforms of HOs: HO-1 and HO-2. The constitutive form, HO-2, is membrane-bound protein found at highest levels in the brain and testis.6 The inducible form, HO-1, is a 32.8-kDa membrane-bound enzyme found at the highest concentrations in the liver and spleen. HO-1 is increased in animal tissues after treatment with its natural substrate heme and various metals, xenobiotics, endocrine factors, and synthetic metalloporphyrins6 as well as several agents that cause oxidative damage.7-9 HO-1 promotes protection through the removal of the dangerous prooxidant “free” heme, generated in stress conditions, and releases biliverdin and bilirubin, which are metabolites with antioxidant properties.3,10
HOs, in particular HO-1, have been extensively studied in hepatocytes and other nonerythroid cells.4,5 Ironically, even though RBCs possess the greatest amounts of heme, virtually nothing is known about the expression of HO-1 in developing RBCs. Moreover, it is unknown whether HO-1 plays any role in hemoglobin synthesis under physiological or pathological conditions.
The aim of our study was to determine whether developing RBCs express HO-1 and, if so, characterize its role in physiology and possibly pathophysiology of erythropoiesis. We have demonstrated that HO-1 is present in mouse erythroid cells (Ter119+ and fetal liver [FL] cells), and show that its expression is enhanced during erythroid differentiation. Interestingly, we have also observed that HO-1 overexpression in murine erythroleukemia (MEL) cells impairs and that its absence, in FL obtained from HO-1−/− mice, enhances hemoglobinization, respectively. Our results indicate that an appropriate level of HO-1 is essential for efficient erythropoiesis.
Material and methods
Animals
FVB.129S2(B6)-Hmox1tm1Poss/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in our animal facility. All animal studies were approved by the McGill Animal Care Committee. Pregnant animals were euthanized on embryonic day 12.5 post coitum and the embryos were genotyped to identify those that were HO-1−/−. Because there is no truly erythroid cell line available, we used primary erythroid cells from control and HO-1−/− mice whenever possible. Livers from appropriate animals were processed as described in the following section. For reticulocytes studies, wild-type (HO-1+/+) and HO-1 knockout (HO-1−/−) mice were injected for 3 days with phenylhydrazine (25 mg/kg). After 2 days of rest, the mice were euthanized and the blood was collected by cardiac puncture. Thiazole Orange staining was performed to determine the percentage of reticulocytes in both HO-1+/+ and HO-1−/− samples.11 The percentage of reticulocytes determined by thiazole orange staining was used to normalize the results.
Cell culture and chemicals
In experiments in which primary cells would not suffice, we settled for using MEL cells. MEL cells were cultured as described in the supplemental Methods on the Blood Web site. All of the experiments were conducted with both uninduced MEL cells (0 hours) as well as cells treated with 1.8% dimethylsulfoxide (DMSO) to induce differentiation and hemoglobinization.12 Primary erythroid cells were cultured as described.13 Briefly, cells were grown from FLs that were obtained from embryonic day 12.5 embryos of wild-type and HO-1 knockout (FVB.129[B6] background) mice. Primary FL cells were kept either in an undifferentiated state (0 hours) or induced to terminal differentiation (24, 48, or 72 hours). The medium composition to maintain the cells in such states is described in further detail in the supplemental Methods. Where indicated, cells were treated with 100 µM of the HO-1 inhibitor, tin-protoporphyrin IX (SnPP) (Frontier Scientific, Logan, UT), and 0.4 mM of heme synthesis inhibitor, succinyl acetone (SA) (Sigma, St Louis, MO).
MEL cells’ stable transfection
We generated MEL cells that overexpress HO-1 as described in supplemental Methods. MEL cell clones showing highest levels of HO-1 expression (MELHO-1) were used in the experiments. MEL cell clones carrying only the plasmid, without HO-1 complementary DNA insert, were used as control.
Cell membrane TfR detection
Nondifferentiated and differentiated cells were washed twice with phosphate-buffered saline/2% fetal calf serum and stained with fluorescence-labeled antibodies against transferrin receptor (TfR) (CD71- fluorescein isothiocyanate; Pharmingen, San Diego, CA). Surface marker expression was analyzed by flow cytometry (FACSCalibur; BD Biosciences, Mississauga, Canada) as shown in supplemental Figure 1. Geometric means were used to quantify the changes in TfR expression in the different conditions tested.
Ter119− and Ter119+ cell sorting
Femora and humeri from wild-type mice were collected and their bone marrows extracted. Bone marrow cells were processed following the protocol described in supplemental Methods. Ter119− and Ter119+ cells were sorted by flow cytometry (FACSVantage SE; BD Biosciences).
Heme and hemoglobin assay
Cellular heme content was assayed as described previously.14 The experiment results were normalized by protein concentration. Hemoglobin assay was performed as previously.15,16 The quantification results were normalized by cell number and cell volume. Both protocols are described in detail in supplemental Methods.
Statistical analysis
Statistical analysis was done using the software SPSS v15.0 (IBM Software, Markham, Canada). Data were evaluated applying analysis of variance and Student t tests. Error bars of graphs represent the standard deviation of 3 independent experiments (n = 3).
Results
HO-1 mRNA and protein are highly expressed in the bone marrow Ter119+ cells
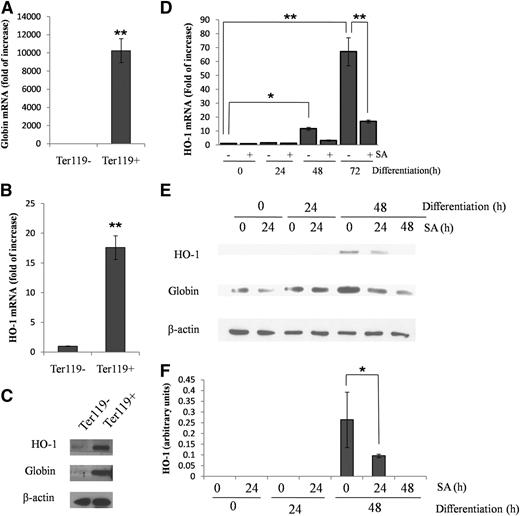
We used the erythroid marker Ter11917 to isolate erythroid cells from mouse bone marrow using a fluorescence-activated cell sorter. As expected, Ter119+ cells showed a high expression of globin messenger RNA (mRNA) and protein, indicating their erythroid origin (Figure 1A,C). We also found that HO-1 mRNA (Figure 1B) and protein (Figure 1C) expressions were increased 20-fold and 2.5-fold, respectively, in Ter119+ cells (representing all cells of erythroid lineage) as compared with Ter119− cells (representing the bone marrow–derived, nonerythroid cells).
HO-1 and globin expression is increased in Ter119+ cells from mouse bone marrow and during the differentiation of FL cells. (A-B) Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) of mouse bone marrow cells, sorted by their expression of Ter119, showing globin and HO-1 mRNA. (C) Western blot of HO-1 and globin protein of Ter119+/− cells. (D) qRT-PCR showing HO-1 mRNA levels in FL cells in a noninduced (0 hours) state or after their differentiation at the indicated time points. SA (0.4 µM), was added 24 hours before harvest. (E) Representative western blot of HO-1 and globin protein of FL cells in a noninduced (0 hours) state or after their differentiation at the indicated time points. (F) Densitometric analysis of HO-1 protein levels normalized to β-actin. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
HO-1 and globin expression is increased in Ter119+ cells from mouse bone marrow and during the differentiation of FL cells. (A-B) Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) of mouse bone marrow cells, sorted by their expression of Ter119, showing globin and HO-1 mRNA. (C) Western blot of HO-1 and globin protein of Ter119+/− cells. (D) qRT-PCR showing HO-1 mRNA levels in FL cells in a noninduced (0 hours) state or after their differentiation at the indicated time points. SA (0.4 µM), was added 24 hours before harvest. (E) Representative western blot of HO-1 and globin protein of FL cells in a noninduced (0 hours) state or after their differentiation at the indicated time points. (F) Densitometric analysis of HO-1 protein levels normalized to β-actin. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
HO-1 mRNA and protein levels increase during erythroid differentiation of FL cells
Using primary erythroid cells isolated from mouse FL cells at 12.5 days, we evaluated HO-1 mRNA levels during erythropoietin (EPO)-induced erythroid differentiation. As shown in Figure 1D, HO-1 mRNA levels significantly increase during 48 and 72 hours of differentiation. Hence, we examined whether heme, whose synthesis is well known to increase throughout erythroid differentiation,1 could be involved in the enhancement of expression of HO-1 in the differentiating FL cells. We demonstrated (Figure 1D) that SA, a specific inhibitor18 of 5-aminolevulinic acid dehydratase (the second enzyme in heme biosynthesis), added 24 hours before cell harvest, blocked the increase in HO-1 mRNA expression at 48 and 72 hours. Moreover, the expression of HO-1 protein, which became detectable by western blot analysis at 48 hours of differentiation, was suppressed by SA (Figure 1E-F). Thus, as in virtually all cell types,19 heme induces HO-1 expression in erythroid cells.
