Key Points
JMJD1C is required for leukemia maintenance.
JMJD1C is a potential therapeutic target in leukemia.
Abstract
Epigenetic regulatory mechanisms are implicated in the pathogenesis of acute myeloid leukemia (AML) and acute lymphoid leukemia (ALL). Recent progress suggests that proteins involved in epigenetic control are amenable to drug intervention, but little is known about the cancer-specific dependency on epigenetic regulators for cell survival and proliferation. We used a mouse model of human AML induced by the MLL-AF9 fusion oncogene and an epigenetic short hairpin RNA (shRNA) library to screen for novel potential drug targets. As a counter-screen for general toxicity of shRNAs, we used normal mouse bone marrow cells. One of the best candidate drug targets identified in these screens was Jmjd1c. Depletion of Jmjd1c impairs growth and colony formation of mouse MLL-AF9 cells in vitro as well as establishment of leukemia after transplantation. Depletion of JMJD1C impairs expansion and colony formation of human leukemic cell lines, with the strongest effect observed in the MLL-rearranged ALL cell line SEM. In both mouse and human leukemic cells, the growth defect upon JMJD1C depletion appears to be primarily due to increased apoptosis, which implicates JMJD1C as a potential therapeutic target in leukemia.
Introduction
Translocations involving mixed lineage leukemia (MLL) gene occur frequently in acute leukemia, especially in childhood and therapy-related leukemia.1,2 Leukemias with MLL translocations are associated with higher resistance to chemotherapy and lower survival rates than other types of leukemia.3,4 In recent years, the understanding of the molecular basis of leukemogenesis driven by MLL fusions has greatly improved: MLL is an H3K4 methyltransferase and is required for transcription of 2% of mammalian genes, including many Hox genes and Wnt-regulated genes.5 In MLL fusions, the H3K4 methyltransferase activity of MLL is lost and the mechanism of MLL fusion–driven leukemogenesis depends on the identity of a fusion partner, most commonly AF4, AF9, and ENL. These recruit MLL into several protein complexes associated with transcriptional elongation, such as the elongation-assisting protein complex, the AF4/ENL/P-TEFb complex, the super elongation complex, and the DOT1L complex (reviewed in Deshpande et al6 ).
In the search for targeted therapy in MLL-rearranged leukemia, several chromatin-associated proteins were found to be required for survival of MLL fusion–driven leukemia: H3K79 methyltransferase DOT1L7,8 ; histone demethylase LSD19,10 ; bromodomain-containing 4 (BRD4)11,12 ; MLL binding partner menin13 ; PRC2 complex components EZH1/EZH2, EED, and SUZ1214,15 ; PRC1 complex member CBX816 ; H2B ubiquitin ligase RNF2017 ; and methylcytosine dioxygenase TET1.18 Small molecule inhibitors to some of these have been published, such as JQ1 and I-BET151, inhibiting the interaction of BRD4 with histones11,12 ; EPZ004777, inhibiting H3K79 methylation by DOT1L19 ; MI-2 and MI-3, inhibiting menin-MLL interaction20 ; GSK126, EPZ-6438, and EI1, inhibiting H3K27 methylation by EZH221-23 ; and ORY-1001, inhibiting H3K4 demethylation by LSD1.24
Pooled short hairpin RNA (shRNA) screens have been successfully used to identify novel oncogenes and tumor suppressors (eg, in liver cancer25 and lymphoma26,27 ). Two shRNA screens in MLL-AF9 leukemia identified potential therapeutic targets: an in vitro screen with shRNAs targeting 243 chromatin-associated factors resulted in the identification of Brd4 as a promising drug target,11 and an in vivo screen with a library of shRNAs targeting 268 established and putative cancer-associated genes revealed integrin β3 (Itgb3) as critical for maintenance of MLL-AF9 acute myeloid leukemia (AML).28 Here, we present an shRNA screen in primary mouse MLL-AF9 AML cells, accompanied by a counter-screen in c-Kit+–enriched mouse bone marrow (BM) cells using an shRNA library targeting 319 known and candidate epigenetic regulators.
One potential drug target candidate identified through our screening approach was Jmjd1c. Interestingly, it has previously been described as a target of MLL-AF9 and MLL-AF4 fusion proteins in mouse and human leukemic cells.7,29,30 JMJD1C was originally identified as a ligand-dependent thyroid receptor-interacting protein31 and an androgen receptor coactivator.32 It was reported to be an H3K9me2/me1 demethylase and transcriptional activator33 ; however, two recent studies failed to observe any JMJD1C histone demethylase activity following extensive cellular and biochemical assays.34,35 In this study, we validate and characterize a role for JMJD1C in maintenance of leukemia.
Methods
Generation of pMLS library
shRNAs were subcloned from pGIPZ (Open Biosystems) into pMLS (MSCV-LTRmir30-SV40-GFP) vector.36 Sequences of shRNA hairpins are listed in supplemental Table 1, available on the Blood Web site.
Pooled shRNA screening
All mouse studies were approved by the Danish Animal Ethical Committee. Mouse MLL-AF9 or c-Kit+–enriched BM cells were transduced with the shRNA library and fluorescence-activated cell sorter (FACS) sorted 2 days later. Genomic DNA was extracted from the reference (day 0), samples were cultured for 14 days, and shRNA hairpins were amplified by polymerase chain reaction (PCR) with oligos carrying Illumina adaptors and barcodes. Illumina HiSeq sequencing was performed at Danish National High-Throughput DNA Sequencing Centre, University of Copenhagen. Sequencing results were demultiplexed and mapped to the shRNA library by using barcodes with checks for cross alignments between barcodes. Alignment was performed with the bowtie alignment program37 on an shRNA library pseudogenome in which up to 2 mismatches were accepted within the trimmed reads. A sum of ranked standardization scores was then calculated for each gene (see supplemental Methods).
Mouse transplantation
c-Kit+–enriched BM cells from B6 (CD45.2+) donor mice were transduced with MSCV-MLL-AF9-neo. After 2 days, cells were plated into methylcellulose media (M3534; STEMCELL Technologies) with G418. Following 2 rounds of replating, preleukemic cells were transplanted into lethally irradiated (900 cGy) B6.SJL (CD45.1+) recipient mice at 1 × 106 cells per recipient. Two × 105 whole B6.SJL BM cells were co-injected as a support. Primary leukemic cells from BM and spleen of sick mice were harvested, analyzed by flow cytometry, and frozen.
For secondary transplants, pMLS-transduced MLL-AF9 spleen leukemic cells were FACS sorted and injected into sublethally irradiated (450 cGy) B6.SJL recipient mice at 1 × 104 cells per recipient.
Virus production
For retrovirus production, Phoenix-Ecotropic cells38 were cotransfected with pMLS or pMSCV vectors and pCL-Eco by using a calcium phosphate transfection method. For lentivirus production, 293FT cells were cotransfected with pLKO.1-puro, pLKO.1-GFP, or pLKO-puro-IPTG-3xLacO (Sigma-Aldrich) and pAX8 and pCMV-VSV by using a calcium phosphate transfection method.39
Generation of JMJD1C antibody
The 1-289aa coding sequence of human JMJD1C variant 2 was transferred into pET-28 vector (Novagen) and expressed in Rosetta 2 (DE3) cells (Novagen). The recombinant protein was purified using TALON metal affinity resin (Clontech), and a Superdex 200 HR 10/30 gel filtration column (GE Healthcare). JMJD1C polyclonal antibodies were generated by immunizing rabbits with the purified recombinant antigen, and the antibodies were affinity purified by using the antigen (GenScript).
IPTG-inducible system
Cells were transduced with pLKO-puro-IPTG-3xLacO lentiviral vectors and selected with 2 μg/mL puromycin (Sigma-Aldrich). For shRNA expression, culture medium was supplemented with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG; Sigma-Aldrich).
Messenger RNA (mRNA) expression analysis
RNA was purified by using an RNeasy Plus RNA kit (Qiagen) and reverse transcribed by using TaqMan Reverse Transcription Reagents (Applied Biosystems). Quantitative reverse transcription PCR (qRT-PCR) was performed with LightCycler 480 SYBR Green I Master and a LightCycler480 System (Roche). Expression was normalized to RPLP0. Primer sequences are listed in the supplemental Methods.
Expression microarray
RNA was extracted with an RNeasy Plus RNA kit (Qiagen). For SEM cells, RNA was hybridized on Affymetrix Human Gene 2.0 ST arrays by the RH Microarray Center at Rigshospitalet, Copenhagen, Denmark. For MLL-AF9 cells, RNA was hybridized on Agilent SurePrint G3 Mouse GE 8 × 60K arrays according to the manufacturer’s protocol. (Microarray accession numbers: GSE54311 [mouse] and GSE50048 [human].)
Gene set enrichment analysis (GSEA)
Murine gene names were mapped to their orthologous human HUGO Gene Nomenclature Committee–approved gene names by using the Mouse Genome Informatics Mouse/Human Orthology dataset (HMD_HumanPhenotype.rpt; ftp://ftp.informatics.jax.org/pub/reports/index.html#homology), previously published gene sets, and the VLOOKUP function in Microsoft Excel 2011. GSEA (http://www.broadinstitute.org/gsea/index.jsp) was performed on knockdown (KD) vs scrambled (Scr) triplicate expression files. Gene sets used, references, and statistics are listed in supplemental Table 2 and at http://www.broadinstitute.org/gsea/msigdb/index.jsp. For all gene sets, 1000 permutations and the Signal2Noise metric were used. Permutations by gene sets were conducted to assess statistical significance. See the supplemental Methods.
Results
Pooled shRNA screens reveal Jmjd1c as a potential drug target in MLL-AF9 leukemia
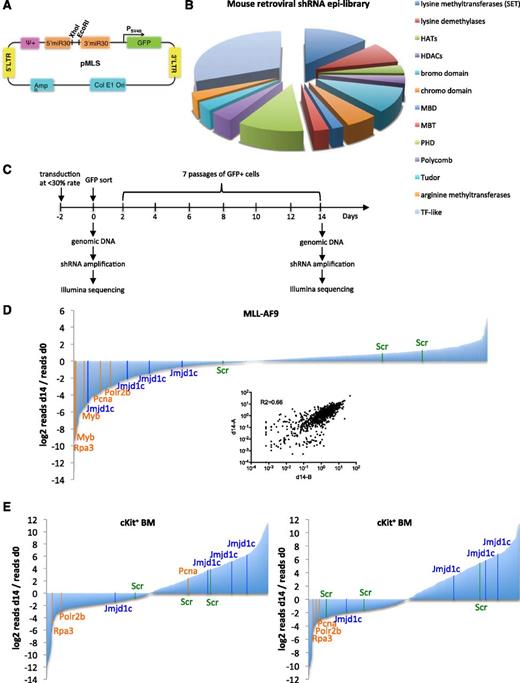
To identify new epigenetic factors involved in AML, we generated a mouse model of human AML induced by the MLL-AF9 fusion oncogene by using a protocol described previously.40 Mice transplanted with preleukemic MLL-AF9 cells developed AML with a median latency of 70 days (supplemental Figure 1A) and of expected myeloid immunophenotype Mac1+Gr1+c-Kit+/−CD3– and B220– (supplemental Figure 1B-C). We generated a retroviral shRNA library targeting epigenetic factors (epi-library), by subcloning selected shRNAs from pGIPZ (Open Biosystems) into pMLS vector36 (Figure 1A). The library contained 898 constructs targeting 319 genes belonging to all major chromatin-associated gene families (Figure 1B and supplemental Table 1).
Pooled shRNA screens with mouse retroviral shRNA epi-library. (A) Schematic map of the pMLS vector. (B) Categories of chromatin-associated factors represented in the mouse retroviral shRNA epi-library. (C) Screening strategy. Mouse primary MLL-AF9 AML cells or c-Kit–enriched mouse BM cells were transduced with the epi-library. Two days after transduction, GFP+ cells were FACS sorted, and cells were harvested at day 0 and day 14 for genomic DNA. shRNA hairpins were amplified by PCR and submitted for sequencing. (D) Screening result in MLL-AF9 AML cells. Data are presented as the ratio of normalized read number at day 14 to normalized read number at day 0 for each shRNA in the library. The result is an average of 2 replicates. Positive controls: orange; negative (nontargeting): green; shRNAs targeting Jmjd1c: blue. Inset shows correlation of normalized reads per shRNA between 2 replicates. (E) Screening result in c-Kit–enriched BM cells. Two independent experiments are presented. Control and Jmjd1c shRNAs are marked as in (D).
Pooled shRNA screens with mouse retroviral shRNA epi-library. (A) Schematic map of the pMLS vector. (B) Categories of chromatin-associated factors represented in the mouse retroviral shRNA epi-library. (C) Screening strategy. Mouse primary MLL-AF9 AML cells or c-Kit–enriched mouse BM cells were transduced with the epi-library. Two days after transduction, GFP+ cells were FACS sorted, and cells were harvested at day 0 and day 14 for genomic DNA. shRNA hairpins were amplified by PCR and submitted for sequencing. (D) Screening result in MLL-AF9 AML cells. Data are presented as the ratio of normalized read number at day 14 to normalized read number at day 0 for each shRNA in the library. The result is an average of 2 replicates. Positive controls: orange; negative (nontargeting): green; shRNAs targeting Jmjd1c: blue. Inset shows correlation of normalized reads per shRNA between 2 replicates. (E) Screening result in c-Kit–enriched BM cells. Two independent experiments are presented. Control and Jmjd1c shRNAs are marked as in (D).
To identify potential drug targets in AML, we performed an in vitro screen revealing shRNAs inhibiting growth of MLL-AF9 cells, and an independent screen in c-Kit+–enriched mouse BM cells. The latter was used as a counterscreen to exclude generally toxic shRNAs. In both screens, the epi-library retrovirus was titrated to ensure transduction efficiency of less than 30%, with the aim of reducing the number of cells infected by more than one shRNA. Transduced cells were FACS sorted and maintained in culture for 14 days (Figure 1C). shRNA hairpins were amplified from genomic DNA isolated from cells at the beginning and at the end of the culture, and abundance of each shRNA was quantified by high-throughput sequencing.
We observed a good correlation between two replicates of the MLL-AF9 in vitro screen; results are therefore presented as an average of 2 experiments (Figure 1D and supplemental Table 3). Control shRNAs inhibiting growth of MLL-AF9 cells such as shRNA targeting Myb, a gene critical for MLL-AF9 leukemia maintenance,41 and shRNAs targeting essential genes Rpa3, Pcna, and Polr2b were strongly depleted at day 14 of MLL-AF9 cell culture compared with day 0. In contrast, 3 nontargeting controls (Scr) were neither strongly depleted nor enriched (Figure 1D). In contrast to the MLL-AF9 screen, the correlation between two replicates of the screen in c-Kit+ BM cells was not high, most likely due to heterogeneity of the c-Kit+ cell population (Figure 1E and supplemental Table 3).
To select hits with the strongest drug-target potential, genes were ranked according to the combined performance of their shRNAs in both screens (supplemental Table 4). Genes with the strongest depletion in MLL-AF9 cells but little depletion in c-Kit+ BM obtained the highest score. Some genes showing a strong depletion in the MLL-AF9 screen—Myb, Tapbp, and Hdac3—show strong depletion in c-Kit+ BM resulting in a relatively low combined score (supplemental Table 4). Brd4, which is known as being required for survival of MLL-AF9 leukemia cells,11 ranks 29th. Jmjd1c is the highest scoring gene. Lack of other known required genes for leukemic cell proliferation, such as Ezh2 or Dot1l, among the top-ranking hits, could be due to poor KD efficiencies of their shRNAs since the library is not validated.
Leukemic cells are more sensitive to Jmjd1c depletion than normal BM cells
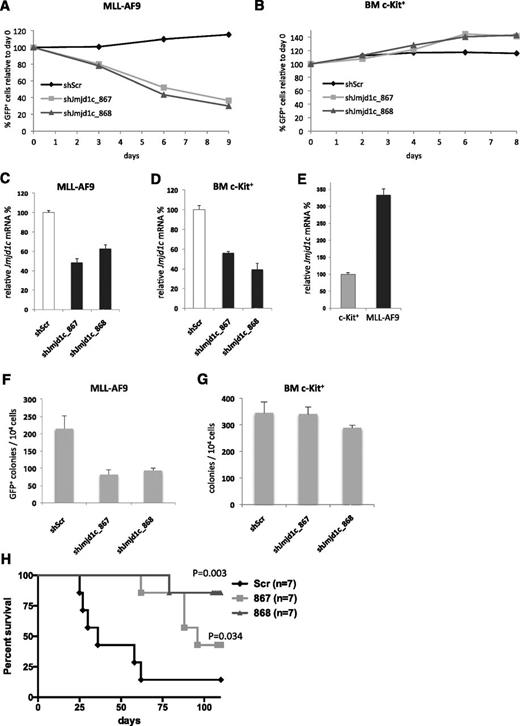
As a first line of validation, we tested the effect of Jmjd1c KD on the proliferation of MLL-AF9 AML and c-Kit+ BM cells in liquid culture. Control cells (shScr) did not show proliferative advantage or disadvantage compared with untransduced cells, whereas MLL-AF9 cells expressing shRNAs targeting Jmjd1c (shJmjd1c_867 or shJmjd1c_868) were gradually depleted over time (Figure 2A). In contrast, c-Kit+ BM cells transduced with shJmjd1c_867 or shJmjd1c_868 were not out-competed by untransduced cells (Figure 2B). Importantly, Jmjd1c transcript levels were depleted to a similar extent in MLL-AF9 and c-Kit+ BM cells upon KD (Figure 2C-D), whereas MLL-AF9 AML cells have higher basal Jmjd1c expression levels compared with the c-Kit+ cell population (Figure 2E). Similar to the results in liquid culture, Jmjd1c-depleted MLL-AF9 cells formed significantly fewer colonies than control cells (Figure 2F), and Jmjd1c-depleted c-Kit+ cells were largely unaffected compared with control BM cells (Figure 2G). Together, these data suggest that Jmjd1c expression is required for survival of MLL-AF9 AML cells, whereas Jmjd1c depletion has no immediate negative effect on normal BM cells in in vitro culture.
Differential sensitivity of mouse MLL-AF9 AML cells and normal BM cells to Jmjd1c KD. (A) Competitive proliferation assay of MLL-AF9 cells transduced with shRNAs targeting Jmjd1c-867 and Jmjd1c-868 or with a nontargeting control (Scr). Graph shows percentage of pMLS-GFP–transduced cells normalized to the percentage observed at day 0 (2 days after transduction). (B) Competitive proliferation assay of c-Kit–enriched BM cells transduced with shRNAs targeting Jmjd1c-867 and Jmjd1c-868 or with a nontargeting control (Scr). Graph shows percentage of pMLS-GFP–transduced cells normalized to day 0 (2 days after transduction). (C) Relative expression of Jmjd1c in MLL-AF9 cells transduced with Scr or Jmjd1c-targeting shRNAs at day 2 after transduction. (D) Relative expression of Jmjd1c in c-Kit+ cells transduced with Scr or Jmjd1c-targeting shRNAs at day 2 after transduction. (E) Relative Jmjd1c mRNA expression in c-Kit+ mouse BM cells and in MLL-AF9 primary mouse leukemic cells. (F) Number of colonies generated by MLL-AF9 cells transduced by pMLS-Scr, pMLS-867, or pMLS-868. Cells were plated in semisolid media 2 days after transduction. Error bars indicate standard deviation of the mean (n = 3). (G) Number of colonies generated by c-Kit+ cells transduced by pMLS-Scr, pMLS-867, or pMLS-868. Cells were plated in semisolid media 2 days after transduction. Error bars indicate standard deviation of the mean (n = 3). (H) Survival curves of sublethally irradiated mice transplanted with 104 MLL-AF9 cells transduced with pMLS-Scr, pMLS-867, or pMLS-868. GFP+ cells were sorted by FACS and transplanted 2 days after transduction.
Differential sensitivity of mouse MLL-AF9 AML cells and normal BM cells to Jmjd1c KD. (A) Competitive proliferation assay of MLL-AF9 cells transduced with shRNAs targeting Jmjd1c-867 and Jmjd1c-868 or with a nontargeting control (Scr). Graph shows percentage of pMLS-GFP–transduced cells normalized to the percentage observed at day 0 (2 days after transduction). (B) Competitive proliferation assay of c-Kit–enriched BM cells transduced with shRNAs targeting Jmjd1c-867 and Jmjd1c-868 or with a nontargeting control (Scr). Graph shows percentage of pMLS-GFP–transduced cells normalized to day 0 (2 days after transduction). (C) Relative expression of Jmjd1c in MLL-AF9 cells transduced with Scr or Jmjd1c-targeting shRNAs at day 2 after transduction. (D) Relative expression of Jmjd1c in c-Kit+ cells transduced with Scr or Jmjd1c-targeting shRNAs at day 2 after transduction. (E) Relative Jmjd1c mRNA expression in c-Kit+ mouse BM cells and in MLL-AF9 primary mouse leukemic cells. (F) Number of colonies generated by MLL-AF9 cells transduced by pMLS-Scr, pMLS-867, or pMLS-868. Cells were plated in semisolid media 2 days after transduction. Error bars indicate standard deviation of the mean (n = 3). (G) Number of colonies generated by c-Kit+ cells transduced by pMLS-Scr, pMLS-867, or pMLS-868. Cells were plated in semisolid media 2 days after transduction. Error bars indicate standard deviation of the mean (n = 3). (H) Survival curves of sublethally irradiated mice transplanted with 104 MLL-AF9 cells transduced with pMLS-Scr, pMLS-867, or pMLS-868. GFP+ cells were sorted by FACS and transplanted 2 days after transduction.
To determine whether depletion of Jmjd1c also has an effect on AML maintenance in vivo, we transplanted 104 control or Jmjd1c-depleted primary MLL-AF9 leukemic cells into secondary recipients. Six of 7 mice transplanted with control cells succumbed to short-latency leukemia. In contrast, mice transplanted with Jmjd1c-depleted cells either did not show any symptoms or developed leukemia with a significantly longer latency compared with the control mice (P = .003; Figure 2H). At the end of the experiment, BM and spleen cells from the remaining mice were GFP− (ie, they have lost the shRNA expressing cells; data not shown). These data indicate that Jmjd1c plays a role in AML maintenance both in vitro and in vivo.
Mouse LSK cells exhibit only mild phenotype after Jmjd1c KD
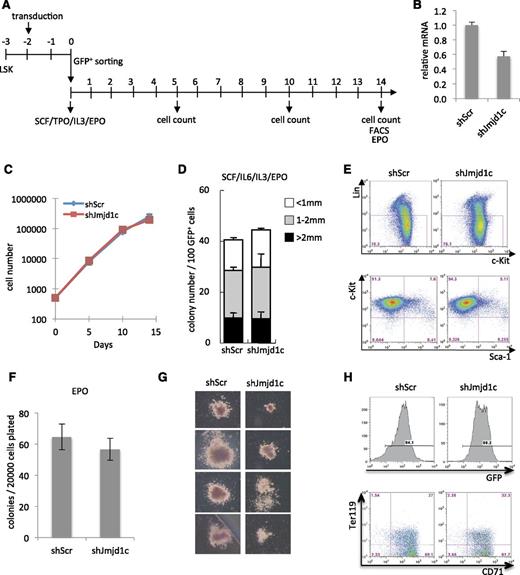
Since silencing of jmjd1c in zebrafish results in impaired erythrocyte and megakaryocyte development,42 and since inhibition or knockout of several genes involved in MLL-rearranged leukemia affect function of hematopoietic stem cells (HSCs) and/or erythroid progenitors (eg, Lsd19 and Myb43 ), we tested the effect of Jmjd1c depletion on survival and function of Lin–Sca-1+c-Kit+ (LSK) cells, with a particular focus on erythroid potential. To this end, we transduced LSK cells with Jmjd1c-targeting shRNA or control shRNA and followed proliferation of transduced cells for 14 days in media supporting self-renewal of HSCs (Figure 3A). Jmjd1c depletion was assessed by using Lin–Sca-1–c-Kit+ cells sorted in parallel to the LSK cells used in the experiment (Figure 3B). We did not observe differences in growth rate, colony size, or number between Jmjd1c-depleted and control cells (Figure 3C-D).
The effect of Jmjd1c KD on growth and differentiation of LSK cells. (A) Experimental overview. (B) Relative expression of Jmjd1c mRNA in Lin–Sca-1–c-Kit+ cells transduced with nontargeting control (shScr) or Jmjd1c-targeting shRNA (sh868) at day 2 after transduction. These cells were sorted in parallel with LSK cells used in the experiment. (C) Proliferation of shScr- and shJmjd1c-transduced LSK cells in liquid culture. (D) Number of hematopoietic colonies generated by shScr- and shJmjd1c-transduced LSK cells in semisolid media supplemented with stem cell factor (SCF), interleukin-6 (IL-6), IL-3 and erythropoietin (EPO). (E) Flow cytometry analysis of lineage markers c-Kit and Sca-1 expression in Jmjd1c-depleted and control cells 14 days after sort. (F) Number of erythroid colonies generated by shScr- and shJmjd1c-transduced LSK cells in semisolid culture supplemented with EPO. (G) Erythroid colony morphology generated by shScr- or shJmjd1c-transduced cells. (H) Flow cytometry analysis of Ter119 and CD71 expression in cultures generated in (F).
The effect of Jmjd1c KD on growth and differentiation of LSK cells. (A) Experimental overview. (B) Relative expression of Jmjd1c mRNA in Lin–Sca-1–c-Kit+ cells transduced with nontargeting control (shScr) or Jmjd1c-targeting shRNA (sh868) at day 2 after transduction. These cells were sorted in parallel with LSK cells used in the experiment. (C) Proliferation of shScr- and shJmjd1c-transduced LSK cells in liquid culture. (D) Number of hematopoietic colonies generated by shScr- and shJmjd1c-transduced LSK cells in semisolid media supplemented with stem cell factor (SCF), interleukin-6 (IL-6), IL-3 and erythropoietin (EPO). (E) Flow cytometry analysis of lineage markers c-Kit and Sca-1 expression in Jmjd1c-depleted and control cells 14 days after sort. (F) Number of erythroid colonies generated by shScr- and shJmjd1c-transduced LSK cells in semisolid culture supplemented with EPO. (G) Erythroid colony morphology generated by shScr- or shJmjd1c-transduced cells. (H) Flow cytometry analysis of Ter119 and CD71 expression in cultures generated in (F).
To also test the quality of Jmjd1c-depleted cells after 14 days of culture, we analyzed them for expression of Sca-1, c-Kit, and lineage markers and plated them in media that supported growth of erythroid progenitors. We did not detect notable differences in immunophenotype at day 14 (Figure 3E) or in the number of erythroid colonies formed by Jmjd1c-depleted and control cells (Figure 3F). However, Jmjd1c-depleted erythroid colonies were generally smaller than control colonies (Figure 3G), despite the fact that they contained similar percentages of Ter119+ erythroid cells (Figure 3H). Together, these data show that Jmjd1c depletion does not have a major impact on hematopoietic progenitors, with Jmjd1c KD efficiency similar to that of MLL-AF9 transformed hematopoietic cells.
Human leukemic cells are sensitive to depletion of JMJD1C
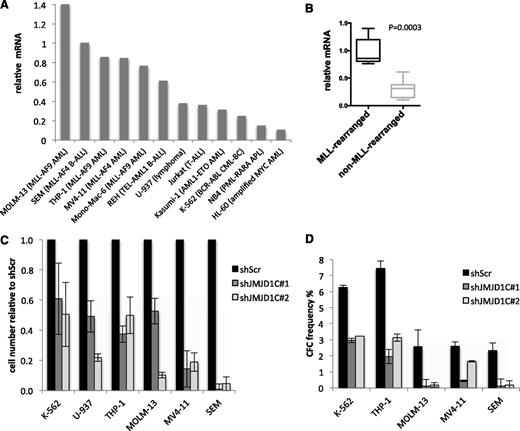
To understand whether JMJD1C is required for human leukemia, we first analyzed the expression of JMJD1C in 5 MLL-rearranged and 7 non–MLL-rearranged human leukemic cell lines. In agreement with studies on primary patient samples,44-48 leukemic cell lines with MLL rearrangements had significantly higher JMJD1C mRNA levels compared with cell lines with other mutations (Figure 4A-B). Despite these differences, both cell lines carrying MLL-AF9 or -AF4 rearrangements and leukemic cells lacking MLL fusions were sensitive to JMJD1C KD (Figure 4C and supplemental Figure 2). Similarly, colony-forming potential of all the cell lines tested was reduced upon JMJD1C depletion (Figure 4D). Importantly, growth of human osteosarcoma cell line U2OS was not affected by JMJD1C KD (supplemental Figure 3). Together, these data show that JMJD1C plays an important function in both human MLL-rearranged and non–MLL-rearranged leukemic cells. Since the strongest effect of JMJD1C depletion was observed in the human MLL-AF4 acute lymphoid leukemia cell line SEM, we chose this cell line for characterization of the JMJD1C KD phenotype. To this end, we generated an inducible system in which the expression of JMJD1C shRNAs is induced by IPTG.
Effect of JMJD1C depletion on human leukemic cells. (A) Relative JMJD1C mRNA levels in a panel of human leukemic cell lines normalized to mRNA levels in SEM cells. (B) Box plots of mean JMJD1C mRNA levels in MLL-rearranged and non–MLL-rearranged cell lines from (A). (C) Relative cell number of the indicated cell lines transduced with shScr, shJMJD1C#1, or shJMJD1C#2 at day 8 after GFP+ sort. Cells were sorted by FACS 4 days after transduction. Error bars indicate standard deviation (n = 3 technical replicates). See also supplemental Figure 2. (D) Number of colonies generated in semisolid media by GFP+ cells transduced with shScr, shJMJD1C#1, or shJMJD1C#2. Error bars indicate standard deviation (n = 3 technical replicates).
Effect of JMJD1C depletion on human leukemic cells. (A) Relative JMJD1C mRNA levels in a panel of human leukemic cell lines normalized to mRNA levels in SEM cells. (B) Box plots of mean JMJD1C mRNA levels in MLL-rearranged and non–MLL-rearranged cell lines from (A). (C) Relative cell number of the indicated cell lines transduced with shScr, shJMJD1C#1, or shJMJD1C#2 at day 8 after GFP+ sort. Cells were sorted by FACS 4 days after transduction. Error bars indicate standard deviation (n = 3 technical replicates). See also supplemental Figure 2. (D) Number of colonies generated in semisolid media by GFP+ cells transduced with shScr, shJMJD1C#1, or shJMJD1C#2. Error bars indicate standard deviation (n = 3 technical replicates).
Growth defect upon JMJD1C depletion is primarily due to increased apoptosis
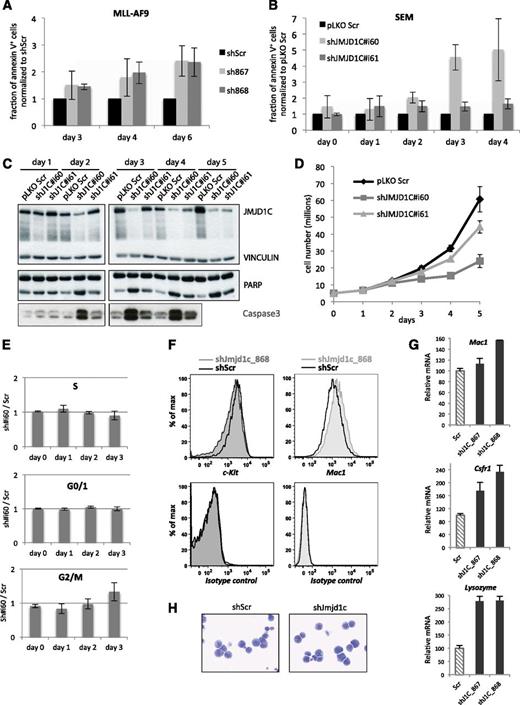
To investigate the growth defect caused by JMJD1C depletion, we measured the fraction of apoptotic annexin V–stained cells by flow cytometric analysis (Figure 5A-B). Mouse MLL-AF9 cells with Jmjd1c KD displayed increasing apoptotic percentages over time as compared with shScr. In SEM cells, induction of JMJD1C KD had a similar effect approximately 1 day after detectable reduction of protein levels by immunoblot (Figure 5B-C). Onset of apoptosis was accompanied by cleavage of caspase 3 and poly ADP ribose polymerase in these cells. These proapoptotic events were detectable at rates inversely correlating with JMJD1C protein levels (Figure 5C) and cell proliferation (Figure 5D). We nonetheless observed no cell cycle progression defects prior to the onset of apoptosis, since there was no accumulation of cells in S, G0/1 or G2/M phases, (Figure 5E). This suggests that apoptosis is not a result of cell cycle arrest but rather a direct effect of JMJD1C depletion in SEM cells.
JMJD1C depletion triggers apoptosis. (A) Relative percentage of annexin V–positive MLL-AF9 cells at 3, 4, and 6 days after transduction with nontargeting control (shScr) or shRNAs targeting Jmjd1c (sh867 and sh868). Average of 3 independent experiments; error bars indicate standard deviation. (B) Relative percentage of annexin V–positive SEM cells over a time course starting from IPTG induction at day 0. Average of 3 independent experiments, error bars indicate standard deviation. (C) Western blot showing JMJD1C, poly ADP ribose polymerase, and caspase 3 levels in control (pLKO Scr) and JMJD1C-depleted SEM cells (shJ1C#i60 and shJ1C#i61). Vinculin was used as a loading control. (D) Number of SEM cells over a time course of JMJD1C KD induction by IPTG. Fresh IPTG was added at days 0, 2, and 4. (E) Cell cycle analysis of JMJD1C-depleted SEM cells (sh#i60) compared with control cells (Scr). Error bars indicate standard deviation (n = 3 for days 0 to 2; n = 2 for day 3). (F) Flow cytometry analysis of c-Kit and Mac1 expression in MLL-AF9 cells with shScr or shJmjd1c. (G) Relative mRNA levels of the indicated genes in cells transduced with shScr or shJmjd1c. (H) Representative images of May-Grünwald-Giemsa–stained MLL-AF9 cells transduced with shScr or shJmjd1c.
JMJD1C depletion triggers apoptosis. (A) Relative percentage of annexin V–positive MLL-AF9 cells at 3, 4, and 6 days after transduction with nontargeting control (shScr) or shRNAs targeting Jmjd1c (sh867 and sh868). Average of 3 independent experiments; error bars indicate standard deviation. (B) Relative percentage of annexin V–positive SEM cells over a time course starting from IPTG induction at day 0. Average of 3 independent experiments, error bars indicate standard deviation. (C) Western blot showing JMJD1C, poly ADP ribose polymerase, and caspase 3 levels in control (pLKO Scr) and JMJD1C-depleted SEM cells (shJ1C#i60 and shJ1C#i61). Vinculin was used as a loading control. (D) Number of SEM cells over a time course of JMJD1C KD induction by IPTG. Fresh IPTG was added at days 0, 2, and 4. (E) Cell cycle analysis of JMJD1C-depleted SEM cells (sh#i60) compared with control cells (Scr). Error bars indicate standard deviation (n = 3 for days 0 to 2; n = 2 for day 3). (F) Flow cytometry analysis of c-Kit and Mac1 expression in MLL-AF9 cells with shScr or shJmjd1c. (G) Relative mRNA levels of the indicated genes in cells transduced with shScr or shJmjd1c. (H) Representative images of May-Grünwald-Giemsa–stained MLL-AF9 cells transduced with shScr or shJmjd1c.
We also explored the possibility that cells were being induced to differentiate. Mouse MLL-AF9 cells were stained with antibodies to c-Kit and Mac1 and analyzed by flow cytometry. The hematopoietic stem and progenitor cell (HSPC) marker c-Kit was slightly decreased in the Jmjd1c-depleted cells in comparison with the control cells (Figure 5F). Mac1 on the other hand was increased in the shJmjd1c-transduced vs the shScr-transduced cell population. In addition, qRT-PCR analysis revealed upregulation of Mac1, Csf1r, and lysozyme 2 transcript levels in Jmjd1c-depleted cells (Figure 5G). However, we did not observe striking differences in morphology between shScr- and shJmjd1c-transduced cells (Figure 5H). Together, these results indicate that Jmjd1c depletion triggers concomitant upregulation of myeloid differentiation markers and downregulation of the HSPC marker c-Kit in murine MLL-AF9 cells, suggestive of altered gene expression programs.
Early gene expression changes after JMJD1C KD in SEM cells
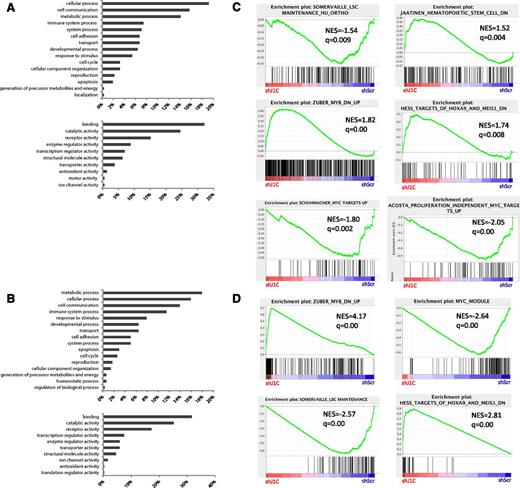
To assess the effect of JMJD1C depletion on transcription, we compared the transcriptome of human SEM and murine MLL-AF9 cells expressing shJMJD1C or shScr. To ensure that early changes would be detected, 48 hours was selected as the earliest time point displaying JMJD1C depletion and detectable phenotype as monitored by poly ADP ribose polymerase and caspase 3 cleavage in SEM cells (Figure 5C). A total of 138 transcripts were detected as changing between the two conditions in SEM cells (false discovery rate [FDR] <0.05) (supplemental Figure 4A-B and supplemental Table 5) and 451 transcripts in MLL-AF9 cells (FDR <0.25) (supplemental Figure 5A-C and supplemental Table 6). Classification of genes into Gene Ontologies (www.pantherdb.org)49 revealed a high percentage of changes in genes related to the same top 3 biologic process categories—cell communication, cellular and metabolic processes, and top 3 molecular functions—with genes coding for products with putative binding, catalytic, and receptor activities (Figure 6A-B). Few of the changes related to cell cycle and apoptosis-associated genes, thus confirming the data previously shown regarding cell cycle progression. Apoptotic-related genes were not expected to be enriched in this analysis, because this process is primarily regulated on a signaling-cascade level.
Gene expression changes upon JMJD1C KD. (A-B) Classification of genes with significant change in expression in (A) SEM cells and (B) MLL-AF9 cells into Gene Ontologies describing cellular function (top) and molecular activity (bottom). (C-D) Enrichment of indicated gene sets in JMJD1C KD vs (C) control SEM or (D) MLL-AF9 cells as revealed by GSEA. NES, normalized enrichment score; q value, false discovery rate. See also supplemental Table 2 and supplemental Figure 4C.
Gene expression changes upon JMJD1C KD. (A-B) Classification of genes with significant change in expression in (A) SEM cells and (B) MLL-AF9 cells into Gene Ontologies describing cellular function (top) and molecular activity (bottom). (C-D) Enrichment of indicated gene sets in JMJD1C KD vs (C) control SEM or (D) MLL-AF9 cells as revealed by GSEA. NES, normalized enrichment score; q value, false discovery rate. See also supplemental Table 2 and supplemental Figure 4C.
To explore the potential perturbation of gene expression programs upon JMJD1C KD, we performed GSEA50 by using previously published gene sets. Although the vast majority of these gene sets were not significantly enriched with JMJD1C KD, several oncogenic and pluripotent programs were revealed to be dependent on JMJD1C expression levels (FDR <0.05; 11.6%; Figure 6C-D and supplemental Table 2), including a leukemic stem cell (LSC) maintenance signature,51 genes downregulated in CD133+ HSCs compared with CD133– cells,52 gene sets upregulated by induction of c-MYC expression in human myelogenous53 and lymphoma cells,54 and genes defining an “Myc core module” in mouse embryonic stem cells.55 A gene set defined upon suppression of Myb in murine MLL-AF9 cells41 is also enriched in the KD of JMJD1C in SEM and MLL-AF9 cells. Moreover, genes that are downregulated in murine hematopoietic precursor cells conditionally expressing Hoxa9 and Meis156 are upregulated in both SEM and MLL-AF9 cells with JMJD1C KD, thus suggesting that JMJD1C levels are important for maintenance of these transformation programs in both species.
However, downregulation of JMJD1C increased expression of genes upregulated in pediatric AML with rearranged MLL compared with AML cases without MLL rearrangements48 and genes associated with MLL fusions irrespective of the lineage of the pediatric acute leukemia,46 suggesting an MLL-rearrangement independent role of JMJD1C. Taken together, these results suggest that by hindering one or several of its functions, JMJD1C suppression perturbs the leukemic expression programs irrespective of lineage and MLL-rearrangement status in mouse and human leukemia.
Ectopic expression of Myb or Myc partially rescues Jmjd1c KD phenotype
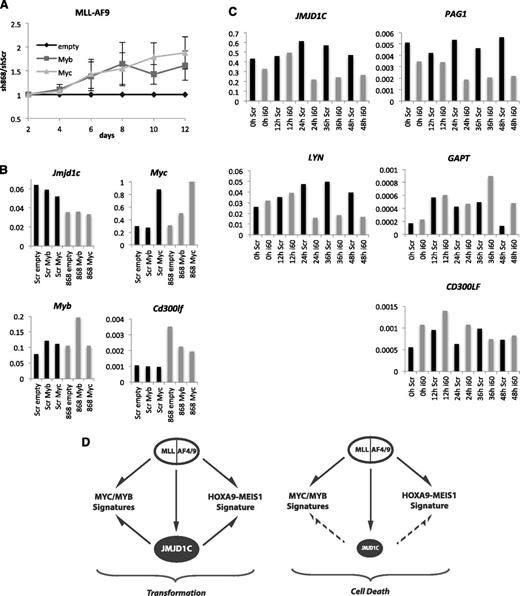
Since the gene expression changes following Jmjd1c KD strongly resemble changes after Myb depletion, we tested whether ectopic expression of Myb or its transcriptional target Myc can rescue the growth defect associated with depletion of Jmjd1c. To this end, we cotransduced murine MLL-AF9 cells with pMLS-YFP carrying either shScr or shJmjd1c and one of the pMSCV-GFP vectors— empty, Myb, or Myc—and followed the percentage of GFP+YFP+ cells over 2 weeks after transduction. While not fully rescuing Jmjd1c-depleted cells, overexpression of Myb or Myc provided a growth advantage over cells cotransduced with an empty vector (Figure 7A-B and supplemental Figure 6).
JMJD1C contributes to MLL-rearranged leukemia maintenance by affecting MYB, MYC, and HOXA9-MEIS1 gene expression programs. (A) MLL-AF9 cells were cotransduced with pMLS-YFP carrying shScr or shJmjd1c (868) and empty vector or pMSCV-GFP vector expressing mouse Myb or Myc complementary DNA. Normalized ratios of GFP+YFP+ cell percentages between shJmjd1c and shScr samples are plotted over a 10-day time course starting from day 2 after transduction. Average of 3 independent experiments. Error bars indicate standard deviation. (B) Relative mRNA levels of the indicated genes in MLL-AF9 cells from one of the experiments in (A). (C) Relative mRNA levels of the indicated genes in SEM cells over a 48-hour JMJD1C inducible KD time course with 12-hour intervals. (D) Model for MLL-rearranged implementation of transformation programs. JMJD1C, MYC, MYB, HOXA9, and MEIS1 are bound and their transcription is maintained by MLL-AF4 and MLL-AF9. In the presence of sufficient levels of JMJD1C, cells remain transformed. However, upon reduction of JMJD1C levels, transformation and stem cell programs are hindered through deregulation of MYC, MYB, and HOXA9-MEIS1 target gene expression, leading to cells displaying an apoptotic phenotype.
JMJD1C contributes to MLL-rearranged leukemia maintenance by affecting MYB, MYC, and HOXA9-MEIS1 gene expression programs. (A) MLL-AF9 cells were cotransduced with pMLS-YFP carrying shScr or shJmjd1c (868) and empty vector or pMSCV-GFP vector expressing mouse Myb or Myc complementary DNA. Normalized ratios of GFP+YFP+ cell percentages between shJmjd1c and shScr samples are plotted over a 10-day time course starting from day 2 after transduction. Average of 3 independent experiments. Error bars indicate standard deviation. (B) Relative mRNA levels of the indicated genes in MLL-AF9 cells from one of the experiments in (A). (C) Relative mRNA levels of the indicated genes in SEM cells over a 48-hour JMJD1C inducible KD time course with 12-hour intervals. (D) Model for MLL-rearranged implementation of transformation programs. JMJD1C, MYC, MYB, HOXA9, and MEIS1 are bound and their transcription is maintained by MLL-AF4 and MLL-AF9. In the presence of sufficient levels of JMJD1C, cells remain transformed. However, upon reduction of JMJD1C levels, transformation and stem cell programs are hindered through deregulation of MYC, MYB, and HOXA9-MEIS1 target gene expression, leading to cells displaying an apoptotic phenotype.
Because the expression of MYB or MYC does not change upon JMJD1C KD, we looked at the expression of several genes potentially contributing to the leukemic phenotype over a JMJD1C KD time course. Expression of Src family tyrosine kinase LYN and c-Src tyrosine kinase (CSK)-binding protein PAG1 was downregulated at 24 hours after induction, coinciding with the earliest decrease in JMJD1C mRNA levels (Figure 7C). Expression of Grb2-binding adaptor protein GAPT, which was reported to inhibit B-cell proliferation,57 was increased starting from 36 hours after induction. CD300LF was upregulated in mouse and human KD cells (supplemental Figures 4A and 5A,C) and in MLL-AF9 cells with Myb KD41 and was shown to mediate cell death in myeloid cells.58 Moreover, Cd300lf levels are partly restored with Myb or Myc overexpression in Jmjd1c-depleted cells, further implicating this gene in an Myb-Myc-Jmjd1c network (Figure 7B). However, the CD300LF upregulation was apparent already before IPTG induction in SEM cells (Figure 7C), which could be due to a potential leakiness of the inducible system and high sensitivity of CD300LF to JMJD1C depletion.
Discussion
In this study, we identify JMJD1C as exerting a key role in leukemia maintenance by using a focused shRNA library in a genetically defined mouse model of human MLL-AF9 leukemia. JMJD1C is a common MLL-AF4 and -AF9 target and is 1.6- to 3.2-fold upregulated in MLL-rearranged vs non–MLL-rearraged leukemias45,48,59 (Figure 4B). Higher expression in murine MLL-AF9 vs c-Kit–enriched cells (Figure 2E) and association with HSC self-renewal and MLL-AF9 transformation60 might suggest a proto-oncogene role of Jmjd1c in transformed blood cells. In contrast, Jmjd1c is not differentially expressed between high vs low LSC frequency MLL leukemia.51 Because of the large size of the JMJD1C coding sequence, we have not been able to ectopically express it in blood cells and address its transformation capability in different genetic backgrounds.
Our data indicate that depletion of Jmjd1c leads to differential growth impairment of normal hematopoietic and leukemic cells (Figures 1D-E, 2A-G, and 3). Lack of effect on nonleukemic cells is in agreement with a recent study reporting lack of overt phenotype in Jmjd1c knockout mice34 and suggests that Jmjd1c is a potential clinically relevant drug target. We performed a panel of assays that revealed apoptosis as being the most prominent effect of Jmjd1c depletion in mouse AML and human acute lymphoid leukemia cells. No effect on cell cycle progression was observed prior to onset of apoptosis, and we detected only mild downregulation of HSCP marker c-Kit in murine MLL-AF9 cells. We observed no increase in differentiated cell frequencies with Jmjd1c KD, thus precluding that the observed growth defect is due to cells terminally differentiating and exiting the cell cycle. Upregulation of myeloid differentiation markers, however, was apparent as measured by qRT-PCR, which implies that in addition to apoptosis onset, self-renewal transcription programs are being lost by the reduction of Jmjd1c levels (Figure 5).
Gene expression analysis in human SEM and murine MLL-AF9 cells enabled the detection of genes deregulated upon JMJD1C depletion. Importantly, these changes strongly correlated with the effect of the suppression of the key leukemia-promoting gene Myb. Correlation of JMJD1C KD with loss of the MLL-rearranged LSC signature and the CD133+ HSC and c-MYC signatures (Figure 6C-D), implicates JMJD1C as having a role in promoting self-renewal and transformation. Although the expression of Myb and Myc does not change upon Jmjd1c KD, the overexpression of either of them partially rescues Jmjd1c-depleted cells (Figure 7A-B), functionally confirming the link between Jmjd1c and the Myb-associated gene expression program. The upregulation of CD300LF expression in Myb-depleted cells41 as well as in human and mouse JMJD1C-depleted cells (supplemental Tables 5 and 6) suggests that it contributes to the JMJD1C suppression phenotype in both human and mouse transformed cells. Indeed, the mRNA levels of Cd300lf were partially rescued with overexpression of Myb or Myc in Jmjd1c-depleted cells (Figure 7B).
Downregulation of Src family kinase LYN and its regulator PAG1 appear to be primary effects of JMJD1C KD in SEM cells (Figure 7C). Whether JMJD1C directly regulates these genes remains unknown, since we have failed to obtain reliable chromatin immunoprecipitation data with several JMJD1C antibodies. In addition, and in agreement with two recent studies,34,35 we have not detected any JMJD1C H3K9 demethylase activity in vitro or by overexpression in HEK293 cells, nor did the KD generate global accumulation of H3K9 methyl marks in SEM cells (supplemental Figure 7). Moreover, a study recently reported a nonhistone target of JMJD1C demethylase activity.61 It is therefore possible that JMJD1C also exerts an indirect function in gene transcription regulation.
The correlation of the JMJD1C KD expression profile in SEM and murine MLL-AF9 cells with several of the expression signatures ties JMJD1C to MLL fusion–dependent transformation programs.41,51,56 JMJD1C function, however, is neither exclusive to nor entirely overlapping with MLL-rearranged leukemia. The KD profile either did not correlate or inversely correlated with defined MLL-rearranged signatures46,48 or an MLL-AF4 target gene set in SEM cells30 (supplemental Figure 4C), and non–MLL-rearranged cell lines are also affected by JMJD1C depletion (Figure 4C-D). We show that in the case of MLL fusion–driven leukemia, JMJD1C expression levels are required for the maintenance of transformation programs (Figure 7D). Taken together, our findings implicate JMJD1C as a crucial gene in leukemia and qualify it as a potential therapeutic target in leukemia subtypes spanning a range of lineages and MLL-rearranged cytogenetic status.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Michael T. Hemann for the pMLS vector, Michael Cleary for the pMSCV-MLL-AF9-neo plasmid, Johannes Zuber for the pMSCV-Myb-PGK-puro-IRES-GFP and pMSCV-Myc-PGK-puro-IRES-GFP plasmids, Jurriaan J. Hölzenspies for help with the Agilent microarrays, Cornelia Steinhauer for assistance with epi-library construction, Anna Fossum for cell sorting, Jens V. Johansen for expression data processing, and Jacob Engelbrecht for library alignment. We thank members of the Helin and Porse laboratories for discussions, technical advice, and support.
P.S. was supported by a Danish Medical Research Council Fellowship, European Molecular Biology Organization Long-Term Postdoctoral Fellowship, and Marie Curie Intra-European Fellowship. The research was supported by the Danish National Research Foundation (DNRF82), the European Union grant BLUEPRINT (FP7/2011, 282510), the Lundbeck Foundation, The Novo Nordisk Foundation, and the Danish Council for Strategic Research (12-110503).
Authorship
Contribution: P.S., V.A.C., J.-P.B., S.M., B.P., and K.H. conceived and designed the experiments; P.S., V.A.C., J.-P.B., and S.M. performed the experiments; P.S., V.A.C., J.-P.B., S.M., F.O.B., and K.H. analyzed the data; P.S., V.A.C., J.-P.B., S.M., J.W., M.B.S., and F.O.B. contributed reagents, materials, and analysis tools; P.S., V.A.C., and K.H. wrote the paper; and J.-P.B. and F.O.B. assisted with writing.
Conflict-of-interest disclosure: K.H. is a cofounder of EpiTherapeutics, works as a consultant for the company, and has shares and warrants in the company. The remaining authors declare no competing financial interests.
Correspondence: Kristian Helin, Biotech Research and Innovation Centre, University of Copenhagen, Ole Maaløes Vej 5, 2200 Copenhagen, Denmark; e-mail: kristian.helin@bric.ku.dk.
References
Author notes
P.S., V.A.C., and J.-P.B. contributed equally to this study.