Key Points
Improvements in splenomegaly and symptoms in patients receiving ruxolitinib occurred regardless of the mutations that were present.
Ruxolitinib relieved the negative impact of prognostically detrimental mutations in myelofibrosis patients from the COMFORT-II study.
Abstract
The JAK1/JAK2 inhibitor ruxolitinib produced significant reductions in splenomegaly and symptomatic burden and improved survival in patients with myelofibrosis (MF), irrespective of their JAK2 mutation status, in 2 phase III studies against placebo (COMFORT-I) and best available therapy (COMFORT-II). We performed a comprehensive mutation analysis to evaluate the impact of 14 MF-associated mutations on clinical outcomes in 166 patients included in COMFORT-II. We found that responses in splenomegaly and symptoms, as well as the risk of developing ruxolitinib-associated anemia and thrombocytopenia, occurred at similar frequencies across different mutation profiles. Ruxolitinib improved survival independent of mutation profile and reduced the risk of death in patients harboring a set of prognostically detrimental mutations (ASXL1, EZH2, SRSF2, IDH1/2) with an hazard ratio of 0.57 (95% confidence interval: 0.30-1.08) vs best available therapy. These data indicate that clinical efficacy and survival improvement may occur across different molecular subsets of patients with MF treated with ruxolitinib.
Introduction
Myelofibrosis (MF),1 either primary (PMF) or evolved from polycythemia vera (PPV-MF) or essential thrombocythemia (PET-MF), is characterized by reduced survival (OS) and disabling symptoms.2-4 Conventional treatment is ineffective; stem cell transplantation is reserved for younger patients with dismal outcome,5 as those included in intermediate-2 (projected survival, 4-5 years) or high-risk (2-2.5 years) category of IPSS or DIPPS-plus prognostic scores.6-8
Activating mutations in JAK2, MPL, and calreticulin are found in ≅60%, 8%, and 15% to 25% of PMF patients, respectively.9-12 Overactivated JAK/STAT signaling is a hallmark of MF independent of mutations.13 Additional mutations14 in genes affecting the epigenetic machinery (TET2, ASXL1, DNMT3A, IDH1, and 2)15,16 and the spliceosome (SRSF2, SF3B1, U2AF)17 are found in 5% to 25% and may have prognostic relevance. We reported that PMF patients harboring mutation in any 1 of EZH2, ASXL1, IDH1/2, and SRSF2 constituted an IPSS- and DIPSS-plus score–independent “high molecular risk” (HMR) category associated with shorter OS and greater risk of leukemia compared with patients lacking the above mutations (“low molecular risk”; LMR).18
Treatment with ruxolitinib, a JAK1 and JAK2 inhibitor, was associated with rapid and durable reductions in splenomegaly and improvement of disease-related symptoms in MF patients compared with placebo (COMFORT-I study)19 or best available therapy (BAT; COMFORT-II).20 A survival benefit in ruxolitinib-treated patients was also demonstrated.21,22 The aim of this study was to analyze the impact of MF-associated mutations on clinical response, hematological toxicity, and survival in a subset of patients receiving ruxolitinib in COMFORT-II.
Study design
In COMFORT-II, MF patients were randomized to receive ruxolitinib (n = 146) or BAT (n = 73). After institutional review board approval and informed written consent, samples were available in 166 cases (ruxolitinib, n = 120; BAT, n = 46; 76% of total; details in supplemental Material, available on the Blood Web site). This study was conducted in accordance with the Declaration of Helsinki. Mutations in 14 genes (JAK2, MPL, EZH2, ASXL1, TET2, IDH1, IDH2, CBL, SRSF2, SOCS1, SOCS2, SOCS3, SH2B3, KRAS) were genotyped in blood DNA at study entry by deep sequencing with Ion Torrent-PGM or Roche-454 platform (described in supplemental Material).
The following end points were retrospectively correlated with mutation status: spleen response, symptomatic improvement, development of ruxolitinib-induced anemia and thrombocytopenia, and survival. Definitions are provided in supplemental Material. We did not include leukemia transformation, because the low number of events (5 in ruxolitinib [3.4%] and 4 in BAT [5.5%] arm) prevented statistical analysis. Relationships between clinical end points and mutations were investigated via descriptive statistics. Survival estimates were obtained with the Kaplan-Meier method; the hazard ratio (HR) was determined using Cox proportional hazards model stratified by baseline IPSS category. Treatment effect and prognostic value of mutations with regard to OS were analyzed using multivariate Cox regression adjusted for IPSS category.
Results and discussion
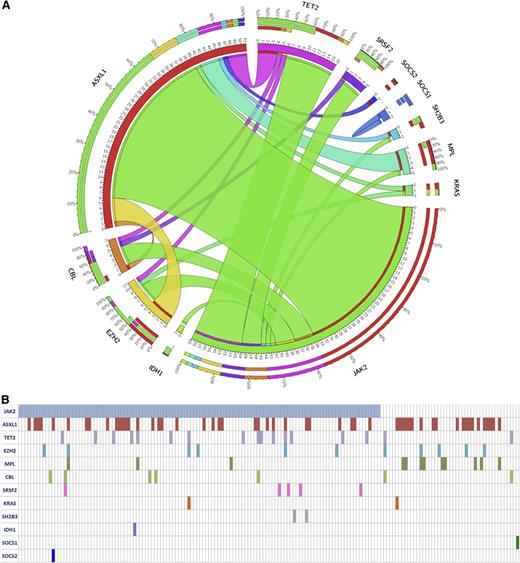
At study entry, patients’ characteristics, according to treatment randomization, were similar regarding MF subtype, hematologic values, spleen volume, symptomatic patients, IPSS risk category, and JAK2V617F mutation, and were comparable to the entire COMFORT-II series (supplemental Table 1). Mutations frequency was: JAK2V617F 75.5%; MPLW515 7.7%; ASXL1 32.5%; TET2 10.7%; EZH2 7.2%; CBL 4.4%; SRSF2 3.0%; SH2B3 1.3%; IDH1 0.7%; IDH2 0.0%; SOCS1 0.7%; SOCS2 0.7%; SOCS3 0.0%; KRAS 1.4% (Figure 1A). These frequencies were comparable in ruxolitinib and BAT arms and were consistent with those previously reported in 171 intermediate-2 and high-risk PMF subjects (supplemental Table 2).18 One hundred fifty patients (90.4%) displayed at least 1 mutation; 57 (34.3%), 5 (3.0%), and 5 (3.0%) patients had 2, 3, and 4 mutations, respectively (Figure 1B). Overall, 46 (38.3%) patients in ruxolitinib and 20 (43.5%) in BAT arm were classified as HMR according to the presence of mutation(s) in any 1 of ASXL1, EZH2, SRSF2, and IDH1-2.
The frequency and the pairwise cooccurrence of mutations in the entire study cohort (n = 166). (A) These are represented by a Circos diagram. Cooccurring mutations are indicated in the clockwise direction; the length of the arc corresponds to the frequency of mutation in the first gene (color coded), and the width of the ribbon corresponds to the frequency of patients who also had a mutation in the second gene. The individual mutated genes for each patients in the whole cohort are indicated in (B).
The frequency and the pairwise cooccurrence of mutations in the entire study cohort (n = 166). (A) These are represented by a Circos diagram. Cooccurring mutations are indicated in the clockwise direction; the length of the arc corresponds to the frequency of mutation in the first gene (color coded), and the width of the ribbon corresponds to the frequency of patients who also had a mutation in the second gene. The individual mutated genes for each patients in the whole cohort are indicated in (B).
In the set of 166 patients, a spleen response was achieved in 31.7% and 35.0% of ruxolitinib arm respectively at 48 and 24 weeks vs 0% and 0% in BAT. Symptomatic improvement, defined from FACT-Lym questionnaire for patients who were symptomatic at baseline (ruxolitinib n = 82, BAT n = 20), was observed in 80.9% and 79.4% in ruxolitinib arm at 48 and 24 weeks compared with 38.8% and 38.8% in BAT. Anemia and thrombocytopenia developed respectively in 72.5% and 35.8% of ruxolitinib and 39.1% and 8.7% of BAT patients. At the median follow-up time of 151 weeks, the mortality rate was 20% (24 of 120) in ruxolitinib and 34.8% (16 of 46) in BAT arm; the median survival was not reached in either. Patients in the ruxolitinib arm had a survival advantage greater than BAT with a relative reduction of risk of death by 47% (HR 0.53; 95% confidence interval (CI), 0.28-0.99). Overall, results in this patient subset were comparable to the entire COMFORT-II series ruling out a selection bias eventually introduced by the availability of DNA samples.
To ascertain correlations between outcomes and mutation pattern, we compared HMR and LMR patients randomized to ruxolitinib. At week 48, a >35% spleen volume reduction was achieved by 26.1% of HMR and 35.0% of LMR patients; at week 24, corresponding figures were 34.8% and 35.0%. The mean spleen volume reduction from baseline at week 48 was −23.5% in HMR vs −30.6% in LMR, and −29.0% vs −29.9% at week 24. No mutation was individually considered correlated with spleen volume reduction. Constitutional symptoms improved in 76.2% (16/21) of HMR vs 83.3% (35/42) of LMR patients at week 48, and at week 24 the proportion was 85.7% (18/21) in HMR vs 76.2% (32/42) in LMR. None of the previously mentioned differences provided a statistically significant result.
We then asked whether a HMR status eventually affected hematologic toxicity associated with ruxolitinib. The percentage of patients developing anemia was 74% and 72% in the HMR and LMR group. Development of anemia was also unrelated to mutations in genes of the JAK2/STAT signaling (JAK2, MPL, SH3B2, CBL, and SOCSs), occurring in 72% of mutated anemic subjects vs 74% of wild type. Ruxolitinib-associated thrombocytopenia occurred at similar rates in HMR (34%) and LMR (36.5%) patients and independently of mutations insisting on JAK2/STAT signaling (35.4% vs 39.1% of wild-type patients).
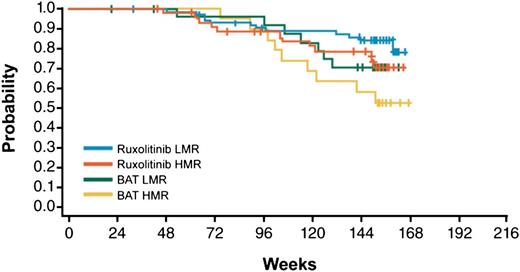
With a median follow-up of 151 weeks, Kaplan-Meier estimates of survival at 144 weeks in patients treated with ruxolitinib were 0.79 for the HMR and 0.85 for the LMR category, compared with 0.58 and 0.71 in BAT arm, therefore indicating a survival benefit of ruxolitinib treatment independent of molecular risk score (Figure 2) and confirming the negative significance of HMR in the control population.18 The survival benefit was confirmed in a multivariate Cox model where the risk of death for HMR patients was reduced by 43% (HR = 0.57, 95% CI: 0.30-1.08) compared with BAT; also, ruxolitinib-treated LMR patients had a reduction in risk compared with HMR by 38% (HR = 0.62, 95% CI: 0.33-1.16). It should be noted, however, that the confidence intervals for hazard ratios include 1, which is likely because of the sample size.
Kaplan-Meier estimate of survival in the study patients’ population stratified by treatment group (BAT vs ruxolitinib) and molecular score (HMR vs LMR).
Kaplan-Meier estimate of survival in the study patients’ population stratified by treatment group (BAT vs ruxolitinib) and molecular score (HMR vs LMR).
In conclusion, we confirmed that previously described mutations comprising a prognostically adverse HMR category in MF patients18 maintained their negative value in this subset of COMFORT-II patients.16 The data presented showed that HMR status did not affect the likelihood of obtaining a >35% spleen volume reduction or symptomatic improvement, nor did it increase the risk of ruxolitinib-associated anemia or thrombocytopenia. Additionally, our findings indicated that ruxolitinib alleviated the negative prognostic impact of mutations and overall suggest that clinical efficacy and survival improvement in MF patients may occur independently of the underlying molecular pattern. There is no mechanistic explanation yet on how these mutations affect survival in MF, and whether ruxolitinib mitigated their significance through anticlonal activity or other mechanisms is the subject of ongoing investigations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a special grant from the Associazione Italiana per la Ricerca sul Cancro—”AIRC 5 per Mille”—to AGIMM, “AIRC-Gruppo Italiano Malattie Mieloproliferative” (#1005) (for a description of the AGIMM project, see www.progettoagimm.it). This work was partially supported by Ministero della Università e Ricerca (MIUR, FIRB project #RBAP11CZLK and PRIN 2010NYKNS7), and was also partially supported by Fondazione Cassa di Risparmio di Carpi (http://www.fondazionecrcarpi.it/) and AIL Modena ONLUS (http://www.ailmodena.org). This work was partially supported by a research grant from Novartis Pharma AG (A.M.V.). The list of COMFORT-II investigators is included in the supplemental Material.
Authorship
Contribution: P.G. and A.M.V. designed the study, analyzed the data, and wrote the manuscript; P.G., F.B., G.R., V.A., L.A., I.B., E. Tenedini, C.M., R.M., and E. Tagliafico performed molecular analysis and analyzed raw sequencing data; P.G., L.P., C.P., R.F., C.H., E.R., M.C., and A.M.V. contributed to clinical patient management and collected samples; V.S. and M.S. contributed to clinical data analysis and manuscript writing; and C.H. and E. Tagliafico contributed to the writing of the manuscript.
Conflict-of-interest disclosure: A.M.V. has served as a member of the advisory board for Novartis and has received a research grant for this study. C.H. has served on advisory boards for Novartis and has received research funding. V.S. and M.S. are employees of Novartis Pharma AG, Basel. The remaining authors declare no competing financial interests.
Correspondence: Alessandro M. Vannucchi, Department of Experimental and Clinical Medicine, University of Florence, Largo Brambilla 3, 50134 Florence, Italy; e-mail: amvannucchi@unifi.it.
References
Author notes
P.G. and F.B. contributed equally to this study.