HO-1 overexpression impairs globin expression and activates heme-regulated eIF2α kinase in differentiating MEL cells
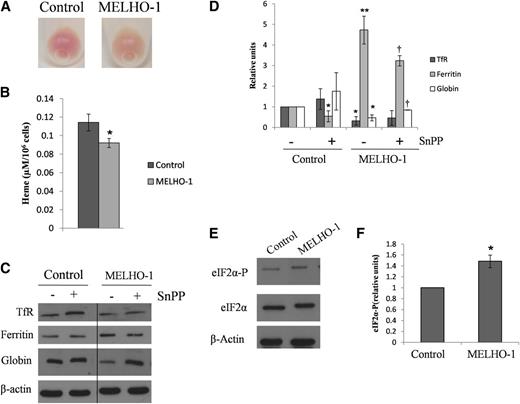
We were surprised that the upregulation of HO-1 expression observed in this experiment (Figure 1D-F) did not interfere with hemoglobinization. To further examine this issue, we generated a MEL cell line stably overexpressing HO-1 (see “Material and methods”). As shown in supplemental Figure 2, MELHO-1 cells express 14-fold higher levels of HO-1 mRNA (supplemental Figure 2A) and 10-fold more HO-1 protein (supplemental Figure 2B) as compared with controls. After 72 hours’ incubation with DMSO, MELHO-1 cell pellets exhibited a lower degree of redness (Figure 2A). Moreover, the HO-1 overexpression resulted in a significant decrease in heme levels after 72 hours of differentiation (Figure 2B). In agreement with these observations, we found that MELHO-1 cells also contain lower levels of globin after 48 hours of differentiation (Figure 2C-D). Interestingly, MELHO-1 ferritin levels increased after 48 hours of differentiation (Figure 2C-D). This increase was partially blocked (Figure 2C-D) by SnPP, an HO-1–specific inhibitor.20
HO-1 overexpression leads to decreases in heme and globin levels and increases ferritin and eIF2α-P protein levels in MEL cells after their differentiation. MEL cells were stably transfected with HO-1 and incubated with DMSO for 72 hours. (A) After DMSO-induced differentiation, MELHO-1 cells exhibited a lower degree of redness than control cells. (B) After 72 hours of incubation with DMSO, MEL cells were harvested and washed, and their heme content was measured. (C) Representative western blot showing TfR, ferritin, and globin protein levels after 48 hours of incubation with DMSO; these cells were treated with and without SnPP (an inhibitor of HO activity). A vertical line has been inserted to indicate a repositioned gel lane. (D) Densitometric analysis of TfR, ferritin, and globin protein levels normalized to β-actin. *,**Statistical significance when compared with control cells without SnPP. †Statistical significance when compared with MELHO-1 cells without SnPP. (E) Representative western blot showing eIF2α-P protein levels in control and MELHO-1 cells after 48 hours of incubation with DMSO. (F) Densitometric analysis of eIF2α-P protein levels normalized to eIF2α-total and β-actin. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *,†P < .05; **P < .001.
HO-1 overexpression leads to decreases in heme and globin levels and increases ferritin and eIF2α-P protein levels in MEL cells after their differentiation. MEL cells were stably transfected with HO-1 and incubated with DMSO for 72 hours. (A) After DMSO-induced differentiation, MELHO-1 cells exhibited a lower degree of redness than control cells. (B) After 72 hours of incubation with DMSO, MEL cells were harvested and washed, and their heme content was measured. (C) Representative western blot showing TfR, ferritin, and globin protein levels after 48 hours of incubation with DMSO; these cells were treated with and without SnPP (an inhibitor of HO activity). A vertical line has been inserted to indicate a repositioned gel lane. (D) Densitometric analysis of TfR, ferritin, and globin protein levels normalized to β-actin. *,**Statistical significance when compared with control cells without SnPP. †Statistical significance when compared with MELHO-1 cells without SnPP. (E) Representative western blot showing eIF2α-P protein levels in control and MELHO-1 cells after 48 hours of incubation with DMSO. (F) Densitometric analysis of eIF2α-P protein levels normalized to eIF2α-total and β-actin. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *,†P < .05; **P < .001.
In erythroid precursors, heme acts as a translational regulator by modulating the activity of the heme-regulated eIF2α kinase, HRI.21 During heme deficiency, HRI is active, which in turn leads to the inhibition of globin translation. We demonstrated that after 48 hours of stimulation of erythroid differentiation (incubation with DMSO), MELHO-1 cells contained increased levels of eIF2α-P compared with controls (Figure 2E-F). This indicates that HO-1 overexpression decreases the regulatory heme pool, which subsequently leads to HRI activation. Moreover, the observed decrease of globin protein levels in differentiated MELHO-1 cells (Figure 2C-D) further supports the conclusion that activated HRI inhibited globin translation.
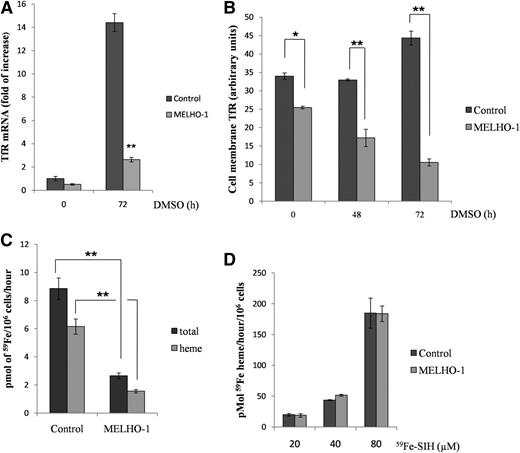
HO-1 overexpression decreases TfR levels and iron uptake in DMSO-treated MEL cells
The rate of iron acquisition from transferrin limits and therefore controls the rate of heme synthesis in erythroid cells.1 The expression of TfRs, the gatekeepers for physiological iron acquisition, is tightly regulated1 during erythroid differentiation. After 72 hours of DMSO-mediated differentiation, TfR mRNA expression was decreased by 5.4-fold in MELHO-1 cells compared with control cells (Figure 3A). Additionally, TfR protein levels were also reduced after 48 hours of differentiation compared with controls (Figure 2C-D). We also found a significant reduction of TfR on MELHO-1 cell membranes compared with control cells at 0 hours as well as 48 and 72 hours of differentiation (Figure 3B). The reduction in TfR on the cell membrane significantly decreased the ability of MELHO-1 cells to take up 59Fe from 59Fe-transferrin (Figure 3C) and incorporate 59Fe into heme (Figure 3C).
