Key Points
VcR-CVAD produced high overall and CR rates in previously untreated MCL patients.
No substantial difference in 3-year PFS or OS was observed in patients receiving ASCT compared with patients receiving maintenance rituximab.
Rituximab, bortezomib, modified hyper-cyclophosphamide, doxorubicin, vincristine, dexamethasone (VcR-CVAD) induction chemoimmunotherapy and maintenance rituximab (MR) were evaluated for efficacy and safety in Eastern Cooperative Oncology Group protocol E1405. Patients with previously untreated mantle cell lymphoma received VcR-CVAD chemotherapy every 21 days for 6 cycles, followed by MR for 2 years. Transplant-eligible patients had the option of autologous stem cell transplantation (ASCT) consolidation instead of MR. The primary end point was the complete response (CR) rate to VcR-CVAD. The secondary end points were overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and toxicities. Seventy-five eligible patients with a median age of 62 (range 40-76) were enrolled. The ORR was 95% and a CR was achieved in 68% of patients. After a median follow-up of 4.5 years, 3-year PFS and OS were 72% and 88%, respectively. No substantial difference in PFS or OS was observed between patients treated with MR (n = 44) vs ASCT (n = 22). There were no unexpected toxicities. VcR-CVAD produced high ORR and CR rates in mantle cell lymphoma. MR after VcR-CVAD induction performed similarly to ASCT and may improve response duration. Randomized clinical trials comparing MR against ASCT should be considered and randomized clinical trials evaluating bortezomib’s contribution to conventional therapy are under way. This study was registered at www.clinicaltrials.gov as #NCT00433537.
Introduction
Mantle cell lymphoma (MCL) remains a therapeutic challenge, with no standard approach to initial therapy. Although the median age at diagnosis for MCL is in the seventh decade, the population of patients with MCL is heterogeneous and encompasses both older adults with diminished tolerance for treatment-related toxicities and younger patients who may be able to tolerate more intensive initial therapy. Traditional chemotherapy regimens such as rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) have been associated with only modest rates of complete responses (CR, 30% to 45%), with median durations of progression-free survival (PFS) of 16 to 29 months.1,-3 Based on the poor quality and durability of remissions with R-CHOP, there has been interest in developing better induction regimens to improve PFS in MCL and achieving this objective with a regimen that can be tolerated by both older and younger adults with MCL.
The modified rituximab, hyperfractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone (R-hyperCVAD) regimen, given with maintenance rituximab (MR), was developed with the goal of less myelosuppression and improved tolerability.4 This regimen included “part A” of conventional R-hyperCVAD chemotherapy, minus the day 11 vincristine and mid-cycle dexamethasone, administered every 28 days, and without administration of alternating cycles of “part B,” containing cytarabine and methotrexate.5 The addition of MR was hypothesized to prolong duration of remission, as has been observed with MR in other indolent non-Hodgkin lymphomas.6,,,-10 The experience with modified R-hyperCVAD and MR (n = 22, median age 64), reported by the Wisconsin Oncology Network, demonstrated an overall response (OR) and CR rate to induction of 77% and 64%, respectively, and a 3-year PFS of 50%, with similar outcomes observed for older and younger patients.4 After a median follow-up of 62 months, the 5-year overall survival (OS) was 62% with a median survival of 70 months.11 These promising results led to the inclusion of modified R-hyperCVAD with MR as a therapeutic option for older adults in the National Comprehensive Cancer Network treatment guidelines for MCL.12
Bortezomib was incorporated into modified the R-hyperCVAD regimen in a follow-up Wisconsin Oncology Network study.13 The new regimen was named VcR-CVAD (in which Vc is Velcade [bortezomib]). Bortezomib, a first-in-class proteasome inhibitor, was a logical choice for inclusion because it has single-agent activity in MCL and inhibits several pathways central to MCL biology, including nuclear factor κB (NFκB) signaling.14,,,-18 In this pilot trial of 30 patients, median age 61, VcR-CVAD with MR demonstrated a CR rate of 77%, an OR rate of 90%, and 3-year PFS and OS of 63% and 86%, respectively.13 These results were considered promising and worthy of additional study. Of note, 7 of the first 14 patients enrolled experienced painful peripheral neuropathy when bortezomib at 1.5 mg/m2 was administered in combination with vincristine at 2 mg. With dose modification of both agents (vincristine 1 mg on day 3, bortezomib 1.3 mg/m2 on days 1 and 4), only 1 in 16 final patients enrolled experienced painful peripheral neuropathy. This study employed MR × 5 years, and 25% of patients were unable to complete the planned MR because of development of hypogammaglobulinemia and recurrent infections.13
With lessons learned regarding bortezomib/vincristine dosing and duration of MR, investigators within the Eastern Cooperative Oncology Group (ECOG) designed a large phase 2 trial (E1405) of VcR-CVAD induction chemotherapy following by MR for 2 years in patients with previously untreated MCL. Recognizing that many clinicians prefer intensive strategies for younger MCL patients, the protocol permitted the triage of patients to autologous stem cell transplantation (ASCT) rather than MR. The primary endpoint of E1405 was the CR rate to VcR-CVAD induction therapy. The major secondary end point was PFS of the regimen, with planned analysis of both MR and ASCT patients.
Methods
Study design
This was a multicenter, open-label phase 2 study of VcR-CVAD induction chemotherapy followed by consolidation therapy with MR in previously untreated MCL. The study was approved by the Human Subjects Committee and institutional review board at each participating institution, and all patients signed an informed consent document describing the investigational nature of the proposed treatment. The study was conducted in accordance with the Declaration of Helsinki and was registered with www.clinicaltrials.gov (#NCT00433537). The study was open from May 1, 2007, to October 24, 2008, with results reported through June 17, 2013.
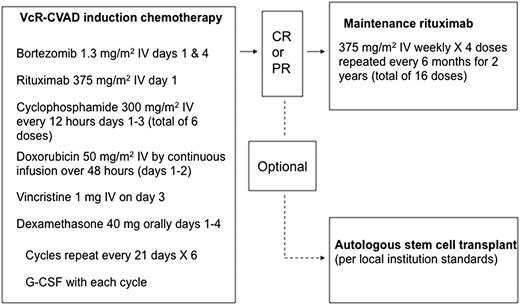
Patients received 6 cycles of induction chemoimmunotherapy (VcR-CVAD regimen) every 21 days (Figure 1). VcR-CVAD induction chemoimmunotherapy consisted of rituximab 375 mg/m2 intravenously (IV) on day 1, bortezomib 1.3 mg/m2 IV on days 1 and 4, cyclophosphamide 300 mg/m2 IV every 12 hours on days 1 through 3 (for a total of 6 doses), doxorubicin 50 mg/m2 IV as a continuous infusion days 1 and 2 (total dose over 48 hours equal to 50 mg/m2), vincristine 1 mg IV day 3, and dexamethasone 40 mg orally on days 1 through 4 of each 21-day cycle. Patients achieving at least a partial response (PR) to induction therapy were then eligible to proceed with MR therapy, 375 mg/m2 weekly × 4 every 6 months for 16 doses, to be initiated 4 to 8 weeks after completion of chemotherapy. Patients received growth factor support with either pegfilgrastim or granulocyte colony-stimulating factor 5 mcg/kg per day beginning on day 5 or 6 of each induction treatment cycle and continuing until absolute neutrophil count was ≥2000/mm3 past the nadir. All appropriate supportive care measures, including tumor lysis syndrome prophylaxis, transfusion support, and antibiotic usage, were employed at the discretion of the treating physician in accordance with local institution standards.
Treatment plan for VcR-CVAD induction and MR. G-CSF, granulocyte colony-stimulating factor.
Treatment plan for VcR-CVAD induction and MR. G-CSF, granulocyte colony-stimulating factor.
Patients determined to be eligible for consolidative ASCT after induction VcR-CVAD were permitted to discontinue protocol therapy and undergo ASCT using the treating institution’s local guidelines (Figure 1). Patients were permitted to undergo stem cell collection, but without administration of additional chemotherapy, after cycles 4, 5, or 6 of VcR-CVAD, or after the completion of VcR-CVAD once the response to induction chemotherapy had been determined. Patients who underwent ASCT continued to be followed for PFS and OS outcomes. Protocol therapy was discontinued in the event of unacceptable toxicity, disease progression, or patient and/or physician discretion.
Patients
Patients ≥18 years of age with histologically proven, previously untreated MCL were eligible for enrollment. Confirmation of the diagnosis of MCL required presence of appropriate morphology and presence of nuclear cyclin D1 by immunohistochemistry, or presence of t(11;14) by fluorescence in situ hybridization, polymerase chain reaction, or conventional karyotyping. Submission of pathologic materials for diagnostic review and classification was required. Ki67 scoring was not required.
Patients were required to have measurable disease and an ECOG performance status ≤2. Hematologic parameters included neutrophil count >1500/mm3 and platelets >100 K/mm3 (unless low counts were attributable to marrow involvement by lymphoma or splenomegaly). Adequate organ function was defined as serum creatinine <2 mg/dL, bilirubin <2 mg/dL (unless resulting from Gilbert disease or lymphomatous involvement of liver), and cardiac ejection fraction >45%.
Patients were excluded if they were pregnant, breastfeeding, or known to have HIV infection or central nervous system involvement by lymphoma. Patients with preexisting grade ≥2 peripheral neuropathy were excluded. Patients with prior malignancies were excluded unless the malignancy was in situ or had been treated with definitive local therapy with a subsequent ≥3-year disease-free survival. Patients were required to have discontinued any adjuvant hormonal therapy at least 3 months before registration.
Assessment of adverse events
All eligible patients were included in safety assessments. Dose modifications for toxicity were specified in the protocol. Toxicities were reported in accordance with the Common Terminology Criteria for Adverse Events, version 3.0.
Efficacy assessment
Response was assessed among eligible patients using the 2007 Revised Response Criteria for Malignant Lymphoma.19 Computed tomography imaging was required for tumor assessment at baseline, after VcR-CVAD cycles 2, 4, and 6, every 6 months for 5 years, and then yearly thereafter. Patients were followed for response until progression and for survival for 10 years from study entry. Positron emission tomography (PET) imaging was required after VcR-CVAD induction for patients with residual masses >1.5 cm. Similarly, PET imaging was required after MR in patients with residual masses >1.5 cm and who were not previously documented to be in CR.
Statistical methods
The primary end point of this study was to determine the CR rate in patients treated with VcR-CVAD. A CR rate >75% with VcR-CVAD would be considered promising, whereas a CR rate <59% would be unacceptable. Patients were enrolled in a 2-stage design. If at least 20 CRs were observed in the first 31 eligible patients, an additional 37 patients (assuming at least 33 were evaluable for response) were to be enrolled in stage 2. With 64 eligible patients, the power to detect a promising CR rate was 90% with 1-sided type I error of 10%. Study accrual continued during the interim analysis. Two-stage confidence intervals (CI) were used to describe the CR rate.20 The study regimen would be considered promising if the 80% CI of CR (corresponding to a test with a 1-sided type I error of 10%) excluded the null hypothesis of 59%.
The analysis of secondary objectives was primarily descriptive. The overall response rate (ORR) to VcR-CVAD was reported with the 95% exact binomial CI. PFS for the whole group was defined as the time from study entry until lymphoma progression or death from any cause. For patients not known to have progressed or died, PFS was censored at last disease assessment date. OS for the whole group was defined as the date of study entry until date of death. Patients not known to have died were censored for OS at last date known alive. The PFS and OS in subgroups of patients receiving MR or undergoing ASCT after VcR-CVAD induction were calculated from the start of MR or ASCT. The PFS and OS were estimated using the Kaplan-Meier method.21 Comparisons of patient characteristics between arms were based on Wilcoxon rank-sum tests for continuous variables and were based on Fisher exact tests for categorical variables. All CIs reported were 2-sided.
Toxicity was monitored continuously throughout the study, with descriptive statistics provided of observed events. Toxicities reported were worst-grade toxicities per patient during the induction period and during the maintenance period.
Results
Patients
Seventy-seven patients were enrolled, with 2 patients excluded following central pathology review. Of the 75 patients included in the final analysis, 65 diagnostic biopsies were confirmed by central pathology review and 10 were included based on review of submitted reports. The median age of the patient population was 62 (range 40-76), with a predominance of men (77%). The majority of patients (96%) had an ECOG performance status ≤1, and 92% had stage III or IV disease. A high Mantle Cell International Prognostic Index (MIPI) score was reported in 22% of patients, and 5% had the blastic subtype of MCL (Table 1).
Baseline characteristics (n = 75)
| . | n . | % . |
|---|---|---|
| Age | ||
| Median (range) | 62 (40-76) | |
| Gender | ||
| Male | 58 | 77 |
| Female | 17 | 23 |
| Ann Arbor stage | ||
| I/II | 6 | 8 |
| III/IV | 69 | 92 |
| Performance status | ||
| 0 | 49 | 65 |
| 1 | 23 | 31 |
| 2 | 3 | 4 |
| MIPI score | ||
| Low risk | 28 | 41 |
| Intermediate risk | 26 | 38 |
| High risk | 15 | 22 |
| Unknown* | 6 | |
| B symptoms | ||
| Present | 17 | 23 |
| Absent | 57 | 77 |
| Unknown | 1 | |
| Lactate dehydrogenase | ||
| Elevated | 30 | 41 |
| Not elevated | 44 | 59 |
| Unknown | 1 | |
| Bone marrow involvement | ||
| Yes | 59 | 80 |
| No | 15 | 20 |
| Unknown | 1 | |
| White blood count (×1000), per mm3 | ||
| Median (range) | 7.2 (3.0-57.7) | |
| Unknown | 5 | |
| . | n . | % . |
|---|---|---|
| Age | ||
| Median (range) | 62 (40-76) | |
| Gender | ||
| Male | 58 | 77 |
| Female | 17 | 23 |
| Ann Arbor stage | ||
| I/II | 6 | 8 |
| III/IV | 69 | 92 |
| Performance status | ||
| 0 | 49 | 65 |
| 1 | 23 | 31 |
| 2 | 3 | 4 |
| MIPI score | ||
| Low risk | 28 | 41 |
| Intermediate risk | 26 | 38 |
| High risk | 15 | 22 |
| Unknown* | 6 | |
| B symptoms | ||
| Present | 17 | 23 |
| Absent | 57 | 77 |
| Unknown | 1 | |
| Lactate dehydrogenase | ||
| Elevated | 30 | 41 |
| Not elevated | 44 | 59 |
| Unknown | 1 | |
| Bone marrow involvement | ||
| Yes | 59 | 80 |
| No | 15 | 20 |
| Unknown | 1 | |
| White blood count (×1000), per mm3 | ||
| Median (range) | 7.2 (3.0-57.7) | |
| Unknown | 5 | |
Five patients had baseline white blood cell count missing and 1 patient had baseline lactate dehydrogenase missing; therefore, the MIPI score could not be calculated.
A comparison of baseline characteristics in patients receiving MR vs ASCT after induction VcR-CVAD is shown in Table 2. The median age is higher in the MR arm, as expected, but there is no difference in MIPI risk scoring or other MIPI factors (performance status, white blood count, or lactate dehydrogenase) between the 2 groups.
Baseline characteristics of patients receiving MR vs ASCT
| . | MR (n = 44) . | ASCT (n = 22) . | P value* . |
|---|---|---|---|
| Age | |||
| Median (range) | 63 (40-76) | 57 (48-68) | .02 |
| WBC (×1000), per mm3 | |||
| Median (range) | 6.7 (4.2-57.7) | 7.5 (3.0-48.8) | .16 |
| Unknown | 2 | 1 | |
| Performance status | |||
| 0 | 26 (59%) | 18 (82%) | .21 |
| 1 | 16 (36%) | 4 (18%) | |
| 2 | 2 (5%) | 0 (0%) | |
| Lactate dehydrogenase | |||
| Elevated | 17 (40%) | 6 (27%) | .42 |
| Not elevated | 26 (60%) | 16 (73%) | |
| Unknown | 1 | 0 | |
| MIPI | |||
| Low risk | 16 (39%) | 10 (48%) | .74 |
| Intermediate risk | 16 (39%) | 8 (38%) | |
| High risk | 9 (22%) | 3 (14%) | |
| Unknown | 3 | 1 | |
| VcR-CVAD response | |||
| CR | 31 (70%) | 20 (91%) | .07 |
| PR | 13 (30%) | 2 (9%) | |
| . | MR (n = 44) . | ASCT (n = 22) . | P value* . |
|---|---|---|---|
| Age | |||
| Median (range) | 63 (40-76) | 57 (48-68) | .02 |
| WBC (×1000), per mm3 | |||
| Median (range) | 6.7 (4.2-57.7) | 7.5 (3.0-48.8) | .16 |
| Unknown | 2 | 1 | |
| Performance status | |||
| 0 | 26 (59%) | 18 (82%) | .21 |
| 1 | 16 (36%) | 4 (18%) | |
| 2 | 2 (5%) | 0 (0%) | |
| Lactate dehydrogenase | |||
| Elevated | 17 (40%) | 6 (27%) | .42 |
| Not elevated | 26 (60%) | 16 (73%) | |
| Unknown | 1 | 0 | |
| MIPI | |||
| Low risk | 16 (39%) | 10 (48%) | .74 |
| Intermediate risk | 16 (39%) | 8 (38%) | |
| High risk | 9 (22%) | 3 (14%) | |
| Unknown | 3 | 1 | |
| VcR-CVAD response | |||
| CR | 31 (70%) | 20 (91%) | .07 |
| PR | 13 (30%) | 2 (9%) | |
WBC, white blood cell.
P values were based on Wilcoxon rank-sum tests for continuous variables and on Fisher exact tests for categorical variables.
Treatment administered
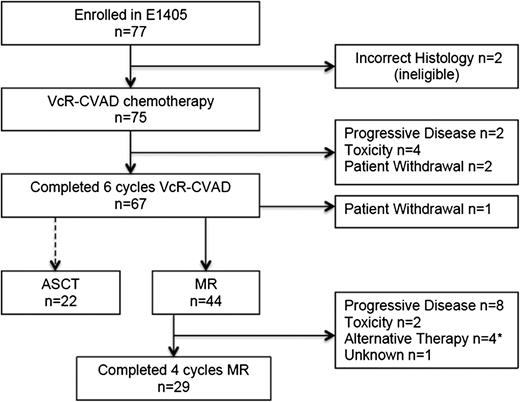
Of the 75 eligible patients treated with VcR-CVAD induction therapy, 67 (89%) completed the full treatment course. Reasons for not completing VcR-CVAD induction (Figure 2) included disease progression (n = 2), adverse events or side effects (n = 4), or patient withdrawal from study treatment (n = 2). Adverse events and toxicities leading to VcR-CVAD discontinuation included neutropenic sepsis after 4 cycles, severe infusion-related reaction with cycle 1 of rituximab, grade 4 syncope after 3 cycles related to severe aortic stenosis requiring urgent repair, and pneumonia/pneumonitis after cycle 3.
Throughput of patients enrolled in E1405. *Includes 1 patient who received ASCT after 1 cycle of MR.
Throughput of patients enrolled in E1405. *Includes 1 patient who received ASCT after 1 cycle of MR.
Twenty-nine (66%) of the 44 patients who initiated MR completed the entire maintenance course (Figure 2). Disease progression was the most common reason for MR discontinuation (n = 8); other reasons included adverse events (n = 2), initiation of an alternative antilymphoma therapy in the absence of progression (n = 3), ASCT after the first MR course (n = 1), and unknown (n = 1). The adverse events leading to MR discontinuation were persistent grade 3 fatigue possibly attributable to rituximab and grade 3 infection. After induction VcR-CVAD chemotherapy, 22 patients elected to undergo ASCT as consolidation therapy. One patient who had completed the induction therapy withdrew from the study.
Responses
Among the 75 eligible patients, 51 achieved CR and 20 achieved PR. Two patients had progressive disease during induction treatment, and another 2 were unevaluable after receiving only 1 treatment cycle as a result of a severe rituximab infusion reaction and patient withdrawal. These 4 patients were counted as nonresponders when calculating response rate. The ORR was 95% (95% CI, 87-99), with a CR rate of 68% (95% CI, 57-79; 80% CI, 61-76). The 80% CI of CR excluded the null hypothesis of 59%, implying that the results met the prespecified criteria for concluding that the study regimen was promising. Of the 20 patients with PRs, 11 were reported as such because of protocol violations. These included missing end-of-induction bone marrow biopsy (n = 5), PET scan (n = 3), or both (n = 3). Using 1999 lymphoma response criteria (computed tomography scan only), the ORR was 95% and the CR/CR unconfirmed rate was 63% (47/75).
Survival outcomes
Survival outcomes are reported after a median duration of follow-up of 4.5 years.
The 3-year PFS from start of MR for the patients receiving MR (n = 44) is 67% (95% CI, 52-85, Figure 3B). Table 3 provides PFS and OS estimates at 2, 3, and 4 years for the eligible patient population from study entry and subgroups treated with MR vs ASCT consolidation from start of MR/ASCT. OS at 3 years was 91% (95% CI, 83-100) for patients treated with MR and was 96% (95% CI, 87-100) for patients who received ASCT (Figure 4B). After adjustment for MIPI prognostic factors and quality of response to VcR-CVAD (CR vs PR), no statistically significant differences in PFS or OS were observed between the 2 arms (supplemental Table 1, available on the Blood Web site). High-risk MIPI scores were associated with inferior OS ( supplemental Table 2).
PFS. (A) PFS from study entry in all eligible patients (n = 75). The median follow-up for PFS is 3.1 years. (B) PFS from start of MR/ASCT by subgroups receiving MR (n = 44) vs ASCT (n = 22). The median follow-up for PFS is 3.4 years for the MR group and 2.6 years for the ASCT group.
PFS. (A) PFS from study entry in all eligible patients (n = 75). The median follow-up for PFS is 3.1 years. (B) PFS from start of MR/ASCT by subgroups receiving MR (n = 44) vs ASCT (n = 22). The median follow-up for PFS is 3.4 years for the MR group and 2.6 years for the ASCT group.
Survival probabilities*
| . | Eligible population (n = 75) . | MR (n = 44) . | ASCT (n = 22)† . |
|---|---|---|---|
| PFS (95% CI) | |||
| 2 years | 0.76 (0.66-0.87) | 0.79 (0.67-0.92) | 0.76 (0.58-1.00) |
| 3 years | 0.72 (0.62-0.84) | 0.67 (0.52-0.85) | – |
| 4 years | 0.46 (0.31-0.69) | 0.59 (0.42-0.83) | – |
| OS (95% CI) | |||
| 2 years | 0.95 (0.90-1.00) | 0.93 (0.86-1.00) | 1.00 (1.00-1) |
| 3 years | 0.88 (0.81-0.96) | 0.91 (0.83-1.00) | 0.96 (0.87-1) |
| 4 years | 0.84 (0.76-0.93) | 0.86 (0.77-0.97) | 0.91 (0.79-1) |
| . | Eligible population (n = 75) . | MR (n = 44) . | ASCT (n = 22)† . |
|---|---|---|---|
| PFS (95% CI) | |||
| 2 years | 0.76 (0.66-0.87) | 0.79 (0.67-0.92) | 0.76 (0.58-1.00) |
| 3 years | 0.72 (0.62-0.84) | 0.67 (0.52-0.85) | – |
| 4 years | 0.46 (0.31-0.69) | 0.59 (0.42-0.83) | – |
| OS (95% CI) | |||
| 2 years | 0.95 (0.90-1.00) | 0.93 (0.86-1.00) | 1.00 (1.00-1) |
| 3 years | 0.88 (0.81-0.96) | 0.91 (0.83-1.00) | 0.96 (0.87-1) |
| 4 years | 0.84 (0.76-0.93) | 0.86 (0.77-0.97) | 0.91 (0.79-1) |
The PFS and OS in the entire population were calculated from study entry, whereas the PFS and OS for the subgroups of patients receiving MR or ASCT after VcR-CVAD induction were calculated from the start of MR or ASCT.
All patients in the ASCT arm had either progressed, died, or were censored within 2 years posttransplant; therefore, the 3- and 4-year PFS cannot be estimated.
OS. (A) OS from study entry in all eligible patients (n = 75). (B) OS from start of MR/ASCT by subgroups receiving MR (n = 44) vs ASCT (n = 22).
OS. (A) OS from study entry in all eligible patients (n = 75). (B) OS from start of MR/ASCT by subgroups receiving MR (n = 44) vs ASCT (n = 22).
Assessment of safety
Toxicities experienced during VcR-CVAD induction were primarily hematologic (Table 4), with grade 3/4 neutropenia (15%/69%) and thrombocytopenia (23%/44%) being the most frequently observed toxicities. Despite the high rates of grade 3/4 neutropenia, events of neutropenic fever and infection were lower. Neutropenic fever occurred in 11% of patients, and neutropenic infections were observed in 6% of patients. Grade 3/4 nausea and vomiting were uncommon (each occurring in 1% of patients). More common constitutional complaints with induction chemotherapy included fatigue (10%, grade 3/4) and anorexia (5%, grade 3). Grade 3 diarrhea and dehydration were each observed in 4% of treated patients. There were no treatment-related deaths observed during induction chemotherapy. There were no reports of grade 3/4 peripheral neuropathy.
VcR-CVAD induction: grade 3-4 toxicities* (% patients), n = 75
| . | Grade 3, % . | Grade 4, % . |
|---|---|---|
| Hematologic | ||
| Neutropenia | 15 | 69 |
| Hemoglobin | 32 | 1 |
| Platelets | 23 | 44 |
| Neutropenic fever | 8 | 3 |
| Neutropenic infection | 5 | 1 |
| Infection without grade 3-4 neutropenia | 7 | 1 |
| Fatigue | 9 | 1 |
| Anorexia | 5 | – |
| Nausea | 1 | – |
| Vomiting | 1 | – |
| Constipation | 1 | – |
| Diarrhea | 4 | – |
| Dehydration | 4 | – |
| Mucositis/stomatitis | 3 | – |
| Abdominal pain | 4 | – |
| Dyspnea | 3 | – |
| Thrombosis/embolism | 3 | 1 |
| Hyperglycemia | 9 | – |
| . | Grade 3, % . | Grade 4, % . |
|---|---|---|
| Hematologic | ||
| Neutropenia | 15 | 69 |
| Hemoglobin | 32 | 1 |
| Platelets | 23 | 44 |
| Neutropenic fever | 8 | 3 |
| Neutropenic infection | 5 | 1 |
| Infection without grade 3-4 neutropenia | 7 | 1 |
| Fatigue | 9 | 1 |
| Anorexia | 5 | – |
| Nausea | 1 | – |
| Vomiting | 1 | – |
| Constipation | 1 | – |
| Diarrhea | 4 | – |
| Dehydration | 4 | – |
| Mucositis/stomatitis | 3 | – |
| Abdominal pain | 4 | – |
| Dyspnea | 3 | – |
| Thrombosis/embolism | 3 | 1 |
| Hyperglycemia | 9 | – |
Toxicities reported are worst-grade toxicities per patient during the induction period among eligible patients.
Toxicities observed during MR are shown in Table 5. Neutropenia was the most common toxicity during MR (11% grade 3, 9% grade 4), with the majority of cases related to delayed neutropenia following rituximab exposure. Grade 3 infections occurred in 11% of patients during MR, although only 2% of events were associated with documented neutropenia.
MR grade 3-4 toxicities* (% patients), n = 44
| . | Grade 3, % . | Grade 4, % . |
|---|---|---|
| Neutropenia | 11 | 9 |
| Hemoglobin | – | 2 |
| Platelets | 2 | – |
| Infections without grade 3-4 neutropenia† | 9 | – |
| Infections with neutropenia | 2 | – |
| Elevated transaminases | 2 | – |
| Hypokalemia | 2 | – |
| Hyponatremia | 2 | – |
| Fatigue | 5 | – |
| Pain | 5 | – |
| Insomnia | 2 | – |
| Syncope | 5 | – |
| Hypoxia | 2 | – |
| Dysphagia | 2 | – |
| . | Grade 3, % . | Grade 4, % . |
|---|---|---|
| Neutropenia | 11 | 9 |
| Hemoglobin | – | 2 |
| Platelets | 2 | – |
| Infections without grade 3-4 neutropenia† | 9 | – |
| Infections with neutropenia | 2 | – |
| Elevated transaminases | 2 | – |
| Hypokalemia | 2 | – |
| Hyponatremia | 2 | – |
| Fatigue | 5 | – |
| Pain | 5 | – |
| Insomnia | 2 | – |
| Syncope | 5 | – |
| Hypoxia | 2 | – |
| Dysphagia | 2 | – |
Toxicities reported are worst-grade toxicities per patient during maintenance period among eligible patients.
Includes 2 respiratory infections for which documentation of neutrophil count was not available.
Discussion
In E1405, the VcR-CVAD induction was well tolerated and highly efficacious in a typical MCL population. The majority of patients assigned to MR remained in remission at 4 years, results comparable to published reports using intensive strategies.5,22,,-25 The findings from this study raise 3 major questions: (1) Does bortezomib add efficacy to conventional cytotoxic chemotherapy? (2) What is the optimal dose and duration of MR? (3) Can nonintensive strategies produce outcomes similar to intensive treatment strategies?
Testing bortezomib in combination with a standard chemoimmunotherapy backbone was warranted. The single-agent activity of bortezomib demonstrated in MCL led to US Food and Drug Administration (FDA) approval for relapsed/refractory MCL in 2006.16 Additionally, bortezomib potentiates chemotherapy in preclinical models of multiple myeloma and MCL.14,26 Bortezomib has been combined with standard agents in other lymphoma subtypes, with encouraging results.27,-29 In MCL, bortezomib has been combined with conventional R-hyperCVAD alternating with rituximab, methotrexate, and cytarabine.30 Twenty patients were evaluated in this phase 1 study, and the toxicity of the combination did not appear different than that of the parent regimen. Bortezomib was also combined with rituximab, vincristine, doxorubicin, dexamethasone, and chlorambucil as part of a frontline strategy for older MCL patients (ages 65-80).31 This phase 2 study from the Groupe Ouest Est d'Etude des Leucémies et Autres Maladies du Sang also generated high CR rates (59%).
The major toxicity from the VcR-CVAD regimen was the expected myelosuppression. Importantly, we did not observe excessive painful peripheral neuropathy using the dose and schedule of vincristine and bortezomib selected for this trial.13 Nearly 90% of patients were able to complete all 6 cycles of induction therapy, compared with just 61% who were able to complete conventional R-hyperCVAD with alternating R-methotrexate and cytarabine in a SWOG study.25 The CR rate of 68% is encouraging and possibly an underestimate of the true CR rate. Eleven of 20 PR patients were coded as such because of missing end-of-treatment PET imaging or bone marrow evaluations (all protocol violations). The CR rate in the 64 completely restaged patients was 80%.
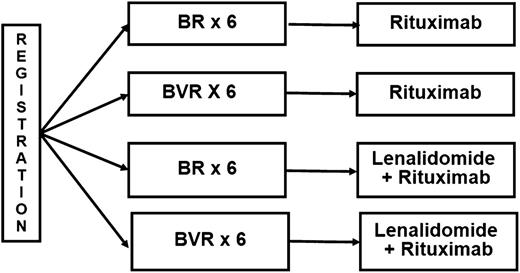
E1405 met the protocol-specified signal of “promising” for the CR rate, suggesting that incorporation of bortezomib adds efficacy to conventional therapy and providing the rationale for further development of bortezomib in frontline MCL regimens. These results informed the design of E1411, an ongoing US intergroup trial (www.clinicaltrials.gov, #NCT01415752) in previously untreated MCL patients age ≥60. E1411 is evaluating the contribution of bortezomib to a bendamustine-rituximab (BR) backbone in a randomized controlled trial (Figure 5).
Schema of E1411. Maintenance therapy is administered for 2 years. B, bendamustine; R, rituximab; V, bortezomib.
Schema of E1411. Maintenance therapy is administered for 2 years. B, bendamustine; R, rituximab; V, bortezomib.
Although not definitive, E1405 adds to existing data supporting the benefit of MR in MCL. Previous reports of MR following the modified R-hyperCVAD induction with or without bortezomib suggested improved PFS.4,11,13 The European MCL consortium has reported clinical benefit for MR in a large randomized clinical trial. In a 2 × 2 design, MCL patients older than age 60 were randomized to fludarabine, cyclophosphamide, and rituximab vs R-CHOP induction with a second randomization to maintenance therapy with rituximab, 1 dose every 2 months, or interferon-α until disease progression.32 This analysis found a significant improvement in sustained 4-year remission in patients receiving MR (58% with MR vs 29% with interferon-α).32 However, questions remain about the optimal dosing and duration of MR therapy, with MR schedules ranging from 4 weekly doses every 6 months × 2 years, to a single dose every 3 months × 5 years, to a single dose every 2 months until disease progression. A prior report using a 5-year MR schedule after a VcR-CVAD induction noted an intolerance rate of >20% resulting from recurrent infections in the setting of neutropenia, and frequently with concurrent hypogammaglobulinemia.13 In the European MCL study cited previously, rates of grade 3/4 neutropenia with MR occurred in 24% of patients, although most infectious complications were grade 1/2 (31% with grade 1/2 infection, 9% with grade 3/4 infections).32 Neutropenia was common with MR in the present study (20% of patients with grade 3/4 neutropenia), although it did not result in infections for the majority of patients. The optimal dosing and duration of MR should be the subject of future research. In the current US intergroup trial (E1411), MR is administered, with or without lenalidomide, as a single dose every 2 months for 2 years.
We did not find a substantial improvement in PFS or OS in patients assigned to ASCT consolidation compared with patients assigned to MR, despite the older age of patients assigned to MR (63 vs 57, P = .02). However, this should be considered hypothesis-generating only because the 2 arms were not randomized, the follow-up time is relatively short, and the study was not powered to detect a difference between arms. Nonetheless, with the increasing role of MR, and as novel agents are developed in frontline MCL regimens, the role of ASCT consolidation in first remission should be the subject of additional study.
The long-term outcomes of patients receiving nonintensive therapy on E1405 compare favorably with other US cooperative group trials testing intensive regimens, despite the E1405 patients being slightly older and at higher risk by MIPI (Table 6). For example, when considering all 75 eligible patients who received VcR-CVAD and censoring the 22 ASCT patients at time of transplant, 3-year PFS and OS were 72% and 86%, respectively, whereas patients treated with conventional R-hyperCVAD with alternating R-methotrexate/cytarabine on SWOG 0213 experienced a 3-year PFS and OS of 66% and 81%, respectively.25 The Cancer and Leukemia Group B reported on an intensive immunochemotherapy regimen followed by ASCT in 78 previously untreated MCL patients and demonstrated a 3-year PFS and OS of 63% and 83%, respectively.23
Recent US cooperative group experience in frontline MCL trials
| Trial . | n . | Median age . | MIPI (%) . | 3 years . | 3 years . |
|---|---|---|---|---|---|
| Low/Int/High . | PFS (%) . | OS (%) . | |||
| ECOG 1405 | 75* | 62 | 41/38/22 | 72 | 86 |
| SWOG 012325 | 49 | 57 | 55/31/14 | 66 | 81 |
| CALGB 5990923 | 78 | 57 | 53/31/15 | 63 | 83 |
| Trial . | n . | Median age . | MIPI (%) . | 3 years . | 3 years . |
|---|---|---|---|---|---|
| Low/Int/High . | PFS (%) . | OS (%) . | |||
| ECOG 1405 | 75* | 62 | 41/38/22 | 72 | 86 |
| SWOG 012325 | 49 | 57 | 55/31/14 | 66 | 81 |
| CALGB 5990923 | 78 | 57 | 53/31/15 | 63 | 83 |
CALGB, Cancer and Leukemia Group B; int, intermediate.
ASCT patients (n = 22) censored at time of transplant.
In recent years, a trend has emerged to investigate intensive therapy strategies in younger patients and nonintensive strategies in older patients.22,32,33 The OS results of the current trial are also comparable to trials testing intensive strategies and limited to patients age 65 and younger. A report by the Groupe d’Etude des Lymphomes de l'Adulte investigated standard R-CHOP alternating with R-DHAP induction before ASCT.34 The Nordic Lymphoma Group reported outcomes after an intensive maxi-CHOP induction including rituximab and high-dose cytarabine, followed by ASCT.22 Both of these trials demonstrated 4-year OS rates of approximately 80%. One trial has observed a borderline significant improvement in OS with the inclusion of high-dose cytarabine into an intensive treatment platform including ASCT.33 If ultimately proven true, high-dose cytarabine inclusion is likely to become standard on regimens designed for younger MCL patients.
Treatment options for MCL are changing rapidly. Bendamustine has emerged as an attractive chemotherapy option for older MCL patients.35,36 Lenalidomide is now FDA approved for relapsed/refractory MCL with prior bortezomib exposure, based upon a single-agent response rate of 26%.37 The combination of lenalidomide and rituximab in MCL appears even more promising with response rates of 57% in a phase 2 clinical trial.38 These data support the randomized comparison of rituximab vs lenalidomide/rituximab as maintenance therapy in E1411. Novel agents targeting phosphatidylinositol-3-kinase-δ and bcl-2 are also showing promise in relapsed/refractory MCL.39,40 Perhaps most promising of all is the discovery of Bruton tyrosine kinase as a therapeutic target in MCL.41 Ibrutinib, an oral Bruton tyrosine kinase inhibitor, demonstrated a single-agent response rate of 68% and median PFS of 13.9 months in patients with relapsed/refractory MCL and is now FDA approved for this population.42
In summary, the VcR-CVAD induction regimen, designed as an induction therapy applicable to virtually all newly diagnosed MCL patients, generated a high ORR (95%) and a promising CR rate (68%) in this US cooperative group trial. The regimen was well tolerated in a typical MCL population with a median age of 62 and patients up to age 76 enrolling in the study. The overall regimen, including MR or ASCT consolidation, has produced encouraging PFS and OS. The results of this trial informed the design of E1411, the current North American intergroup trial for MCL. Patients are randomized to BR or BR plus bortezomib for 6 cycles followed by MR for 2 years, given with or without lenalidomide (Figure 5). Accrual to E1411 is ongoing and may define a new standard of care, particularly in older individuals with MCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, chair) and supported in part by Public Health Service Grants (CA21115, CA23318, CA66636, CA27525, CA21076, and CA14958) and from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authorship
Contribution: J.E.C. wrote and edited the manuscript; H.L. provided data analysis and wrote and edited the manuscript; M.R.S., E.M.P. and S.J.H. provided protocol development and edited the manuscript; R.D.G. and D.T.Y. provided pathology review and edited the manuscript; R.H.A. provided patient enrollment and edited the manuscript; and B.S.K. provided protocol development, data analysis, and wrote and edited the manuscript.
Conflict-of-interest disclosure: J.E.C. received research funding from Celgene and Genentech. M.R.S. received consulting fees from Millennium and Genentech/Roche. R.D.G. received consulting fees from Genentech/Roche. R.H.A. received Research funding from Genentech/Roche. S.J.H. is employed by Genentech/Roche. B.S.K. received consulting fees and research funding from Genentech/Roche and Millennium. The remaining authors declare no competing financial interests.
Correspondence: Brad S. Kahl, University of Wisconsin School of Medicine and Public Health, 1111 Highland Ave, 4059 Wisconsin Institute for Medical Research, Madison, WI 53705; e-mail: bsk@medicine.wisc.edu.