Key Points
Occurrence of t-AML/MDS after Hodgkin lymphoma is a rare event correlating with the intensity of first-line chemotherapy.
Allogeneic stem cell transplantation appears to improve the generally poor prognosis of patients with t-AML/MDS after Hodgkin lymphoma.
Therapy-related acute myeloid leukemia and myelodysplastic syndromes (t-AML/MDS) represent severe late effects in patients treated for Hodgkin lymphoma (HL). Because more recent data are scarce, we retrospectively analyzed incidence, outcome, and risk factors for the development of t-AML/MDS after HL. A total of 11 952 patients treated for newly diagnosed HL within German Hodgkin Study Group trials between 1993 and 2009 were considered. At a median follow-up of 72 months, t-AML/MDS was diagnosed in 106/11 952 patients (0.9%). Median time from HL treatment to t-AML/MDS was 31 months. The median age of patients with t-AML/MDS was higher than in the whole patient group (43 vs 34 years, P < .0001). Patients who received 4 or more cycles of BEACOPPescalated had an increased risk to develop t-AML/MDS when compared with patients treated with less than 4 cycles of BEACOPPescalated or no BEACOPP chemotherapy (1.7% vs 0.7% vs 0.3%, P < .0001). The median overall survival (OS) for all t-AML/MDS patients was 7.2 months. However, t-AML/MDS patients proceeding to allogeneic stem cell transplantation had a significantly better outcome with a median OS not reached after a median follow-up of 41 months (P < .001).
Introduction
Because of improved treatment strategies, Hodgkin lymphoma (HL) has become one of the malignancies with the best cure rates in adult oncology. At present, more than 80% of patients achieve long-term remission and can be considered cured when receiving adequate first-line treatment consisting of chemotherapy optionally combined with radiotherapy (RT). Because remission and cure rates can hardly be further improved, prevention of treatment-related late effects without compromising efficacy has become a major challenge in clinical HL research.1
Therapy-related acute myeloid leukemia and myelodysplastic syndromes (t-AML/MDS) represent particularly severe treatment-related late sequelae. They typically occur 2 to 8 years after initial HL treatment and were previously reported to have a very poor prognosis.2,,-5
The current standard protocols for the treatment of HL include drugs that are known to be leukemogenic. In particular, alkylating agents and topoisomerase-II inhibitors are associated with an increased risk for the development of t-AML/MDS.4,5
Because data on incidence, outcome, and risk factors for the development of t-AML/MDS among HL patients treated with modern approaches are scarce, we retrospectively analyzed 11 952 patients who had received treatment of newly diagnosed HL within German Hodgkin Study Group (GHSG) trial protocols between 1993 and 2009. Particular attention was paid to the treatment of t-AML/MDS since the use of allogeneic stem cell transplantation (aSCT) has become increasingly common. A relevant proportion of patients developing t-AML/MDS after other malignancies achieved long-term remission when treated with aSCT.6,7
Patients and methods
Patients and HL treatment
A total of 11 952 patients aged 16 to 75 with newly diagnosed HL and treated within GHSG trials between 1993 and 2009 were included in this retrospective analysis. According to their clinical stage and the presence or absence of the clinical risk factors, risk factor a was considered a large mediastinal mass larger than one-third of the maximum intrathoracic diameter detected by chest radiography, risk factor b extranodal disease, risk factor c elevated erythrocyte sedimentation rate (ESR) >50 mm/hour in patients without B symptoms and >30 mm/h in patients with B symptoms, and risk factor d involvement of 3 or more nodal areas, patients were allocated to 3 risk groups: early favorable stages (stages I/II without risk factors), early unfavorable stages (stages I/IIA with risk factors a-d, stage IIB with risk factors c-d), and advanced stages (stage IIB with risk factors a-b, stage III/IV). Patients with early favorable stages were treated within the trials HD7 (1993-1998), HD10 (1998-2003), and HD13 (2003-2009); patients with early unfavorable stages were enrolled in HD8 (1993-1998), HD11 (1998-2003), or HD14 (2003-2009); patients with advanced stages were treated within the HD9 (1993-1998), HD12 (1998-2003), HD15 (2003-2008), PROFE (2004-2007), or BEACOPP-14 pilot (1997-2000) trials. Except for those randomized into the HD7 RT arm alone, all patients received chemotherapy consisting of Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), ABVD variants (AV, ABV, AVD), cyclophosphamide, vincristine, procarbazine, prednisone (COPP)/ABVD or bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) variants (BEACOPPbaseline, BEACOPPescalated, BEACOPP-14) either alone or followed by RT (Table 1). All trials were conducted in accordance with the Declaration of Helsinki and are described in detail elsewhere.8,,,,,,,,,-18 This is a retrospective analysis including data of patients initially treated within prospective clinical studies. These studies were approved by institutional review boards before beginning study enrolment.
Distribution of patients according to the chemotherapy and radiotherapy received; cumulative doses of leukemogenic drugs contained in the chemotherapy protocols used
| . | Total . | . | |
|---|---|---|---|
| n . | % . | Cumulative chemotherapy doses . | |
| Chemotherapy received | |||
| None | 316 | 2.6 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× AV | 152 | 1.3 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× ABV | 190 | 1.6 | C: 0 mg/m2, P. 0 mg/m2, E: 0 mg/m2 |
| 2× AVD | 409 | 3.4 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× ABVD | 1 333 | 11.2 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× COPP/ABVD | 1 113 | 9.3 | C: 2 600 mg/m2, P: 2 800 mg/m2, E: 0 mg/m2 |
| 4× ABVD | 2 058 | 17.2 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 4× COPP/ABVD | 277 | 2.3 | C: 5 200 mg/m2, P: 5 600 mg/m2, E: 0 mg/m2 |
| 4× BEACOPPbaseline | 680 | 5.7 | C: 2 600 mg/m2, P: 2 800 mg/m2, E: 1 200 mg/m2 |
| 2× BEACOPPescalated + 2× ABVD | 759 | 6.4 | C: 2 500 mg/m2, P: 1 400 mg/m2, E: 1 200 mg/m2 |
| 8× BEACOPPbaseline | 483 | 4.0 | C: 5 200 mg/m2, P: 5 600 mg/m2, E: 2 400 mg/m2 |
| 6× BEACOPPescalated | 694 | 5.8 | C: 7 500 mg/m2, P: 4 200 mg/m2, E: 3 600 mg/m2 |
| 8× BEACOPP-14 | 777 | 6.5 | C: 5 200 mg/m2, P: 5 600 mg/m2, E: 2 400 mg/m2 |
| 4× BEACOPPescalated + 4× BEACOPPbaseline | 784 | 6.6 | C: 7 600 mg/m2, P: 5 600 mg/m2, E: 3 600 mg/m2 |
| 8× BEACOPPescalated | 1 927 | 16.1 | C: 10 000 mg/m2, P: 5 600 mg/m2, E: 4 800 mg/m2 |
| Total | 11 952 | 100.0 | |
| Radiotherapy received | |||
| None | 3 200 | 26.8 | |
| <EF | 7 567 | 63.3 | |
| EF | 1 185 | 9.9 | |
| Total | 11 952 | 100.0 | |
| . | Total . | . | |
|---|---|---|---|
| n . | % . | Cumulative chemotherapy doses . | |
| Chemotherapy received | |||
| None | 316 | 2.6 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× AV | 152 | 1.3 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× ABV | 190 | 1.6 | C: 0 mg/m2, P. 0 mg/m2, E: 0 mg/m2 |
| 2× AVD | 409 | 3.4 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× ABVD | 1 333 | 11.2 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 2× COPP/ABVD | 1 113 | 9.3 | C: 2 600 mg/m2, P: 2 800 mg/m2, E: 0 mg/m2 |
| 4× ABVD | 2 058 | 17.2 | C: 0 mg/m2, P: 0 mg/m2, E: 0 mg/m2 |
| 4× COPP/ABVD | 277 | 2.3 | C: 5 200 mg/m2, P: 5 600 mg/m2, E: 0 mg/m2 |
| 4× BEACOPPbaseline | 680 | 5.7 | C: 2 600 mg/m2, P: 2 800 mg/m2, E: 1 200 mg/m2 |
| 2× BEACOPPescalated + 2× ABVD | 759 | 6.4 | C: 2 500 mg/m2, P: 1 400 mg/m2, E: 1 200 mg/m2 |
| 8× BEACOPPbaseline | 483 | 4.0 | C: 5 200 mg/m2, P: 5 600 mg/m2, E: 2 400 mg/m2 |
| 6× BEACOPPescalated | 694 | 5.8 | C: 7 500 mg/m2, P: 4 200 mg/m2, E: 3 600 mg/m2 |
| 8× BEACOPP-14 | 777 | 6.5 | C: 5 200 mg/m2, P: 5 600 mg/m2, E: 2 400 mg/m2 |
| 4× BEACOPPescalated + 4× BEACOPPbaseline | 784 | 6.6 | C: 7 600 mg/m2, P: 5 600 mg/m2, E: 3 600 mg/m2 |
| 8× BEACOPPescalated | 1 927 | 16.1 | C: 10 000 mg/m2, P: 5 600 mg/m2, E: 4 800 mg/m2 |
| Total | 11 952 | 100.0 | |
| Radiotherapy received | |||
| None | 3 200 | 26.8 | |
| <EF | 7 567 | 63.3 | |
| EF | 1 185 | 9.9 | |
| Total | 11 952 | 100.0 | |
C, cyclophosphamide; E, etoposide; EF, extended-field; P, procarbazine.
Follow-up
Follow-up examinations were conducted regularly at the participating trial centers. According to the GHSG recommendations, follow-up visits took place every 3 months for the first half year, every 6 months until the fourth year, and once a year thereafter. Besides medical history and clinical examination, a laboratory analysis including full blood cell count, ESR, and blood chemistry was mandatory at every follow-up visit. Results including the possible detection of hematological or solid secondary malignancies were sent to the GHSG trial coordination center. For patients diagnosed with t-AML/MDS, date of diagnosis with t-AML/MDS, age at diagnosis, and time between HL treatment and the occurrence of t-AML/MDS were recorded. Cytomorphology of t-AML/MDS was described according to the French-American-British/World Health Organization classifications. Cytogenetic and molecular genetic features were documented if available. The same is true for the treatment modality applied for t-AML/MDS, the result of the antileukemic treatment and the clinical outcome.
With respect to the completeness of follow-up information, 74% of patients (78% of patients with HL progression or relapse in the course of follow-up; 73% of patients without HL recurrence in the course of follow-up) considered for this retrospective study had their last reported follow-up visit within the past 2 years before the most recent analysis of the GHSG trial protocol within which they were treated. The median time interval between the last reported follow-up and the most recent analysis of the respective study was 3.7 years among patients developing t-AML/MDS after HL treatment and 1.1 years for patients who did not develop t-AML/MDS. This difference can be explained by the significantly higher death rate among the t-AML/MDS patients in comparison with those without t-AML/MDS; when the calculations are restricted to survivors, the median time interval was 1.3 years for patients with t-AML/MDS and 1.0 years for patients not developing t-AML/MDS. Thus, follow-up quality and t-AML/MDS do not seem to be strongly associated.
Statistical methods
Cumulative incidences of t-AML/MDS and their variances were estimated, and differences between treatment groups were tested using the method of Pepe and Mori.19 Differences in overall survival (OS) after t-AML/MDS diagnosis between patients undergoing aSCT and patients not undergoing aSCT were tested using the log-rank test.
Multivariate analysis of risk of t-AML/MDS was performed using a Cox proportional hazards model.20 The dependent variable was time to any t-AML/MDS; a sensitivity analysis was performed on time to t-AML/MDS as the first event (censoring at HL progression or relapse or another secondary malignancy prior to t-AML/MDS). All analyses were stratified according to GHSG stage and treatment group (early favorable stages, early unfavorable stages, advanced stages). First, a limited number of plausible explanatory variables were fitted: age (linear), sex, clinical stage (I to IV, linear), amount of BEACOPP chemotherapy (none, BEACOPP chemotherapy with less than 4 cycles of BEACOPPescalated, 4 or more cycles of BEACOPPescalated), and amount of RT (none, less than extended-field RT [EF-RT], EF-RT). Second, a larger set of possible explanatory variables, namely those listed previously plus B symptoms (present, not present), performance status, elevated ESR, elevated lactate dehydrogenase, and the remaining International Prognostic Index criteria low serum albumin (<4 g/dL), low hemoglobin (<10.5 g/dL), leukocytosis (≥15 000/mm3), and lymphocytopenia (<600/mm3 or <8% of the white blood cell count or both) (each yes/no), were fitted.21
Results
Patient characteristics
A total of 11 952 patients enrolled in 11 GHSG trials for newly diagnosed HL were considered for this retrospective study. Median follow-up was 72 months. Within the follow-up period, 106 patients were diagnosed with t-AML/MDS resulting in an overall t-AML/MDS rate of 0.9%. Of these 106 patients, 12 had had HL progression or relapse and 2 had had a secondary malignancy other than t-AML/MDS before the diagnosis of t-AML/MDS.
Median time from HL treatment to diagnosis of t-AML/MDS was 31 months, with most cases occurring between 12 and 36 months after initial treatment (Table 2). The median age of patients developing t-AML/MDS was significantly higher than in the whole patient group (43 years vs 34 years, P < .0001). The distribution according to the HL risk groups also differed between patients with t-AML/MDS and the whole patient group. Although 25%, 34%, and 41% of all patients had been treated for early favorable, early unfavorable, and advanced HL, respectively, only 5% of those who developed t-AML/MDS had had treatment of early favorable HL, 20% had been treated for early unfavorable HL, and 75% had received treatment of advanced-stage disease (Table 3).
Time from HL treatment to t-AML/MDS diagnosis
| Time from HL to t-AML/MDS, years . | n . | % . | Cumulative n . | Cumulative % . |
|---|---|---|---|---|
| <1 | 21 | 19.81 | 21 | 19.81 |
| 1-<3 | 43 | 40.57 | 64 | 60.38 |
| 3-<5 | 19 | 17.92 | 83 | 78.30 |
| 5-<7 | 13 | 12.26 | 96 | 90.57 |
| 7-<10 | 10 | 9.43 | 106 | 100.00 |
| Time from HL to t-AML/MDS, years . | n . | % . | Cumulative n . | Cumulative % . |
|---|---|---|---|---|
| <1 | 21 | 19.81 | 21 | 19.81 |
| 1-<3 | 43 | 40.57 | 64 | 60.38 |
| 3-<5 | 19 | 17.92 | 83 | 78.30 |
| 5-<7 | 13 | 12.26 | 96 | 90.57 |
| 7-<10 | 10 | 9.43 | 106 | 100.00 |
Patient characteristics and amount of treatment received for HL
| . | Patients with t-AML/MDS (n = 106) . | All patients (n = 11 952) . |
|---|---|---|
| Sex | ||
| Male | 53% | 56% |
| Age | ||
| Median (range) | 43 (16-71) | 34 (16-75) |
| HL risk group | ||
| Early favorable | 5% | 25% |
| Early unfavorable | 20% | 34% |
| Advanced | 75% | 41% |
| First-line chemotherapy for HL | ||
| No BEACOPP | 23% | 49% |
| <4 cycles BEACOPPescalated | 19% | 23% |
| ≥4 cycles BEACOPPescalated | 58% | 28% |
| Radiotherapy for HL | ||
| No RT | 45% | 27% |
| <EF | 41% | 63% |
| EF | 14% | 10% |
| . | Patients with t-AML/MDS (n = 106) . | All patients (n = 11 952) . |
|---|---|---|
| Sex | ||
| Male | 53% | 56% |
| Age | ||
| Median (range) | 43 (16-71) | 34 (16-75) |
| HL risk group | ||
| Early favorable | 5% | 25% |
| Early unfavorable | 20% | 34% |
| Advanced | 75% | 41% |
| First-line chemotherapy for HL | ||
| No BEACOPP | 23% | 49% |
| <4 cycles BEACOPPescalated | 19% | 23% |
| ≥4 cycles BEACOPPescalated | 58% | 28% |
| Radiotherapy for HL | ||
| No RT | 45% | 27% |
| <EF | 41% | 63% |
| EF | 14% | 10% |
Impact of treatment and other factors on the risk of t-AML/MDS
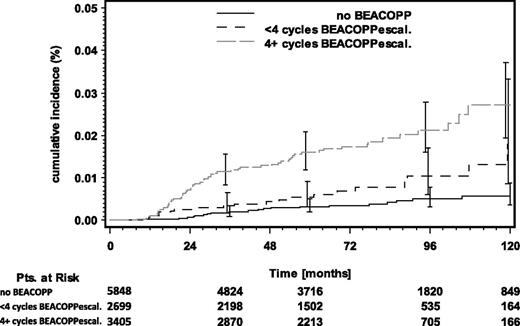
To better evaluate the correlation between the intensity of first-line HL chemotherapy and the risk of developing t-AML/MDS, patients were divided into 3 groups: (1) patients who had not been treated with BEACOPP chemotherapy; (2) patients receiving BEACOPP chemotherapy containing less than 4 cycles of BEACOPPescalated; and (3) patients receiving treatment containing 4 or more cycles of BEACOPPescalated. Among the 11 952 patients included in this analysis, 49% had not received BEACOPP chemotherapy, 23% had received BEACOPP chemotherapy with less than 4 cycles of BEACOPPescalated, and 28% had been treated with 4 or more cycles of BEACOPPescalated. In contrast, among the 106 t-AML/MDS patients considered, 23% had received no BEACOPP chemotherapy, 19% had received BEACOPP chemotherapy with less than 4 cycles of BEACOPPescalated, and 4 or more cycles of BEACOPPescalated had been applied in 58% of cases (Table 3). The cumulative incidence rate (CIR) of t-AML/MDS 6 years after HL treatment differed significantly among the 3 groups. While the CIR for patients who had been treated without BEACOPP chemotherapy or with less than 4 cycles of BEACOPPescalated was 0.3% and 0.7%, respectively, patients treated with 4 or more cycles of BEACOPPescalated had a CIR of 1.7% (P < .0001) (Figure 1).
Cumulative incidence rates of t-AML/MDS grouped by the amount of BEACOPP chemotherapy received. Pts, patients.
Cumulative incidence rates of t-AML/MDS grouped by the amount of BEACOPP chemotherapy received. Pts, patients.
Among the 14 patients with either HL relapse or a second malignancy other than t-AML/MDS before t-AML/MDS diagnosis, 12 had received BEACOPPbaseline (n = 3), BEACOPP-14 (n = 1), or BEACOPPescalated (n = 8) chemotherapy as first-line treatment of advanced HL, whereas 2 patients had been treated with COPP/ABVD for early unfavorable HL. Eight patients with HL recurrence had had high-dose chemotherapy followed by autologous stem cell transplantation (ASCT), conventional chemotherapy had been applied in 3 patients, and 1 patient had received localized RT only as salvage therapy. Generally, patients with HL progression or relapse had a higher risk for the development of t-AML/MDS than patients without HL recurrence (1.5% vs 0.8%). However, final conclusions regarding the contributions of first-l and second-line treatments are difficult to draw on the basis of the present data because of the variable treatments applied at initial diagnosis and at relapse, respectively. Both cases of secondary malignancies before t-AML/MDS diagnosis had been non-Hodgkin lymphoma (NHL). Treatment of NHL had consisted of bendamustine and mitoxantrone in 1 patient, whereas the second patient had not received additional treatment after splenectomy.
Demographic and clinical variables were tested in a multivariate analysis for a possible association with the development of t-AML/MDS. These variables included age, sex, clinical stage, the number of BEACOPP cycles (no BEACOPP, BEACOPP chemotherapy with less than 4 cycles of BEACOPPescalated, 4 or more cycles of BEACOPPescalated), and the extent of RT (no RT, less than EF-RT, EF-RT). As a result, older age (P < .0001), treatment containing 4 or more cycles of BEACOPPescalated (P = .024), and EF-RT (P = .0001) were identified as factors associated with an increased risk of developing t-AML/MDS. When compared with the group of patients not treated with BEACOPP chemotherapy, hazard ratios for the subsequent development of t-AML/MDS were 1.95 (P = .14) and 3.35 (P = .011) for patients who had received less than 4 cycles of BEACOPPescalated and 4 or more cycles of BEACOPPescalated, respectively. Similar results were obtained when times to t-AML/MDS were censored at HL progression or relapse or another secondary malignancy before t-AML/MDS diagnosis. Inclusion of additional possible explanatory variables in the model also did not change the results appreciably, but 2 further unexpected variables were significantly associated with an elevated t-AML/MDS risk: low hemoglobin (P = .0042) and lack of B symptoms (P = .012) at initial HL diagnosis.
Characteristics, treatment, and outcome of patients with t-AML/MDS after HL
The results of cytogenetic and/or molecular genetic evaluations were available for 61 of 106 patients: 7/61 (11%) had a normal karyotype; abnormalities in chromosome 5 and/or chromosome 7 were found in 8/61 patients (13%); mixed-lineage leukemia (MLL) rearrangement was detected in 19/61 patients (31%); 14/61 patients (23%) had a complex karyotype; and 13/61 patients (21%) had other alterations (Table 4).
Characteristics of t-AML/MDS patients, genetic alterations, and response to induction chemotherapy in 95 patients undergoing and not undergoing aSCT (11 patients with unclear transplantation status excluded)
| . | Allogeneic transplantation (n = 45) . | No allogeneic transplantation (n = 50) . |
|---|---|---|
| Sex | ||
| Male | 50% | 64% |
| Age at t-AML/MDS diagnosis | ||
| Median (range) | 41 (20-68) | 55 (18-74) |
| Time from HL treatment to diagnosis of t-AML/MDS | ||
| Median (range), months | 30 (11-101) | 28 (7-124) |
| Cytogenetics/molecular genetic alterations reported | 39 (87%) | 20 (40%) |
| Normal karyotype | 6 | 0 |
| Alterations of chromosomes 5/7 | 8 | 0 |
| MLL rearrangement | 13 | 5 |
| Complex karyotype | 3 | 11 |
| Other genetic alterations | 9 | 4 |
| Response to induction therapy reported | 35 (78%) | N/A |
| CR | 23 | |
| Blast reduction, but no CR | 3 | |
| Refractory/relapsed | 7 | |
| No induction therapy | 2 |
| . | Allogeneic transplantation (n = 45) . | No allogeneic transplantation (n = 50) . |
|---|---|---|
| Sex | ||
| Male | 50% | 64% |
| Age at t-AML/MDS diagnosis | ||
| Median (range) | 41 (20-68) | 55 (18-74) |
| Time from HL treatment to diagnosis of t-AML/MDS | ||
| Median (range), months | 30 (11-101) | 28 (7-124) |
| Cytogenetics/molecular genetic alterations reported | 39 (87%) | 20 (40%) |
| Normal karyotype | 6 | 0 |
| Alterations of chromosomes 5/7 | 8 | 0 |
| MLL rearrangement | 13 | 5 |
| Complex karyotype | 3 | 11 |
| Other genetic alterations | 9 | 4 |
| Response to induction therapy reported | 35 (78%) | N/A |
| CR | 23 | |
| Blast reduction, but no CR | 3 | |
| Refractory/relapsed | 7 | |
| No induction therapy | 2 |
N/A, not available.
Information on the treatment applied for t-AML/MDS was available for 95 patients. Among these 95 patients, 45 patients underwent aSCT and 50 patients received conventional chemotherapy or supportive care only (Table 4).
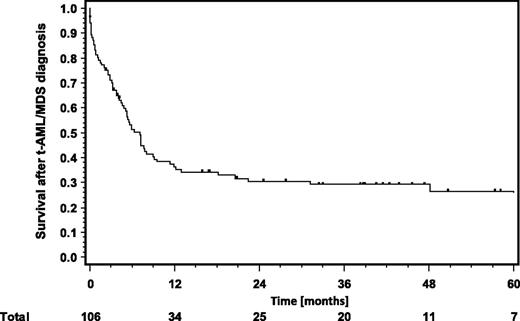
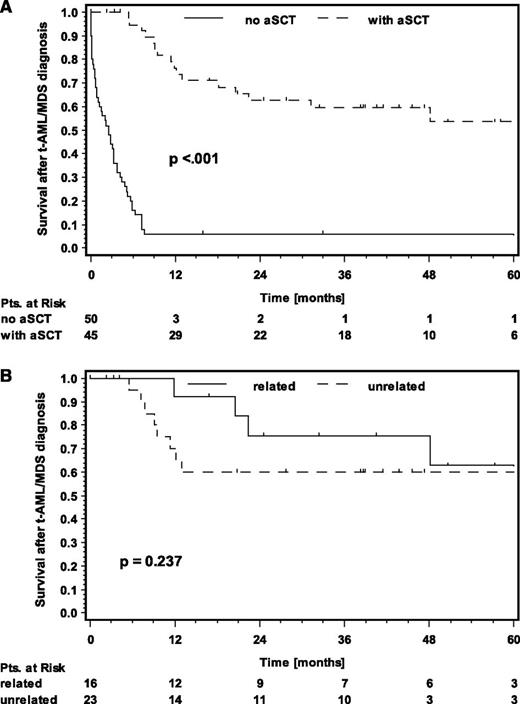
In general, OS for patients with t-AML/MDS was poor, with a median of 7.2 months (Figure 2). The major cause of death was the secondary malignancy itself. However, t-AML/MDS patients who underwent aSCT had a significantly better outcome with a median OS not reached after a median follow-up of 41 months (P < .001) (Figure 3). This correlates with the finding that patients younger than 40 years at t-AML/MDS diagnosis have a significantly better prognosis than those age 40 years or older, because patients undergoing aSCT were significantly younger than those treated with conventional chemotherapy or receiving supportive care only (39 years vs 54 years, P = .0002) (supplemental Figure 1, available on the Blood Web site, Table 4). No significant OS differences were seen between patients with related or unrelated donors (P = .237) (Figure 3). The amount of BEACOPP chemotherapy and the sex did also not influence the outcome of t-AML/MDS.
OS. (A) OS after t-AML/MDS diagnosis in patients undergoing aSCT (dashed line) or not undergoing aSCT (solid line); (B) OS after t-AML/MDS diagnosis in patients undergoing aSCT with an unrelated donor (dashed line) or a related donor (solid line).
OS. (A) OS after t-AML/MDS diagnosis in patients undergoing aSCT (dashed line) or not undergoing aSCT (solid line); (B) OS after t-AML/MDS diagnosis in patients undergoing aSCT with an unrelated donor (dashed line) or a related donor (solid line).
Information on the response to induction chemotherapy before conditioning treatment and aSCT was available for 35 of 45 patients (78%) undergoing this treatment modality. Cytomorphologically, 23/35 patients (66%) had complete remission (CR), 3/35 patients (9%) had blast reduction but no CR, 7/35 patients (20%) were refractory or had relapsed disease, and 2 patients had not received induction chemotherapy before conditioning treatment was initiated (Table 4).
Discussion
To our knowledge, this is the largest analysis on incidence, outcome, and risk factors for the development of t-AML/MDS among HL patients treated within controlled clinical studies. It is also the first study on t-AML/MDS after HL including a larger number of patients receiving intensive BEACOPP-containing first-line HL therapy. Overall, 11 952 patients treated within GHSG trials for newly diagnosed HL were considered. The major findings were as follows: t-AML/MDS after HL was a rare event; there was a significant association between the intensity of chemotherapy for HL and the risk of developing t-AML/MDS; t-AML/MDS mostly presented with unfavorable cytogenetic and molecular genetic features; although the overall outcome of t-AML/MDS after HL was still poor, patients proceeding to aSCT had a significantly improved outcome when compared with former analyses.
At the time of HL diagnosis, patients who subsequently developed t-AML/MDS were significantly older than the whole patient group (43 years vs 34 years). This observation is in line with previous studies such as a large population-based study including 35 511 HL patients treated between 1970 and 2001. This study reported a significantly higher risk for the development of t-AML/MDS among patients diagnosed with HL age 35 or older as compared with patients younger than age 35 at HL diagnosis.22
Among the patients included in this analysis, 60% of t-AML/MDS cases occurred within the first 3 years after HL treatment. Median time from HL treatment to t-AML/MDS diagnosis was 31 months. However, more than 20% of t-AML/MDS cases were diagnosed 5 to 10 years after HL treatment, a fact underscoring the necessity of long-term follow-up in HL patients. Overall, the latency period observed in the present study was relatively short in comparison with older analyses on t-AML/MDS after HL such as 2 Italian studies from 1986 and 1998. These studies including patients mainly treated with mechlorethamine, vincristine, procarbazine, and prednisone (MOPP) and ABVD-based protocols had reported median times of 55 months and 60 months from HL treatment to the diagnosis of t-AML.23,24 The difference between our study and the Italian analyses may at least in part be due to the more frequent use of the etoposide-containing BEACOPP protocol among the patients included in the present study. Etoposide and other topoisomerase-II inhibitors typically induce t-AML with a short latency period of about 2 years after exposure; in contrast, t-AML/MDS induced by alkylating agents usually occur 5 to 8 years after exposure.4 In addition to the latency periods, t-AML/MDS can often be attributed to either alkylating agents or topoisomerase-II inhibitors on the basis of characteristic genetic alterations. Alkylating agents typically induce alterations of chromosome 5 and/or chromosome 7, t-AML after exposure to topoisomerase-II inhibitors is associated with a rearrangement of the MLL gene in many cases. Among the 61 t-AML/MDS patients with known genetic characteristics included in this analysis, MLL rearrangement indeed was the genetic alteration most often observed (19/61 patients); alterations of chromosome 5 and/or chromosome 7 and complex karyotypes were documented in 8/61 and 14/61 patients, respectively. A normal karyotype was found in 7/61 patients. These findings are consistent with a recent analysis on patients diagnosed with t-AML after different primary malignancies reporting a significantly increased proportion of patients with unfavorable genetic alterations as compared with de novo AML.7
Our study revealed an increased risk of developing t-AML/MDS for patients treated with 4 or more cycles of BEACOPPescalated when compared with patients receiving less than 4 cycles of BEACOPPescalated or no BEACOPP chemotherapy. This finding is not unexpected because the cumulative doses of leukemogenic drugs appear to correlate with the risk of developing t-AML/MDS. As shown in a recent analysis from Stanford reporting incidence and outcome of t-AML/MDS among 754 HL patients treated within 3 trial generations between 1974 and 2003, reduction of the cumulative doses of alkylating agents resulted in a significant decrease of the t-AML/MDS rate. The reduction of RT fields and doses over decades probably also had an impact on the lower t-AML/MDS incidence among patients treated for HL after 1989.25 In an older analysis, patients receiving consolidating RT after MOPP chemotherapy had a significantly higher risk for developing t-AML/MDS when compared with patients who had received chemotherapy alone.23 Another report evaluating the role of the extent of the RT field revealed a significantly increased t-AML/MDS risk for patients who had more extended RT after MOPP chemotherapy in comparison with those who had limited RT after the same chemotherapy.26 Within GHSG studies for newly diagnosed advanced HL, the proportion of patients receiving consolidating RT after chemotherapy could be reduced from 71% in the HD9 trial to 11% in the HD15 trial. This reduction correlated with a substantial decrease of the 5-year t-AML/MDS rate.16,27 Within the HD15 trial, only 0.3% of patients randomized into arm B and treated with 6 cycles of BEACOPPescalated followed by localized RT of metabolically active residual lymphoma larger than 2.5 cm developed t-AML/MDS at a median follow-up of 48 months.16 Therefore, 6 cycles of BEACOPPescalated followed by localized RT of metabolically active residual lymphoma has become our standard of care in patients up to 60 years with newly diagnosed advanced HL, although chemotherapy with 4 or more cycles of escalated BEACOPP appears to be associated with an increased risk to develop t-AML/MDS. In our opinion, this increased risk is outweighed by the improved efficacy of BEACOPPescalated. Three smaller prospective randomized trials have consistently shown a significantly better tumor control with a tendency toward a superior OS and a recent network meta-analysis including 9993 patients revealed a significantly superior OS for BEACOPPescalated when compared with ABVD with a 5-year advantage of 10%.28,,-31
Within the present study, the overall outcome after t-AML/MDS diagnosis was poor with a median OS of 7.2 months and thus not significantly different from a previous GHSG analysis on t-AML/MDS including HL patients mainly treated with COPP/ABVD-based proocols between 1981 and 1998.3 However, while 44/46 patients with t-AML/MDS included in the older GHSG analysis had died after 24 months irrespective of the treatment modality applied, the present study included a relevant number of young patients responding to chemotherapy and ultimately proceeding to aSCT who enjoyed a significantly improved OS. This finding is in agreement with analyses on t-AML/MDS performed by other groups. A German study reported a 4-year OS rate of 42.6% for t-AML/MDS patients undergoing aSCT or other intensive consolidation therapy in first CR; the 2-year OS rate after aSCT was 37% among t-AML/MDS patients included in an Italian analysis.7,32 The largest study on allogeneic transplantation for t-AML/MDS came from the United States. Outcome of 868 patients undergoing allogeneic bone marrow transplantation or aSCT for t-AML/MDS after different solid tumors and hematological malignancies was analyzed. At 1 and at 5 years, OS rates were 37% and 22%, respectively. Transplantation at age 35 years or younger was associated with an improved outcome as compared with patients older than 35 years.6 These results are consistent with those from the present study, which also revealed a favorable clinical outcome particularly for younger patients treated with aSCT.
In conclusion, t-AML/MDS represent rare but severe late sequelae of HL treatment still associated with a poor prognosis, although selected patients can be rescued by aSCT. Thus, novel treatment strategies for HL should aim at a reduction of chemotherapy and RT in order to reduce the risk for the development of t-AML/MDS. Several trials evaluating such a treatment reduction on the basis of interim positron emission tomography have been initiated, and results from some of these studies recently became available.16,33 In addition, further optimization of aSCT may increase cure rates among patients diagnosed with t-AML/MDS after HL who are eligible for this treatment modality.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.A.E., I.T., H.H., J.F., P.B., and A.E. designed research and analyzed and interpreted data; D.A.E., I.T., K.B., T.H., B.K., V.D., S.S., A.R., M.F., B.B., B.v.T., P.B., and A.E. provided study material or patients; D.A.E., H.H., J.F., V.D., M.F., P.B., and A.E. provided administrative support; D.A.E., I.T., J.F., and A.E. wrote the paper; and all authors collected and assembled data and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financials interests.
Correspondence: Andreas Engert, First Department of Internal Medicine, University Hospital Cologne, Kerpener Strasse 62, D-50937 Cologne, Germany; e-mail: a.engert@uni-koeln.de.