HO-1 overexpression decreases TfR expression and iron uptake in differentiated MEL cells. (A) qRT-PCR showing TfR mRNA of MEL (control) and MELHO-1 cells before (0 hours) and after (72 hours) induction with DMSO. (B) Flow cytometry analysis showing cell membrane TfR levels of DMSO-treated controls and induced MELHO-1 cells. (C) MEL cells were differentiated for 72 hours and, 3 hours before harvest, incubated with 59Fe-Tf, after which heme and non-heme fractions were isolated (see the “Materials and methods” section). (D) MEL cells were differentiated for 72 hours and 3 hours before harvest; they were then incubated with increasing amounts of 59Fe-SIH. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
HO-1 overexpression decreases TfR expression and iron uptake in differentiated MEL cells. (A) qRT-PCR showing TfR mRNA of MEL (control) and MELHO-1 cells before (0 hours) and after (72 hours) induction with DMSO. (B) Flow cytometry analysis showing cell membrane TfR levels of DMSO-treated controls and induced MELHO-1 cells. (C) MEL cells were differentiated for 72 hours and, 3 hours before harvest, incubated with 59Fe-Tf, after which heme and non-heme fractions were isolated (see the “Materials and methods” section). (D) MEL cells were differentiated for 72 hours and 3 hours before harvest; they were then incubated with increasing amounts of 59Fe-SIH. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
HO-1 could interfere with the formation of heme and; therefore, we tested the integrity of heme biosynthesis by supplying cells with 59Fe-salicylaldehyde isonicotinoyl hydrazone (59Fe-SIH), a membrane-permeable iron chelate that has the ability to supply iron for ferrochelatase22-24 via bypassing the physiological transferrin-TfR pathway. There was no difference in iron incorporation into heme between MELHO-1 and control cells when the physiological iron delivery pathway was bypassed using 59Fe-SIH (Figure 3D). Hence, these data indicate that HO-1 overexpression leads to a diminished iron supply for heme biosynthesis as a result of decreased TfR expression. Because in erythroid cells heme is needed to maintain a high rate of TfR synthesis,25 the hemoglobinization defect in MELHO-1 is likely caused by the reduction in TfR levels. Additionally, we observed a slight decrease in the iron-regulatory protein 1 (IRP1) RNA-binding activity in MELHO-1 with no difference in IRP2 levels when compared with control (supplemental Figure 3). These data suggest only a small contribution, if any, of the iron-responsive element/IRP system to the reduction in TfR levels and the increase in ferritin levels in MELHO-1 cells (Figures 3A-B and 2C-D).
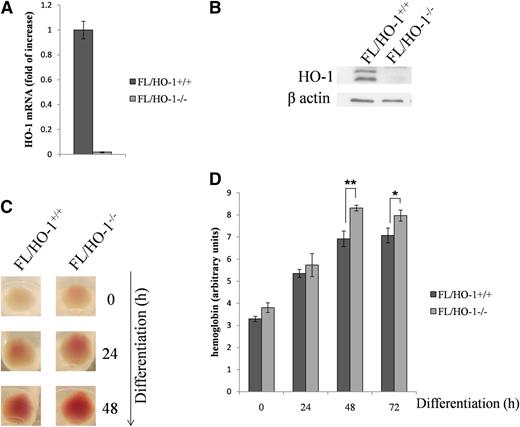
Differentiating FL/HO-1−/− cells contain higher hemoglobin levels
The results of the preceding experiments led us to examine several parameters of erythroid differentiation in FL cells isolated from HO-1 knockout embryos (FL/HO-1−/−). Figure 4A-B show that, in FL/HO-1−/− cells, HO-1 was not detectable at mRNA or protein levels. Interestingly, FL/HO-1−/− cell pellets exhibited a higher degree of redness than wild-type cell pellets at 0, 24, and 48 hours of differentiation (Figure 4C). Indeed, erythroid cells lacking HO-1 have a higher hemoglobin content, as determined by its direct measurement (Figure 4D).
FL HO-1 knockout cells accumulate excess hemoglobin when induced to differentiate with EPO. FL cells were isolated from mouse embryos between days 12.5 and 13.5 and kept either in a nondifferentiated state (0 hours) or induced to differentiate during the indicated intervals. (A-B) FL/HO-1−/− cells do not express HO-1 at mRNA or protein levels as determined by qRT-PCR and western blot analysis, respectively. (C) The color of cell pellets demonstrates that FL/HO−/− cells exhibited a higher degree of redness than wild-type cells at 0 hours as well as at 24 and 48 hours of differentiation. (D) Measurement of hemoglobin levels in FL/HO-1−/− cells before and after differentiation. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
FL HO-1 knockout cells accumulate excess hemoglobin when induced to differentiate with EPO. FL cells were isolated from mouse embryos between days 12.5 and 13.5 and kept either in a nondifferentiated state (0 hours) or induced to differentiate during the indicated intervals. (A-B) FL/HO-1−/− cells do not express HO-1 at mRNA or protein levels as determined by qRT-PCR and western blot analysis, respectively. (C) The color of cell pellets demonstrates that FL/HO−/− cells exhibited a higher degree of redness than wild-type cells at 0 hours as well as at 24 and 48 hours of differentiation. (D) Measurement of hemoglobin levels in FL/HO-1−/− cells before and after differentiation. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
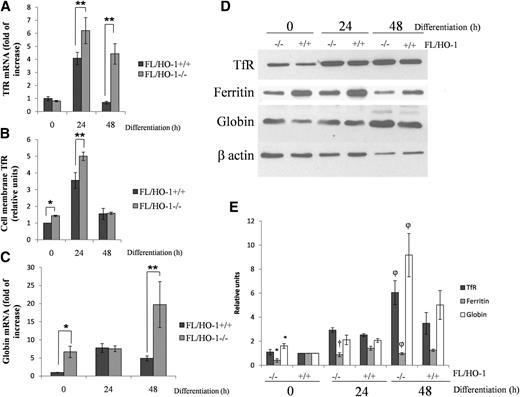
Differentiating FL/HO-1−/− cells show increased TfR and globin expression reduced ferritin expression
As expected, we found that FL/HO-1−/− cells express higher amounts of TfR mRNA, in particular at 24 and 48 hours (Figure 5A), and exhibit a slight increase in TfR protein levels (Figure 5D-E). Flow cytometry analysis revealed an increase in TfR presence on the membrane of FL/HO-1−/− cells, in particular at 0 and 24 hours after EPO treatment (Figure 5B). Figure 5C shows a significant increase in globin mRNA in FL/HO-1−/− cells at 0 and 48 hours. The equal levels of globin mRNA at 24 hours can likely be explained by the presence of overriding, heme-independent transcription factors that drive globin gene expression during the early stages of differentiation in both cell types equally. FL/HO-1−/− cells contain more globin protein (Figure 5D-E), although the differences in its levels are smaller than in the case of globin mRNAs. This probably indicates that translation is limiting in the overall process of globin gene expression. On the other hand, ferritin protein levels were reduced in FL/HO-1−/− cells (Figure 5D-E), suggesting that the increased flux of iron into heme leads to a decrease in ferritin translation. An additional factor that could cause a decrease in ferritin synthesis would be a decrease in “free” iron levels caused by HO-1 deficiency.
FL/HO-1−/− cells express higher levels of TfR and globin and contain less ferritin after EPO-induced differentiation. (A) qRT-PCR showing TfR mRNA levels in wild-type FL and FL/HO-1−/− cells. (B) Flow cytometry analysis showing cell membrane TfR levels of wild-type FL and FL/HO-1−/− cells at 0, 24, and 48 hours (h) of EPO-induced differentiation. (C) qRT-PCR showing globin mRNA levels in wild-type FL and FL/HO-1−/− at 0, 24, and 48 hours of EPO-induced differentiation. (D) Representative western blot showing TfR, ferritin, and globin protein levels of wild-type FL and FL/HO-1−/− cells at 0, 24, and 48 hours of EPO-induced differentiation. (E) Densitometric analysis of TfR, ferritin, and globin protein levels normalized to β-actin. *Statistical significance (P < .05) when compared with FL/HO-1+/+ cells at the time point 0 hours. †Statistical significance (P < .05) when compared with FL/HO-1+/+ cells at time point 24 hours. φStatistical significance (P < .05) when compared with FL/HO-1+/+ cells at time point 48 hours. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). **P < .001.
FL/HO-1−/− cells express higher levels of TfR and globin and contain less ferritin after EPO-induced differentiation. (A) qRT-PCR showing TfR mRNA levels in wild-type FL and FL/HO-1−/− cells. (B) Flow cytometry analysis showing cell membrane TfR levels of wild-type FL and FL/HO-1−/− cells at 0, 24, and 48 hours (h) of EPO-induced differentiation. (C) qRT-PCR showing globin mRNA levels in wild-type FL and FL/HO-1−/− at 0, 24, and 48 hours of EPO-induced differentiation. (D) Representative western blot showing TfR, ferritin, and globin protein levels of wild-type FL and FL/HO-1−/− cells at 0, 24, and 48 hours of EPO-induced differentiation. (E) Densitometric analysis of TfR, ferritin, and globin protein levels normalized to β-actin. *Statistical significance (P < .05) when compared with FL/HO-1+/+ cells at the time point 0 hours. †Statistical significance (P < .05) when compared with FL/HO-1+/+ cells at time point 24 hours. φStatistical significance (P < .05) when compared with FL/HO-1+/+ cells at time point 48 hours. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). **P < .001.
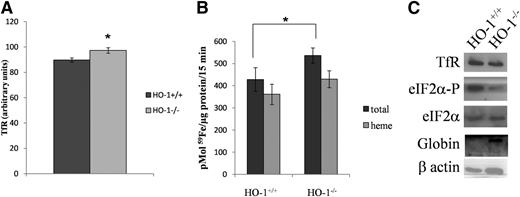
HO-1−/− reticulocytes display increased TfR levels and iron uptake and contain lower levels of eIF2α phosphorylation
Reticulocytes were isolated from both HO-1+/+ and HO-1−/− mice injected with phenylhydrazine. When analyzed by flow cytometry, HO-1−/− reticulocytes showed a significant increase in the levels of TfR present on the cell membrane (Figure 6A). Moreover, HO-1−/− reticulocytes took up significantly more 59Fe-Tf compared with HO-1+/+ reticulocytes after 15 minutes of incubation (Figure 6B). Additionally, eIF2α-P levels were decreased in HO-1−/− reticulocytes (Figure 6C), suggesting an increased heme level and consequent reduction in HRI activity. In agreement with that, globin protein levels were higher in HO-1−/− reticulocytes compared with HO-1+/+ (Figure 6C).
HO-1−/− reticulocytes express more TfR at the cell membrane, have higher iron uptake, and exhibit decreased levels of phosphorylated eIF2α. Wild-type (HO-1+/+) and HO-1 knockout (HO-1−/−) mice were injected with phenylhydrazine (25 mg/kg) for 3 consecutive days. After 2 days of rest, reticulocytes from HO-1+/+ and HO-1−/− mice were collected. (A) Flow cytometry analysis showing cell-membrane TfR levels of reticulocytes HO-1+/+ and HO-1−/− reticulocytes. (B) HO-1+/+ and HO-1−/− reticulocytes were incubated with 59Fe-Tf for 15 minutes, after which heme and non-heme fractions were isolated. (C) Representative western blot showing TfR, eIF2α-P, and globin protein levels of HO-1+/+ and HO-1−/− reticulocytes. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
HO-1−/− reticulocytes express more TfR at the cell membrane, have higher iron uptake, and exhibit decreased levels of phosphorylated eIF2α. Wild-type (HO-1+/+) and HO-1 knockout (HO-1−/−) mice were injected with phenylhydrazine (25 mg/kg) for 3 consecutive days. After 2 days of rest, reticulocytes from HO-1+/+ and HO-1−/− mice were collected. (A) Flow cytometry analysis showing cell-membrane TfR levels of reticulocytes HO-1+/+ and HO-1−/− reticulocytes. (B) HO-1+/+ and HO-1−/− reticulocytes were incubated with 59Fe-Tf for 15 minutes, after which heme and non-heme fractions were isolated. (C) Representative western blot showing TfR, eIF2α-P, and globin protein levels of HO-1+/+ and HO-1−/− reticulocytes. Error bars of graphs represent standard deviation of 3 independent experiments (n = 3). *P < .05; **P < .001.
Discussion
HO-1 has been extensively studied in hepatocytes and other nonerythroid cells.4,6,26 In contrast, the literature on HO-1 in erythroid cells is extremely scarce. It is quite surprising that there is so little known about the role of the enzyme that catabolizes heme (HO-1) in erythroid cells that make, by far, the most heme in mammals. We were able to identify only 2 rather noninstructive studies that examined HO-1 mRNA expression in erythroid cells.27,28 Fujita and Sassa observed a decrease in HO-1 mRNA levels in the first 12 hours of differentiation, at which point hemoglobin synthesis barely commences. In a recently published study, Alves et al were unable to detect HO-1 in K562 cells. However, there K562 cells were ill-defined cells derived from a patient with chronic myelogenous leukemia, exhibiting only a low expression of embryonic and fetal types of hemoglobin.29 Their report also claims an absence of HO-1 expression in bone marrow “erythroid precursors” that were isolated using cell-surface markers, which are not appropriate for detecting hemoglobinized cells. Additionally, bone marrow used in their study was derived from patients with hematological malignancies. In this context, a recent study by Contag and coworkers30 reported disturbed erythropoiesis in mouse spleens after the transplantation of bone marrow cells isolated from HO-1+/− mice. It is likely that that decreased macrophage HO-1 will diminish heme iron recycling from senescent erythrocytes and cause abnormal erythropoiesis in transplanted mice. Moreover, these authors did not investigate HO-1 expression in erythroblasts of normal or transplanted mice. Because this study did not differentiate between erythroid and macrophage HO-1 function, it is not possible to relate the findings of Contag et al to our current report.
In the present work, we have demonstrated that HO-1 is present in erythroid cells and that its expression is upregulated during erythroid differentiation. When overexpressed in MEL cells, HO-1 impairs the hemoglobin synthesis pathway by inhibiting TfR expression and consequently iron uptake, heme synthesis, and globin expression. The key element in this chain of events is the TfR, whose expression in erythroid cells requires appropriate levels of heme for its transcription and likely also translation25 by modulating HRI activity. On the other hand, HO-1 absence leads to an enhancement of hemoglobin synthesis that is likely caused by increased TfR transcription and translation and, consequently, iron uptake.
Ter119, a molecule associated with glycophorin A, is expressed on erythroid cells at the differentiation stages from early proerythroblast to mature erythrocytes.17 We demonstrated that the expression of both HO-1 mRNA (Figure 1B) and protein (Figure 1C) was increased 20-fold and 2.5-fold, respectively, in Ter119+ cells compared with Ter119− cells. We have also found that HO-1 increases after 48 and 72 hours of differentiation of FL cells (Figure 1D-F) and that this upregulation can be dramatically inhibited by the heme synthesis inhibitor SA (Figure 1D-F). This finding strongly suggests that the enhancement of HO-1 levels in FL cells is mediated by heme, a well-known inducer of this enzyme in numerous cell types.5,26,31 This is the first report showing that this mechanism operates in erythroid cells as well. Heme is not only the essential component of hemoglobin, it is also a key regulatory molecule involved in the erythroid differentiation process. Considering these facts, it is surprising that erythroid cells not only contain, but even upregulate, HO-1 during their differentiation. Thus, HO-1 appears to be part and parcel of the physiological erythroid differentiation program, but does not seem to, under physiological conditions, catabolize heme associated with hemoglobin.
The increase in ferritin levels in MELHO-1 cells (Figure 2C-D) is likely caused by a more efficient translation of this protein resulting from iron liberated from heme as an outcome of HO-1 overexpression. This conjecture was confirmed by our finding that the HO-1 inhibitor, SnPP, decreased ferritin levels in MELHO-1 cells (Figure 2C-D). Intriguingly, SnPP decreased ferritin levels also in control of MEL cells (Figure 2C-D), suggesting that both the synthesis of heme and its catabolism are involved in maintaining appropriate levels of heme in erythroid cells. It is tempting to speculate that, in control cells, HO-1 regulates the level of “uncommitted” heme pool, whereas unphysiologically high levels of HO-1 can also attack heme associated with hemoglobin or, at least, earmarked for globin binding. This hypothesis is supported by our finding that hemoglobin and heme levels decrease in MELHO-1 cells (Figure 2A-B).
We deemed it important to also investigate iron metabolism indices and some aspects of erythroid differentiation in erythroid cells lacking HO-1. We demonstrated that FL cells, obtained from FL/HO-1−/− mice, accumulated more hemoglobin (Figure 4C-D) when induced to differentiate with EPO. Moreover, after 24 and 48 hours of differentiation, FL/HO-1−/− had significantly increased TfR mRNA (Figure 5A) and protein (Figure 5D-E) levels; the presence of TfR at the cell membrane increased at 0 and 24 hours of differentiation (Figure 5B). The likely explanation for the enhanced expression of FL/HO-1−/− cells’ TfR is their heme-mediated increase at both transcriptional and translational levels (reviewed in Ponka and Lok 25 ). There is a tendency for increased uptake of iron and its incorporation into heme in FL/HO-1−/− cells (supplemental Figure 4), but this difference is statistically significant only at 0 hours. At 0 and 48 hours of differentiation, globin mRNA expression was also significantly higher in FL/HO-1−/− cells (Figure 5C); this increase was associated with enlarged globin protein levels (Figure 5D-E).
These results suggest that an expansion of an “uncommitted” or “regulatory” heme pool leads to an increase in TfR levels (Figure 5A-B,D-E) and consequently enhanced globin expression (Figure 5C-E). Heme can be expected not only to promote the efficiency of globin translation21,32 but also its transcription.33,34 Similarly, TfR levels and iron uptake were increased in HO-1 knockout reticulocytes (Figure 6A-B). We also observed a trend of increased iron incorporation into heme in HO-1 knockout reticulocytes compared with wild-type reticulocytes (Figure 6B). Additionally, eIF2α-P levels were reduced in HO-1 knockout reticulocytes (Figure 6C). Therefore, the effects of HO-1 deficiency on erythroid iron metabolism and heme synthesis are preserved up to the very late stages of the terminal erythroid differentiation.
Compared with control cells, ferritin protein expression is dramatically reduced in both nondifferentiated and differentiated FL/HO-1−/− cells (Figure 5D-E). This finding strongly suggests that in the absence of HO-1, there is a reduction in the cellular labile iron pool, which is highly likely as a result of increased heme stability.
HO-1–deficient mice developed anemia associated with abnormally low serum iron levels.35 HO-1 deficiency in humans appears as an extremely rare condition, with only 2 live births reported.36,37 Both patients presented severe anemia.36,37 Hence, our results showing that FL/HO-1−/− cells have their hemoglobin synthesis pathway induced (Figures 4C-D and 5A-E) appear to be contradictory to the previous reports.35-37 However, both mice and human HO-1–deficient phenotypes derive from the essential role of this enzyme in the recycling of hemoglobin iron from senescent erythrocytes by reticuloendothelial macrophages.35,38 Macrophages recycle approximately 25 mg of iron daily, most of which is then transported to the bone marrow for hemoglobin synthesis in developing erythroid cells.39 Hence, it is not appropriate to compare the phenotype occurring in humans and mice lacking functional HO-1 with our results conducted on FL/HO-1−/− cells.
The next question is whether developing RBCs could benefit from having a higher than physiological rate of hemoglobin synthesis; we surmise that they would not. First, hyperchromia of erythrocytes would predictably lead to abnormal membrane deformability.40 Second, it was shown that there is a critical mean corpuscular hemoglobin concentration, approximately 22 g/dL, above which DNA replication is not possible.41 This means that the number of cell divisions of erythroblasts with an artificially enhanced rate of hemoglobinization would be lower; this could disturb erythropoiesis. We have recently embarked on the generation of conditional erythroid-specific Hmox1−/− mice with the aim of further assessing the role of HO-1 in erythropoiesis.
The results reported here have important ramifications for our understanding of erythroid differentiation, both in normal and some pathological conditions. First, it has been proposed that most mammalian cells contain a “free” or “uncommitted” heme pool, serving both precursor and regulatory functions.1 This study strengthens the idea that such a pool is also present in erythroid cells42,43 by demonstrating that the inhibition of HO-1 activity decreases ferritin levels in MEL cells (Figure 2C-D and previous discussion). It has been estimated that the concentrations of “free” heme in young rat reticulocytes are 10 μM or lower.43 As mentioned, the “regulatory” pool of heme is essential for synthesis of globin chains at both the transcriptional and translational levels. Additionally, in erythroid cells, heme serves as a positive feedback regulator that maintains high TfR levels.25
Second, the aberrant expression of HO-1 may play a role in the pathophysiology of thalassemia. In the normal assembly of adult hemoglobin (α2β2), the synthesis of globin chains is very tightly coordinated. However, in β-thalassemia, production of β-globin decreases and excess α-globin accumulates; in α-thalassemia, this process occurs in reverse.44,45 Unpaired globin chains that accumulate in both types of thalassemic erythroblasts have heme molecules attached to them. α-subunits, which accumulate in β-thalassemia, are highly unstable and susceptible to autoxidation, likely liberating the heme that would be expected to induce HO-1. Oxidative stress is a key pathogenic factor involved in damaging β-thalassemic erythroblasts,46 which also exhibit a significantly higher level of apoptosis.47 Indeed, we found that MELHO-1 cells generate significantly higher levels of reactive oxygen species than control cells (supplemental Figure 5A) and display elevated apoptosis (supplemental Figure 5B), suggesting that elevated levels of HO-1 could play a role in the pathophysiology of β-thalassemic erythroblasts. Additionally, our finding of elevated apoptosis in MELHO-1 cells is congruent with an earlier report that the inhibition of heme synthesis induces apoptosis in human erythroid progenitor cells.48 Also, membranes of β-thalassemic erythrocytes,49,50 and likely also erythroblasts, contain iron—a substrate for a Fenton reaction—that can be derived from heme because of the enhanced activity of HO-1.
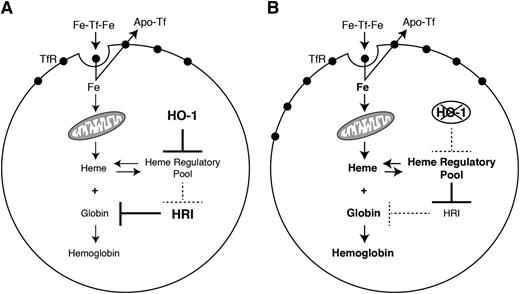
In conclusion, our results bring to light the importance of HO-1 expression for erythroid development and expand our knowledge about the fine regulation of hemoglobin synthesis in erythroid cells. Our results also indicate that HO-1 plays an important role as a coregulator of the erythroid differentiation process. Moreover, HO-1 expression must be tightly regulated during RBC development, because imbalance of its expression may lead to serious consequences for the hemoglobin biosynthesis pathway (Figure 7A-B).
HO-1 controls heme regulatory pool in erythroid cells. (A) When overexpressed, HO-1 causes depletion of heme in the heme regulatory pool. This leads to a secondary decrease in TfR expression and iron uptake by the cell. Moreover, reduction in heme levels results in increased HRI activity that, in turn, represses globin translation. (B) On the other hand, when HO-1 is absent, an expansion of the heme regulatory pool results in a mainly transcriptional activation of TfR expression and an enhanced iron uptake by the cell. The increase in the heme levels also represses HRI activity, leading to augmented globin translation. Under physiological conditions, appropriate levels of HO-1 guarantee optimal hemoglobinization rates.
HO-1 controls heme regulatory pool in erythroid cells. (A) When overexpressed, HO-1 causes depletion of heme in the heme regulatory pool. This leads to a secondary decrease in TfR expression and iron uptake by the cell. Moreover, reduction in heme levels results in increased HRI activity that, in turn, represses globin translation. (B) On the other hand, when HO-1 is absent, an expansion of the heme regulatory pool results in a mainly transcriptional activation of TfR expression and an enhanced iron uptake by the cell. The increase in the heme levels also represses HRI activity, leading to augmented globin translation. Under physiological conditions, appropriate levels of HO-1 guarantee optimal hemoglobinization rates.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Marc Mikhael for his valuable suggestions and advice. We are also grateful to 4 anonymous reviewers for their valuable comments.
This work was supported in part by the Canadian Institutes for Health Research (D.G.-S., P.P., A.S., M.M.J.), the Czech Science Foundation (P305/11/1745) (M.H.), and by the Education for Competitiveness Operational Programme of the Czech Ministry of Education, Youth and Sports (CZ.1.07/2.3.00/20.0164).
Authorship
Contribution: D.G.-S. and M.S. performed research, analyzed data, and wrote the manuscript; M.H. and M.M.J. performed research and analyzed data; J.A.B.C. analyzed data; A.S. analyzed data and wrote the manuscript; and P.P. conceived the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prem Ponka, Lady Davis Institute for Medical Research, Jewish General Hospital, and the Department of Physiology, McGill University, 3755 Chemin Cote-Ste-Catherine, Montréal, QC, H3T 1E2, Canada; e-mail: prem.ponka@mcgill.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal