Key Points
Estrogen receptor β (ERβ) activation inhibits lymphoma growth, vascularization, and dissemination in vivo.
ERβ activation may mechanistically explain differences in gender incidence and prognosis and contribute to new therapies of lymphomas.
Abstract
Most lymphomas show an increased incidence and poorer prognosis in males vs females, suggesting endocrine regulation. We have previously shown that tumor growth in vivo of a murine T-cell–derived lymphoma is repressed following activation of estrogen receptor β (ERβ, ESR2). By using ERβ-deficient mice, we now demonstrate that this inhibition is mediated via a direct effect on the tumor cells and not on the microenvironment. Furthermore, we show that the growth-suppressing effects of ERβ agonist are also valid for human B-cell lymphomas as demonstrated in tumors derived from Granta-519 mantle cell lymphoma (MCL) and Raji Burkitt lymphoma (BL) cells. In Granta-519 MCL tumors, activation of ERβ reduced expression of BAFF and GRB7, 2 important molecules involved in B-cell proliferation and survival. Importantly, activation of ERβ inhibited angiogenesis and lymphangiogenesis, possibly mediated by impaired vascular endothelial growth factor C expression. Furthermore, using disseminating Raji BL cells, we show that ERβ activation reduces dissemination of grafted Raji BL tumors. We also show by immunohistochemistry that ERβ is expressed in primary MCL tissue. These results suggest that targeting ERβ with agonists may be valuable in the treatment of some lymphomas, affecting several aspects of the malignant process, including proliferation, vascularization, and dissemination.
Introduction
Several epidemiological studies show a clear gender difference in most B- and T-cell lymphomas and lymphoid leukemias, with a higher incidence and poorer prognosis in males compared with females.1,2 Furthermore, lymphoid malignancies are likely to be under estrogen influence, because epidemiological data in females show an association between reproductive hormonal factors and oral contraceptives, with a reduced risk for non-Hodgkin lymphomas of up to 50%.3,4 However, lymphomas are not generally considered sex-hormone– or endocrine-regulated cancers, and mechanisms explaining the gender difference in lymphoma incidence and prognosis are lacking. As an experimental support for an estrogen-dependent influence on lymphoma growth in vivo, we have recently shown that when grafting mice with a murine T-cell lymphoma, male mice developed larger tumors compared with female mice, a difference that was abolished following ovariectomy.5
Estrogen signaling is mediated via estrogen receptor α (ERα, ESR1) and β (ERβ, ESR2). ERα and ERβ have been shown to elicit opposite effects on proliferation and apoptosis. Signaling via ERα is known to stimulate proliferation in reproductive organs and tissues.6-8 In contrast, ERβ seems to inhibit proliferation. In experiments using cells expressing ERβ following transfection, a ligand-independent inhibition of cell proliferation was demonstrated,9,10 and studies in ERβ−/− mice have revealed inhibitory effects of ERβ on proliferation and differentiation.11 With regard to apoptosis, proapoptotic effects of ERβ have been demonstrated in prostate and ovarian cancer cells.12,13 In addition, using allograft experiments, we have previously shown that treatment with an ERβ-selective agonist inhibits proliferation and activates apoptosis in a T-cell–derived murine lymphoma that expresses ERβ.5
Previous studies have shown that ERβ is the dominant ER expressed in normal lymphoid cells at the protein level.14 Furthermore, our studies have demonstrated ERβ expression in Burkitt lymphoma (BL) cell lines5 and in B cells from chronic lymphocytic leukemia (CLL) patients,15 which suggests that human B-cell lymphoid malignancies also express ERβ and may be a target for ERβ-agonist treatment.
In this study, we investigated whether ligand-activated ERβ-mediated effects on lymphoma growth inhibition involve effects on cells in the microenvironment. Furthermore, we investigate the impact of ERβ activation on vascularization of lymphoma in vivo. We also identify genes regulated following ERβ activation that are known to influence B-cell proliferation and survival. We focus on human mantle cell lymphoma (MCL), which shows a high ratio of male-to-female incidence.1 Furthermore, because MCL is a lymphoma with a poor prognosis (3-year median survival time),16 new therapeutic targets that could improve disease outcome are needed. Finally, we studied the effect of ERβ-selective agonist treatment on B-cell lymphoma dissemination in a mouse model using disseminating Raji BL cells.17
Methods
Cell lines
Murine mouse T-cell lymphoma line EG7,18 human MCL cell line Granta-519,19 human BL line Raji,17 and human breast cancer MDA-MB-23120 cells were maintained in RPMI 1640 medium (EG7, Granta-519, Raji) or Dulbecco’s modified Eagle’s medium (MDA-MB-231) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 IU penicillin/mL, and 100 µg/mL streptomycin at 37°C in 5% CO2.
Mice
C57Bl/6J mice (8-10 weeks of age) were purchased from Charles River (Lille Skensved, Denmark). Nonobese diabetic severe combined immunodeficiency (NOD-SCID) γ (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ)21 and ERβ−/− (BERKO)22 mice were bred at the Department of Biosciences and Nutrition, Karolinska Institutet, Stockholm, Sweden. Mice were housed in 12-hour light/12-hour darkness cycles at a temperature of 21°C to 22°C and a relative humidity of 50% to 62%, with free access to fresh water and food, at the pathogen-free animal facility of Karolinska University Hospital (Huddinge, Sweden). Animal care was in accordance with the guidelines of Karolinska Institutet.
Tumor grafting, in vivo treatments, and tumor growth measurements
For lymphoma grafting, male mice (8-12 weeks of age) were subcutaneously injected in the right flank with 2.5 × 105 EG7 cells or 15 × 106 Granta-519 lymphoma cells or 5 × 106 Raji lymphoma cells in 100 µL sterile phosphate-buffered saline. In vivo treatments with diarylpropionitrile (DPN), ICI 182.780, KB099520, propyl pyrazole triol (PPT), and estradiol and tumor growth measurements were performed as previously described,5 starting from the day lymphomas began to develop. For short-term treatment experiments, tumors were grown until they reached the size of 500 mm3. The animals were then injected with DPN (12.5 μmol/kg body weight) twice 8 or 24 hours before euthanasia.
For the experiment comparing treated and untreated tumors of the same size, tumor-bearing mice were injected with DPN (12.5 μmol/kg body weight) or vehicle. When tumors in the vehicle-treated group reached the size of 500 mm3, the mice were euthanized, while treatment of the DPN-treated group continued until tumors reached the same size of 500 mm3 before euthanasia. The experiments were repeated 3 times with reproducible results.
Messenger RNA (mRNA) isolation, complementary DNA synthesis, and quantitative real-time polymerase chain reaction (PCR) were performed as described previously,23 and the primers used are given in supplemental Table 1 on the Blood Web site. Primers for the 60S acidic ribosomal, large, protein P0 housekeeping gene RPLP0 (36B4) were used to normalize the results. The specificity of PCR product was examined by dissociation curves and results calculated by the 2−ΔΔCT method.
Immunofluorescence/immunohistochemistry and primary MCL biopsies
Immunofluorescence was performed as described previously.5 Lymph node biopsy specimens were obtained from 4 MCL patients at Karolinska University Hospital. The patients were men 75 to 76 years of age. A t(11;14)(q13;q32) translocation had previously been verified by fluorescence in situ hybridization. Further determination of the lymphomas as MCL was by Cyclin D1 immunohistochemistry. The staining procedure for ERβ and Cyclin D1 in primary MCL samples, normal tonsil, and Granta-519 tumors is detailed in supplemental Methods.
Quantification of LYVE-1 staining was performed by counting the number of positively stained lymphatic vessels and measuring the length and width of positively stained lymphatic vessels from 5 randomly chosen nonoverlapping fields (at original magnification ×200) using ImageJ software. Quantification of vascular endothelial growth factor C (VEGF-C) and Ki67 (MKI67) expression was performed by counting the number of positively stained cells with a distinct visible nuclear staining pattern in 5 randomly chosen nonoverlapping fields (at original magnification ×200) from each tumor.
Antibodies
Antibodies to VEGF-C (H-190) from Santa Cruz Biotechnology (Santa Cruz, CA), ERβ (PPG5/10) and Cyclin D1 (SP4) from Dako (Carpinteria, CA), LYVE-1 (ab33682) from Abcam (Cambridge, MA), and Ki67 (NB110-89717) from Novus Biologicals (Littleton, CO) were used for immunostaining. Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Statistical analysis
Statistical analysis was performed using unpaired Student t tests. Mean values ± standard deviations are presented. P values of <.05 were considered significant.
Ethical aspects
Animal care was in accordance with the guidelines of Karolinska Institutet, and all animal experiments were performed in accordance with ethical committee approvals (Stockholm södra djurförsöksetiska nämnd S218-11, S28-13). Analysis of the MCL samples was done according to ethical approvals (NN-689/03, 2011/1053-3). This study was conducted in accordance with the Declaration of Helsinki.
Results
Inhibition of lymphoma growth by ERβ agonists is mediated via a direct effect on lymphoma cells and not on tumor microenvironment
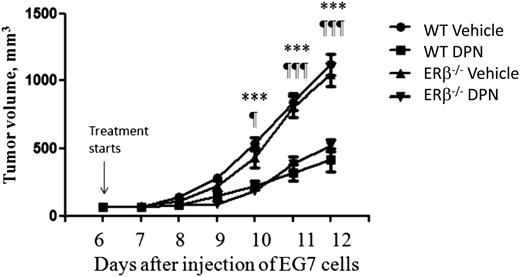
Tumor growth is a complex process involving tumor cells, the host microenvironment, and their interaction.24 This involves, for example, infiltration of immune and endothelial cells and fibroblasts into the tumor. Previously, we have shown that ERβ-selective agonist treatment inhibits murine EG7 T-cell lymphoma growth in vivo.5 Because ERβ is expressed in both EG7 tumor cells and normal host immune and endothelial cells,25 the tumor-inhibiting effect of ERβ agonists may be mediated by direct effects on tumor cells and/or the tumor microenvironment. In order to investigate the relative impact of ERβ agonist on the tumor cells vs the microenvironment, we engrafted wild-type and ERβ−/− (BERKO) mice with ERβ-positive EG7 lymphoma cells and treated them with the ERβ-selective agonist DPN. A significant reduction of lymphoma growth was observed in both wild-type and ERβ−/− mice treated with DPN compared with control groups treated with vehicle (Figure 1). There was no significant difference in response to DPN between wild-type and ERβ−/− mice. These results demonstrate that suppression of lymphoma growth by an ERβ-selective agonist is mediated mainly via direct effects on tumor cells and not on the tumor microenvironment.
Similar effects of ERβ agonist treatment on lymphoma growth in wild-type and ERβ−/−mice. Male C57/Bl6J wild-type and ERβ−/− (BERKO) mice were subcutaneously grafted with 2 × 106 ERβ-positive EG7 lymphoma cells. Starting from day 7 after tumor cell grafting, animals were treated subcutaneously daily with the ERβ-selective agonist DPN (12.5 μmol/kg per day) or vehicle. The groups consisted of 5 vehicle-treated wild-type (●), 6 DPN-treated wild-type (▪), 6 vehicle-treated ERβ−/− (▼), and 5 DPN-treated ERβ−/− (▲) mice. For wild-type mice, vehicle vs DPN: ***P < .001. For ERβ−/− mice, vehicle vs DPN: ¶P < .05 and ¶¶¶P < .001. No significant difference was observed between DPN-treated wild-type and ERβ−/− mice or between wild-type and ERβ−/− vehicle-treated mice. WT, wild-type.
Similar effects of ERβ agonist treatment on lymphoma growth in wild-type and ERβ−/−mice. Male C57/Bl6J wild-type and ERβ−/− (BERKO) mice were subcutaneously grafted with 2 × 106 ERβ-positive EG7 lymphoma cells. Starting from day 7 after tumor cell grafting, animals were treated subcutaneously daily with the ERβ-selective agonist DPN (12.5 μmol/kg per day) or vehicle. The groups consisted of 5 vehicle-treated wild-type (●), 6 DPN-treated wild-type (▪), 6 vehicle-treated ERβ−/− (▼), and 5 DPN-treated ERβ−/− (▲) mice. For wild-type mice, vehicle vs DPN: ***P < .001. For ERβ−/− mice, vehicle vs DPN: ¶P < .05 and ¶¶¶P < .001. No significant difference was observed between DPN-treated wild-type and ERβ−/− mice or between wild-type and ERβ−/− vehicle-treated mice. WT, wild-type.
ERβ-selective agonists inhibit human lymphoma growth in vivo in xenograft experiments
In order to investigate whether the inhibiting effect on lymphoma growth by ERβ agonists could also be seen on human lymphoma subtypes, we analyzed the effects of ERβ agonist on human B-cell lymphomas in xenograft experiments. In humans, lymphomas are overwhelmingly of B-cell origin. We focused our studies on MCL (represented by human Granta-519 lymphoma cells), because this lymphoma subtype is one that shows the highest male-to-female incidence ratio.1 Granta-519 cells express ERβ at the mRNA and protein levels (Figure 2A and supplemental Figure 1a), whereas ERα mRNA expression seems to be absent or very low (Figure 2A). The specificity of the antibody used for ERβ staining was confirmed using ERβ-negative MDA-MB-231 cells, which only showed a clear nuclear staining following transfection with an ERβ expression vector (supplemental Figure 1b-c).
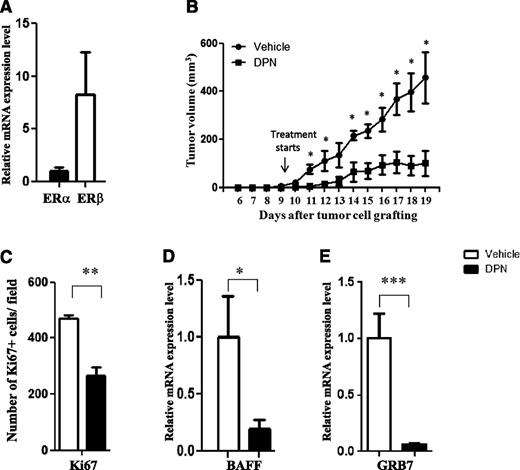
The selective ERβ agonist DPN inhibits growth of Granta-519 MCL tumors in vivo. (A) The expression of ERα and ERβ mRNA in Granta-519 MCL was measured by quantitative real-time PCR. (B) Male NOD-SCID mice were subcutaneously grafted with 15 × 106 Granta-519 MCL cells. Starting from day 9 after tumor cell grafting, animals were treated subcutaneously daily with the ERβ-selective agonist DPN (12.5 μmol/kg per day) (▪) or vehicle (●). Both groups consisted of 5 mice. (C) Ki67 expression in sectioned Granta-519 MCL tumors from mice was significantly lower in tumors from mice treated with DPN than in those from vehicle-treated control mice. Treatment with DPN also inhibited the expression of BAFF (D) and GRB7 (E) in Granta-519 MCL lymphomas. For vehicle vs DPN: *P < .05, **P < .01, and ***P < .001. Data are representative of at least 2 independent experiments.
The selective ERβ agonist DPN inhibits growth of Granta-519 MCL tumors in vivo. (A) The expression of ERα and ERβ mRNA in Granta-519 MCL was measured by quantitative real-time PCR. (B) Male NOD-SCID mice were subcutaneously grafted with 15 × 106 Granta-519 MCL cells. Starting from day 9 after tumor cell grafting, animals were treated subcutaneously daily with the ERβ-selective agonist DPN (12.5 μmol/kg per day) (▪) or vehicle (●). Both groups consisted of 5 mice. (C) Ki67 expression in sectioned Granta-519 MCL tumors from mice was significantly lower in tumors from mice treated with DPN than in those from vehicle-treated control mice. Treatment with DPN also inhibited the expression of BAFF (D) and GRB7 (E) in Granta-519 MCL lymphomas. For vehicle vs DPN: *P < .05, **P < .01, and ***P < .001. Data are representative of at least 2 independent experiments.
To test the antiproliferative effect of ligand-activated ERβ on human MCL in vivo, Granta-519 cells were engrafted subcutaneously to male NOD-SCID mice, followed by daily subcutaneous injections with the selective ERβ agonist DPN or vehicle from the first day a palpable tumor could be detected. Treatment with DPN clearly suppressed lymphoma growth of Granta-519 MCL (Figure 2B). Inhibition of Granta-519 tumor growth was also seen following treatment with another highly selective ERβ agonist, KB099520 (supplemental Figure 2).5,26,27 Furthermore, the tumor-inhibiting effect of DPN was abolished if DPN was coadministered with the ER antagonist ICI 182.780 or impaired when coadministered with estradiol (supplemental Figures 3 and 4a). Note that estradiol is only a weak partial agonist when it comes to inhibition of lymphoma growth in vivo (supplemental Figure 4b),5 suggesting that the tumor-inhibiting effect indeed was ER-mediated. Because the selective ERα agonist PPT had no effect on MCL growth (supplemental Figure 4b), the dose of DPN used does not activate ER,28 and the expression of ERα is low in Granta-519 MCL (Figure 2A), the effect is very likely ERβ-mediated. Furthermore, DPN treatment also suppressed growth of BL tumors in vivo when Raji (see Figure 5B) or Ramos cells (data not shown) were grafted to NOD-SCID mice.
Because the ERβ agonists significantly inhibited lymphoma growth in vivo, we specifically looked for genes related to tumor proliferation that support B-cell survival and growth. Ki67, a common marker of proliferation, was significantly downregulated by DPN in Granta-519 MCL tumors following treatment (Figure 2C). In addition, we found that B-cell activating factor (BAFF, also called TNSF13B) expression was downregulated following DPN treatment of Granta-519 MCL tumors (Figure 2D). Interestingly, and in relation to B-cell lymphomas, BAFF regulates the survival and maturation of B cells.29,30 In addition, it has been shown that BAFF supports CLL B-cell survival.31 We also analyzed the effect of DPN on the expression of growth factor receptor–bound protein 7 (GRB7), because overexpression of GRB7 has been linked to tumor cell proliferation and tumor invasion.32,33 Furthermore, a positive correlation between GRB7 expression and progression of CLL, including increased accumulation of lymphoid cells in the bone marrow, has been reported.34 Here, we show that GRB7 expression was downregulated by DPN treatment (Figure 2E). These results suggest that the antiproliferative effect on MCL in vivo following ERβ agonist treatment may occur by regulating important genes involved in B-cell survival and proliferation.
DPN inhibits angiogenesis and lymphangiogenesis in MCL
Tumor-stimulated angiogenesis and lymphangiogenesis are important factors for tumor growth and dissemination. To investigate whether tumor-stimulated angiogenesis and lymphangiogenesis are affected by selective ERβ agonists, we analyzed by quantitative PCR or immunohistochemistry the expression of vasculogenic growth factors and markers of angiogenesis and lymphangiogenesis in human Granta-519 MCL tumors treated with DPN. With regard to vascularization, vascular endothelial growth factors (VEGFs) have been shown to be key regulators of both angiogenesis and lymphangiogenesis.35 When analyzing the effect of DPN on VEGF expression in human Granta-519 MCL tumors, VEGF-C was found to be the main VEGF significantly repressed by DPN treatment on both the mRNA (Figure 3A) and protein levels, as shown by immunochemistry (Figure 3B and supplemental Figure 5). This finding suggested a suppressive effect of DPN on tumor vascularization, because VEGF-C has been shown to induce tumor-associated angiogenesis and lymphangiogenesis.36 Furthermore, it was found that DPN significantly downregulated the expression of angiogenin, an additional factor involved in tumor angiogenesis (Figure 3A).37 Analysis of the expression of CD34, a marker of blood vessel endothelial cells,38 showed that it was downregulated in the DPN-treated Granta-519 MCL tumors, confirming reduced angiogenesis (Figure 3A).
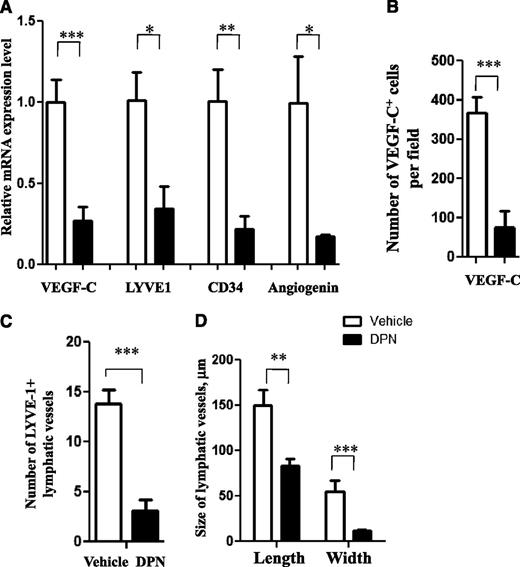
DPN inhibits the expression of genes regulating vascularization in MCL. (A) DPN inhibits expression of VEGF-C, LYVE-1, CD34, and angiogenin mRNA in Granta-519 MCL tumors. (B-D) The number of VEGF-C–positive cells, as determined by immunostaining (B), the number of LYVE-1-positive lymphatic vessels (C), and the size of the lymphatic vessels, as determined by immunostaining (D), were decreased in DPN-treated Granta-519 MCL lymphomas compared with control. Each group consisted of 5 mice. For vehicle vs DPN: *P < .05, **P < .01, and ***P < .001. Data are representative of 3 independent experiments.
DPN inhibits the expression of genes regulating vascularization in MCL. (A) DPN inhibits expression of VEGF-C, LYVE-1, CD34, and angiogenin mRNA in Granta-519 MCL tumors. (B-D) The number of VEGF-C–positive cells, as determined by immunostaining (B), the number of LYVE-1-positive lymphatic vessels (C), and the size of the lymphatic vessels, as determined by immunostaining (D), were decreased in DPN-treated Granta-519 MCL lymphomas compared with control. Each group consisted of 5 mice. For vehicle vs DPN: *P < .05, **P < .01, and ***P < .001. Data are representative of 3 independent experiments.
In order to investigate the effect of ERβ-selective agonist treatment on the formation of lymphatic vessels, we evaluated the expression of LYVE-1, which is a specific and widely used lymphatic endothelial marker.39 The expression of LYVE-1 mRNA was significantly downregulated in DPN-treated human Granta-519 lymphomas (Figure 3A). Downregulation of LYVE-1 expression in Granta-519 MCL tumors was confirmed at the protein level, and this was reflected in a reduced number and size of lymphatic vessels, as assessed by immunofluorescence (Figure 3C-D and supplemental Figure 6). DPN also reduced VEGF and LYVE-1 mRNA expression in BL Raji and murine EG7 T-cell lymphoma tumors, suggesting that the vascularization-inhibiting effect of ERβ agonists may operate in several lymphoma types (supplemental Figure 7).
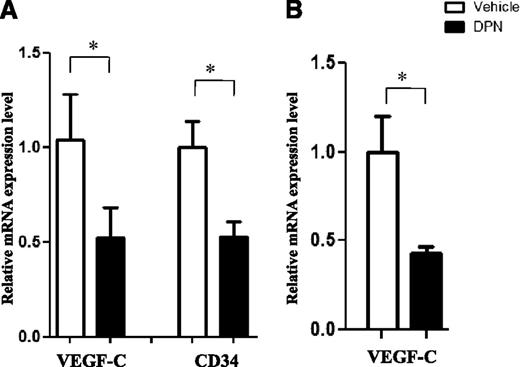
To exclude the possibility that the reduced angiogenesis was not just related to a difference in tumor size following ERβ agonist treatment, we compared the expression of VEGF-C and CD34 mRNA in control and DPN-treated Granta-519 MCL tumors grown to the same size before analysis (see “Methods”). A significant downregulation of both VEGF-C and CD34 mRNA expression in DPN-treated Granta-519 MCL was observed in comparison with vehicle-treated tumors of the same size, suggesting that the reduced angiogenesis was an effect of the DPN treatment and not just of tumor size (Figure 4A). Furthermore, a short-term 24-hour treatment with DPN of in vivo–established Granta-519 lymphoma tumors was sufficient to reduce expression of VEGF-C mRNA (Figure 4B).
Reduced expression of vasculogenic markers is an effect of DPN treatment. (A) The expression of VEGF-C and CD34 mRNA was analyzed in control and DPN-treated Granta-519 MCL tumors grown to the same size before analysis, and inhibition of VEGF-C and CD34 by DPN was observed. (B) Treatment with DPN for 24 hours inhibited the expression of VEGF-C. Each group consisted of 5 mice. For vehicle vs DPN, *P < .05. Data are representative of 2 independent experiments.
Reduced expression of vasculogenic markers is an effect of DPN treatment. (A) The expression of VEGF-C and CD34 mRNA was analyzed in control and DPN-treated Granta-519 MCL tumors grown to the same size before analysis, and inhibition of VEGF-C and CD34 by DPN was observed. (B) Treatment with DPN for 24 hours inhibited the expression of VEGF-C. Each group consisted of 5 mice. For vehicle vs DPN, *P < .05. Data are representative of 2 independent experiments.
Lymphoma dissemination is inhibited by ERβ agonist treatment
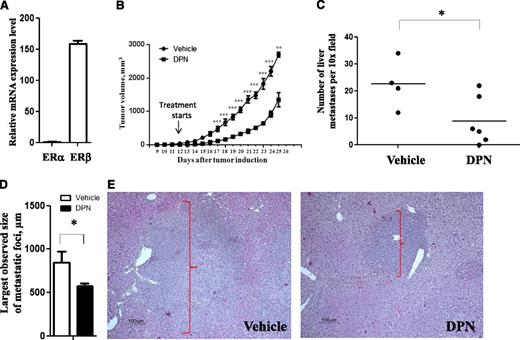
Tumor vascularization is an important mechanism supporting tumor dissemination. Because our results showed reduced angiogenesis and lymphangiogenesis following ERβ agonist treatment (see above), we hypothesized that ligand-mediated activation of ERβ may repress lymphoma dissemination. To test whether treatment with ERβ agonist will inhibit lymphoma dissemination in vivo, we engrafted NOD-SCID mice subcutaneously with Raji BL cells, which previously have been shown to be able to disseminate to the liver.17 As in the case of Granta-519 MCL tumors, Raji BL cells mainly expressed ERβ (Figure 5A), and treatment with DPN significantly inhibited growth of Raji BL in grafting experiments (Figure 5B). This demonstrated that the lymphoma growth-inhibiting effect of ERβ agonist seems to be of a more general nature, affecting various lymphoma subtypes. More importantly, microscopic inspection of the liver sections stained with hematoxylin and eosin clearly revealed a smaller number and size of disseminated tumor loci in DPN-treated mice compared with the vehicle-treated group (Figures 5C-E and supplemental Figure 8). This clearly demonstrated that ERβ agonist treatment significantly suppressed lymphoma dissemination in vivo.
Selective ERβ agonist inhibits growth and dissemination of Raji BL cells in vivo. (A) Expression of ERα and ERβ mRNA in Raji BL was measured by quantitative real-time PCR. (B) Male NOD-SCID mice were subcutaneously grafted with 5 × 106 Raji BL cells. Starting from day 12 after tumor cell grafting, animals were treated subcutaneously daily with the ERβ-selective agonist DPN (12.5 μmol/kg per day) (▪) or vehicle (●). The number (C) and size (D-E) of tumor foci of Raji cell dissemination in liver were significantly reduced in DPN-treated mice with Raji lymphomas as evaluated using hematoxylin and eosin staining. Original magnification ×200. The groups consisted of 4 (vehicle-treated) and 6 (DPN-treated) mice. For vehicle vs DPN: *P < .05, **P < .01, and ***P < .001. Data are representative of at least 2 independent experiments.
Selective ERβ agonist inhibits growth and dissemination of Raji BL cells in vivo. (A) Expression of ERα and ERβ mRNA in Raji BL was measured by quantitative real-time PCR. (B) Male NOD-SCID mice were subcutaneously grafted with 5 × 106 Raji BL cells. Starting from day 12 after tumor cell grafting, animals were treated subcutaneously daily with the ERβ-selective agonist DPN (12.5 μmol/kg per day) (▪) or vehicle (●). The number (C) and size (D-E) of tumor foci of Raji cell dissemination in liver were significantly reduced in DPN-treated mice with Raji lymphomas as evaluated using hematoxylin and eosin staining. Original magnification ×200. The groups consisted of 4 (vehicle-treated) and 6 (DPN-treated) mice. For vehicle vs DPN: *P < .05, **P < .01, and ***P < .001. Data are representative of at least 2 independent experiments.
ERβ is expressed in lymph node biopsy specimens from MCL patients
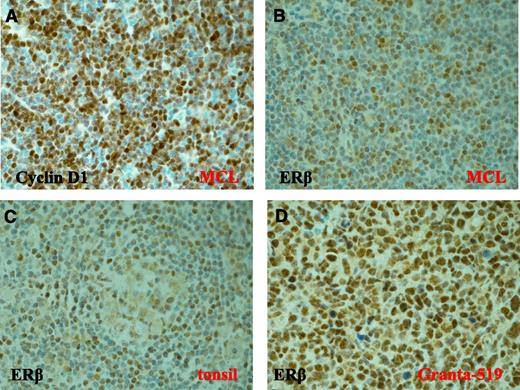
In order to test whether ERβ is expressed in primary lymphoma samples and thus could act as a drug target in lymphoma therapy, we analyzed lymph node biopsy specimens from a few MCL patients for ERβ expression by immunohistochemistry. The diagnosis of MCL of tumor samples was confirmed by high expression of Cyclin D1 (Figure 6A) and the presence of the t(11;14(q13;q32)) CCND1 translocation (data not shown). Immunohistochemical analysis using the verified ERβ antibody (see above and supplemental Figure 1b-c) showed clear nuclear staining of ERβ in lymphoma biopsy sections from 4 out of 4 examined MCL patients (Figure 6B). Nuclear ERβ staining was also found in sections of normal lymphoid tissue (tonsil), mainly in germinal centers and mantle zones of B-cell follicles (Figure 6C). High ERβ expression was seen in the Granta-519 MCL (Figure 6D). These results suggest that ERβ expression, at least in certain clinical lymphomas, is preserved during transformation of normal lymphoid cells to malignant lymphoid cells.
Expression of ERβ in primary MCL tissue. Clear expression of Cyclin D1 (A) and nuclear ERβ (B-D) was detected by immunohistochemistry in MCL patient samples (B), in normal tonsil (C), and in Granta-519 MCL tumors (D). Original magnification ×600. The 4 MCL patient samples showed similar staining intensity.
Expression of ERβ in primary MCL tissue. Clear expression of Cyclin D1 (A) and nuclear ERβ (B-D) was detected by immunohistochemistry in MCL patient samples (B), in normal tonsil (C), and in Granta-519 MCL tumors (D). Original magnification ×600. The 4 MCL patient samples showed similar staining intensity.
Discussion
Our study has shown that ligand-activated ERβ suppresses the growth of different types of human lymphomas using a xenograft model and that ligand-mediated activation of ERβ suppresses both vascularization and dissemination of lymphomas. A reduction in VEGF and LYVE-1 mRNA expression following DPN treatment was also seen in Raji BL and murine T-cell lymphoma EG7 tumors, suggesting that the vascularization-inhibiting effect of ERβ agonists may be more general and operate in several lymphoma types. Importantly, the inhibitory effect on lymphoma growth by the ERβ agonist DPN was also seen using a structurally different ERβ selective agonist and was blocked when the ERβ agonist was combined with an ER antagonist. This suggests that the effect is mediated via ERβ. That the tumor-inhibiting effect by DPN is ERβ and not ERα dependent is further supported by the fact the expression of ERα was very low in Granta-519 MCL and that tumor growth inhibition was not seen following treatment with the ERα-selective agonist PPT. In addition, the doses of the selective ERβ agonists used did not activate ERα, because they did not affect uterus size or weight in the treated mice.5,28
Growth of lymphomas in vivo requires contact between tumor cells and the cells of the tumor microenvironment, such as immune, endothelial, and stromal cells, which provide multiple regulatory signals to lymphoma cells.40 Although previous studies have shown expression of ERβ in immune5,14 and endothelial25 cells, the effect of ERβ on the cells forming the tumor microenvironment remains to be investigated. Using ERβ−/− mice, we have tested whether treatment with an ERβ agonist directly affects the cells of the tumor microenvironment, contributing to the tumor reducing effect. However, in vivo comparison of the responses to an ERβ selective-agonist in wild-type and ERβ−/− mice grafted with ERβ-positive murine EG7 lymphoma cells did not reveal any significant differences between these 2 groups. This observation allowed us to conclude that the direct effects of an ERβ-selective agonist on tumor microenvironment in lymphoma in vivo models used in our study are minor and do not significantly affect tumor growth. Whether ERβ expression in cells of the microenvironment has a role in lymphomas other than murine EG7 tumors is unclear and could not be investigated, because immunocompromised mice deficient in ERβ expression are not presently available. Despite this, proper 3-dimensional organization or matrix interactions are most likely necessary for the gene-expression changes to occur, because corresponding gene regulation and effects on cell proliferation were not observed in Granta-519 in vitro cell culture (data not shown). The mechanistic contribution of these tumor-stroma interactions to proper gene regulation in vivo is still unclear.
In vivo experiments using MCL cells identified several genes regulating tumor proliferation affected by the ERβ-selective agonist DPN. Downregulation of Ki67 expression clearly indicated the suppressive effect of DPN on MCL proliferation. In addition, the expression of the genes BAFF and GRB7, which are known to regulate tumor progression,29,32 was downregulated. Whether these genes are direct targets of ERβ or indirectly downregulated is still unclear. BAFF is known to regulate B-cell survival and is associated with the transformation of the tumors to a more aggressive phenotype.31 Overexpression of GRB7 has been connected to tumor cell proliferation and invasion.32,33 In addition, expression of GRB7 was shown to positively correlate with progression of CLL.34 Overexpression of Cyclin D1 is a key feature in the majority of MCL cells, including Granta-519 cells.19,41 However, the expression of Cyclin D1, one of the most specific markers of MCL development, was not affected by DPN treatment (data not shown). Although previous studies have demonstrated that estrogens activate the expression of Cyclin D1 via ERα but inhibit via ERβ in HeLa cells in vitro,42 the mechanisms of the inhibitory effects of ERβ agonist in lymphoid cells on tumor cell proliferation do not seem to involve Cyclin D1. Furthermore, in contrast to murine EG7 cell tumors,5 apoptosis was not affected in Granta-519 MCL tumors (data not shown). This may reflect a difference in the relative importance of different growth inhibitory signaling pathways in response to ERβ activation in MCL vs murine T-cell lymphoma.
A novel mechanism identified was that signaling via ERβ inhibited vascularization, both angiogenesis and lymphangiogenesis, in the MCL engrafted tumor. It is well established that vascularization supports tumor survival and proliferation. Multiple studies have shown that angiogenesis also plays an important role in tumor progression and metastasis.43 The family of VEGFs are central regulators of angiogenesis and lymphangiogenesis.35 Moreover, and related to our study, VEGFs are suggested to have a major role in the development and progression of hematologic malignancies such as acute leukemia, chronic leukemia, non-Hodgkin lymphoma, and multiple myeloma.44 In human lymphoma tissues (Granta-519 MCL and Raji BL), we found that VEGF-C was the main VEGF downregulated following DPN treatment, whereas in murine EG7 lymphoma it was vegf-d. In addition, other markers of angiogenesis and blood vessel endothelial cells, angiogenin, and CD34, respectively, were significantly downregulated in the studied Granta-519 MCL tumors. Importantly, the reduced VEGF-C expression was not an indirect effect due to smaller tumor size following DPN treatment but was also seen in comparison with tumors of the same size in vehicle-treated mice. Furthermore, regulation of VEGF-C was seen within 24 hours following treatment, indicating that inhibition of VEGF-C expression occurs relatively early in response to ERβ agonist treatment. However, direct proof that VEGF-C and the other genes identified to be regulated by DPN are a direct ERβ target has been hard to establish due to lack of good ERβ immunoprecipitating antibodies working on endogenous ERβ in a chromatin immunoprecipitation assay and poor/missing regulation of the identified target genes in cell cultures of the lymphoma cells used.
In addition to angiogenesis, we show that LYVE-1, a marker of lymphatic vessels, is downregulated by selective ERβ agonist DPN treatment in the lymphoma engrafting models studied. VEGF-C induces lymphangiogenesis via VEGFR-3 in normal development and has also been shown to be lymphangiogenic in tumors.35 Lymphangiogenesis also plays a crucial role in cancer metastasis45 and has been found to be related to poor prognosis in diffuse large B-cell lymphoma patients.46 Furthermore, the mean survival rates were significantly shorter in cases expressing high levels of VEGF-A and VEGF-C.47 Moreover, it has been shown that targeting lymphangiogenesis by antilymphangiogenic agents might be a novel way to prevent lymphatic dissemination of tumors,48 and inhibition of VEGF signaling and angiogenesis has already been successfully established for the treatment of different cancers.49 Because activation of ERβ reduced vascularization of the engrafted lymphoma tumor in our experimental model, we investigated whether this would lead to a reduced number of disseminated tumor foci. For this purpose, we used a Raji BL cell line that, in contrast to the Granta-519 MCL, previously has been shown to disseminate to the liver following subcutaneous engrafting.17 In line with a reduced vascularization, DPN treatment led to a lower number and smaller size of tumor loci in the liver. The liver dissemination is in accordance with the pattern and mechanisms of B-cell lymphoma dissemination previously described.50,51 However, further studies are required to identify genes and mechanisms regulating lymphoid cell dissemination that are affected by ligand-activated ERβ.
Despite significant progress in MCL research, the understanding of MCL pathogenesis is not complete, and considering the poor prognosis, new therapeutic approaches are needed.41 With regard to the clinical importance of our findings, we demonstrate nuclear expression of ERβ protein in biopsy sections from MCL patients. This confirms that ERβ expression is maintained in tumor cells of MCL patients. Furthermore, these results suggest that ERβ agonists may be useful for MCL therapy by targeting ERβ expressed in the lymphoma cells.
In summary, we have found that selective ERβ agonists inhibit the proliferation, vascularization, and dissemination of lymphoid tumors in vivo. Importantly, although the inhibition of proliferation and vascularization seems to occur in the different lymphoma types tested, the molecular mechanisms may vary or contribute to various extents in different lymphomas, considering their heterogeneity. Altogether, the inhibitory effect of ligand-activated ERβ on lymphoma tumor proliferation, vascularization, and dissemination suggests that the administration of selective ERβ agonists could be useful in the treatment of several lymphomas through novel mechanisms not targeted by currently available therapies. This could be particularly valuable in lymphomas with poor survival, as in the case of MCL. Furthermore, our results may contribute to a mechanistic understanding of the difference in gender incidence and prognosis of lymphomas.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Jose Inzunza and Jan-Åke Gustafsson, Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden, for providing ERβ−/− mice and Stefan Nilsson for valuable discussions.
This project was supported by the Swedish Cancer Society, AFA Insurance, and Karo Bio Research Funds (S.O.).
Authorship
Contribution: K.Y. designed and performed the research, analyzed data, and wrote the manuscript; M.S.H. and J.G. performed experiments and analyzed data; M.P.C. provided disseminating Raji cells, provided critical support of their use, and commented on the manuscript; B.S. provided clinical materials, stained them, and provided critical comments on the manuscript; and S.O. designed and led the research, provided research support, and made critical comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sam Okret, Department of Biosciences and Nutrition, Karolinska Institutet, Novum, SE-141 83 Huddinge, Sweden; e-mail: sam.okret@ki.se.