In this issue of Blood, Yakimchuk and colleagues show that estrogen receptor β (ERβ) signaling can act tumor-suppressive predominantly through the regulation of genes by ERβ in the tumor, not in the microenvironment, and point out new therapeutic strategies.1
An intriguing discovery in hemato-oncology has been that women display a lower incidence of B- and T-cell lymphomas than men and female patients have a better prognosis than male patients.2,3 Although female reproductive hormones were suspected early to play a role in this phenomenon, the precise underlying mechanisms remained enigmatic. Lymphomas are still not generally perceived as hormone-controlled; however, several strong hints now point at a protective function of estrogens in lymphomagenesis. Clues came initially from epidemiological studies.2 For example, a population-based case-control study on female non-Hodgkin lymphoma (NHL) patients that had taken or not taken oral contraceptives or estrogen-containing lactation suppressants documented that estrogen exposure essentially cut the risk of NHL in half.3
In the estrogen-signaling pathway the predominant estrogen 17β-estradiol acts through the binding and activation of 2 cellular proteins belonging to the steroid hormone receptor family of transcription factors, ERα and ERβ, which then homo- or heterodimerize to regulate a plethora of cellular genes.4,5 Lymphoid cells primarily express ERβ which in contrast to ERα seems to mostly act antiproliferative and proapoptotic through the execution of a specific and complex gene expression program, a hallmark of many tumor-suppressive transcriptional regulators. Several isoforms of the ERs exist; they result from alternative splicing, yet most of these do not bind to ligand and seem to act primarily through modifying the full-length wild-type proteins. Previous work by Yakimchuk and colleagues showed that grafted T-cell lymphomas grew faster in male mice than in female mice and that female protection was abolished by ovariectomy while tumor size reduction could be achieved by the treatment with ERβ agonists such as diarylpropionitrile (DPN). Tumor suppression by these compounds was apparently the result, at least in part, of inhibited proliferation and elevated apoptosis.6 The authors also reported the expression of ERβ in peripheral blood mononuclear cells (PBMCs) of patients suffering from chronic lymphocytic leukemia (CLL) and suggested that ERβ stimulation might be a useful therapeutic strategy against these neoplasms.7
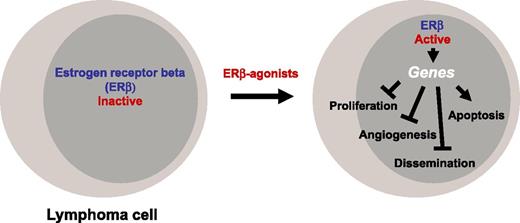
One of the interesting questions was of course whether hormone sensitivity of lymphomas reflects primarily the sensitivity of the tumor itself or rather the sensitivity of the microenvironment (or both). ERβ overproduction has been shown in Burkitt lymphoma6 and in PBMCs from CLL patients,7 in accordance with the suggestion that estrogens may affect lymphoma (and leukemia) cells directly. Along the same line, when the ligand-independent inhibitory ERβ splice variant ERβ2 was overproduced in certain tumors, the patients had a reduced overall survival.8 It was clear though that in order to gain further insight into the relative importance of ERβ expression in the tumor vs microenvironment, knockout mouse models would be useful. ERβ-deficient mice are prone to myeloproliferative disease.9 The current article by Yakimchuk and colleagues reports on these ERβ-deficient mice.1 The main achievement of the work is to document that (predominantly) the activation of the ERβ in the tumor, not in the microenvironment, acts tumor-suppressive, and that ERβ-mediated tumor suppression may be primarily communicated through the modulation of genes in the tumor cells to cause reduction of proliferation and survival, as well as reduction of dissemination and vascularization (see figure). However, it seems fair to conclude that the jury is still out on a (supportive) role of ERβ signaling in the tumor microenvironment as well because proper analysis of this compartment is still lacking and will almost certainly require the study of 3-dimensional cell structures (see figure).
Where, then, do these discoveries lead us? One obvious question was whether the results obtained with mice can be extrapolated to human patients. Yakimchuk et al showed by immunocytochemistry that 4 of 4 primary lymphoma samples from patients with mantle cell lymphoma (MCL) indeed expressed ERβ in the cell nuclei.1 Furthermore, ERβ dependence of the tumor-suppressive effects in their experimental systems was strongly indicated by the efficacy of low-dose selective ERβ agonist DPN, as well as by the efficacy of a structurally different ERβ-selective agonist, and in addition was suggested by the relatively low expression levels of ERα in lymphoma cells. It will be important in the future to work out precisely which ERβ-regulated target genes are mainly responsible for the tumor-suppressive phenotype. For example, vascular endothelial growth factors (VEGFs) produced by tumor cells are well known to be important regulators of angiogenesis and lymphangiogenesis.10 Are VEGFs under the control of ERβ in lymphomas, and is their inhibition central to the inhibition of vascularization by ERβ agonists? Good chromatin immunoprecipitation–grade ERβ-specific antibodies should be helpful in addressing this and related questions concerning the identification of relevant target genes. Moreover, can anti-VEGF signaling be a full therapeutic substitute for stimulated ERβ signaling in those lymphomas that are sensitive to inhibition of vascularization? Altogether, the work presented by Yakimchuk and colleagues instils hope that selective ERβ agonists are useful as therapeutic agents in lymphoma patients, in particular in those suffering from cancers with poor prognosis such as MCL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal